Published online Mar 14, 2014. doi: 10.3748/wjg.v20.i10.2641
Revised: November 28, 2013
Accepted: January 2, 2014
Published online: March 14, 2014
Processing time: 202 Days and 17.4 Hours
AIM: To investigate the effects of sodium alginate (AL-Na) on indomethacin-induced small intestinal lesions in rats.
METHODS: Gastric injury was assessed by measuring ulcerated legions 4 h after indomethacin (25 mg/kg) administration. Small intestinal injury was assessed by measuring ulcerated legions 24 h after indomethacin (10 mg/kg) administration. AL-Na and rebamipide were orally administered. Myeloperoxidase activity in the stomach and intestine were measured. Microvascular permeability, superoxide dismutase content, glutathione peroxidase activity, catalase activity, red blood cell count, white blood cell count, mucin content and enterobacterial count in the small intestine were measured.
RESULTS: AL-Na significantly reduced indomethacin-induced ulcer size and myeloperoxidase activity in the stomach and small intestine. AL-Na prevented increases in microvascular permeability, superoxide dismutase content, glutathione peroxidase activity and catalase activity in small intestinal injury induced by indomethacin. AL-Na also prevented decreases in red blood cells and white blood cells in small intestinal injury induced by indomethacin. Moreover, AL-Na suppressed mucin depletion by indomethacin and inhibited infiltration of enterobacteria into the small intestine.
CONCLUSION: These results indicate that AL-Na ameliorates non-steroidal anti-inflammatory drug-induced small intestinal enteritis via bacterial translocation.
Core tip: Sodium alginate ameliorates small intestinal enteritis via bacterial translocation.
-
Citation: Yamamoto A, Itoh T, Nasu R, Nishida R. Sodium alginate ameliorates indomethacin-induced gastrointestinal mucosal injury
via inhibiting translocation in rats. World J Gastroenterol 2014; 20(10): 2641-2652 - URL: https://www.wjgnet.com/1007-9327/full/v20/i10/2641.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i10.2641
It is well-known that non-steroidal anti-inflammatory drugs (NSAIDs) damage the stomach. Small bowel injuries from these drugs have also been reported. Patients who are on long-term NSAIDs treatment develop mucosal injuries of the small intestine, including bleeding, erosion, and ulcers[1-4]. In rats, indomethacin, one of the most commonly used NSAIDs, causes significant gastrointestinal damage[5,6], and several enteropathic consequences have been identified, including bacterial translocation[7], neutrophil activation[8], oxidative stress[9], and mucin deficiency[10].
Proton pump inhibitors (PPIs) significantly improve NSAIDs-induced pathologies of the gastrointestinal tract[11]. However, PPI may not demonstrate a therapeutic effect on NSAIDs-induced small bowel disease because gastric acid contributes little to small intestinal ulceration[12,13]. Moreover, it has been reported that PPIs exacerbate NSAIDs-induced small intestinal injury in rats by causing dysbiosis[14]. Therefore, drugs that protect against NSAID-induced gastrointestinal injury are needed. Rebamipide, a mucosal protective agent, has been reported to suppress NSAIDs-induced intestinal injury[15,16]. However, the development of a better therapeutic agent is needed. It was reported that rebamipide, one of mucosal protective agent, suppressed NSAIDs-induced intestinal injury[15,16]. But, the development of more therapeutic agent is demanded.
Sodium alginate (AL-Na) is a polysaccharide with homopolymeric blocks of (1-4)-linked β-D-mannuronate, and its C-5 epimer α-L-glucuronate residues are widely distributed in the cell walls of brown algae. AL-Na elicits a muco-protective effect by covering the surface of the gastrointestinal tract. Therefore, it may be useful for the treatment of gastric and esophageal ulcers and bleeding[17-22]. Recently, it was reported that AL-Na may be an efficacious, non-erosive treatment for reflux disease[23], and it may also be a useful in the treatment of upper gastrointestinal disease. Moreover, Humphreys et al[24] reported that AL-Na was poorly absorbed, primarily through the gastrointestinal tract, and was excreted in the feces. Therefore, AL-Na may be effective in both the upper and lower gastrointestinal tracts. Additionally, AL-Na is reportedly an effective treatment for experimental colitis[25,26] and for radiation-induced colon damage[27,28]. Therefore, we hypothesised that AL-Na may ameliorate small intestinal damage and evaluated its effects on indomethacin-induced small intestinal injuries in rats.
Six-week-old male Sprague-Dawley rats (body weights of 160-200 g) were purchased from Japan SLC, Shizuoka, Japan. Animals were maintained in an air-conditioned room with controlled temperature (24 °C ± 2 °C) and humidity (55% ± 15%). They were housed in steel cages with a 12 h light-dark cycle (lights on from 0700 to 1900 h). Food and water were freely available except during test periods. All animal handling procedures were conducted in accord with the guidelines for Animal Experiments of Sakai Chemical Industry.
Animals were fasted for 18 h, orally administered indomethacin (Wako, Japan) at a dose of 25 mg/kg and sacrificed after 6 h. Stomachs were removed, inflated by injecting 10 mL of 2% formalin for 10 min to fix the tissue walls, and opened along the greater curvature. Visible hemorrhagic lesions were examined, and the areas (mm2) of visible lesions were calculated using Image J software.
Animals were not fasted, orally administered indomethacin at a dose of 10 mg/kg and sacrificed after 24 h under deep ether anesthesia. The small intestines were removed, and the organ length and wet weight were measured. Evans blue (1 mL; Sigma Aldrich Corp., St. Louis, MO, United States) was intravenously injected into the animals 30 min before sacrifice. The small intestines were opened along the anti-mesenteric attachment and examined for lesions, and the areas (mm2) of visible lesions were calculated using Image J software. Blood sampling was conducted before Evans blue injection, and hematocyte numbers were determined using an automated blood cell counter (KX-21NV; Sysmex, Japan).
AL-Na was obtained from Kyosei pharmaceutical (Japan), and low molecular weight AL-Na was provided by Kaigen (Japan). AL-Na and low molecular weight AL-Na were dissolved in distilled water. Rebamipide (Mucosta; Otsuka Pharmaceutics, Japan) was suspended in a 0.5% carboxymethylcellulose (CMC-Na; Wako) solution. In the small intestinal studies, AL-Na (250 and 500 mg/kg), low molecular weight AL-Na (500 mg/kg), rebamipide (100 mg/kg), and CMC-Na (250 mg/kg) were orally administered 30 min before and 6 h after treatment with indomethacin[29]. Indomethacin-treated control animals were administered distilled water at the same time.
Myeloperoxidase (MPO) activity in the stomach and small intestine were measured. The animals were sacrificed under deep ether anesthesia, and the stomachs and small intestines were removed. After rinsing the tissues with saline, the mucosa was scraped, weighed, and homogenised in 50 mmol/L phosphate buffer containing 0.5% hexadecyl trimethyl ammonium bromide (HTAB, pH 6.0; Sigma). Homogenised samples were frozen, thawed twice and then centrifuged at 8000 g for 20 min at 4 °C. Supernatants were then assayed using a fluorometric detection kit (Assay Designs, Taiwan). Changes in fluorescence were measured using a plate reader with 545 nm excitation and 590 nm emission filters (ARVOsx; Wallac, United States).
Microvascular permeability was evaluated in intestinal mucosa following treatment with indomethacin by measuring the amount of extravasated Evans blue dye.
After the removal of ileal tissue, the tissue was diluted with 1N KOH (0.7 mL) at 37 °C for 24 h. Acetone-phospholic acid was then added, and the sample was shaken. The sample was prepared after deposit filtration. The quantity of accumulated dye in the sample dilution was measured after 30 min using a spectrophotometer (JASCO; V-560, Japan) at 620 nm and was expressed as μg per 100 mg wet tissue.
After rinsing the ileal tissues with saline, the mucosa was scraped, weighed, and homogenised in 50 mmol/L phosphate buffer containing 5 mmol/L Tris-HCl buffer solution (pH 7.4), 5 mmol/L EDTA, and 1 mmol/L 2-mercaptoethanol. After centrifugation at 15000 g for 20 min at 4 °C, the superoxide dismutase (SOD) content of the supernatants was determined using a superoxide dismutase ELISA kit (Northwest Life Science Specialties; NWLSS, United States). Subsequently, the glutathione peroxidase (GPx) and catalase activities were measured using a glutathione peroxidase assay kit (NWLSS) and a catalase activity assay kit (BioVision, United States). The absorbance was measured at 450, 340, and 570 nm using a plate reader (iEMS reader MF; Labsystems, Finland).
Rats were anesthetised with diethyl ether, and the small intestines were excised. The luminal contents were collected by flushing with 15 mL of ice-cold PBS (pH 7.4) containing 0.02 mol/L sodium azide and the same volume of air. The contents were freeze-dried and stored for luminal mucin analysis. Total freeze-dried samples were suspended in a sodium chloride solution (0.15 mol/L) containing 0.02 mol/L sodium azide at 4 °C. The samples were homogenised for 1 min and immediately centrifuged at 10000 g for 30 min to obtain the supernatant. Mucin was recovered as a 60% ethanol precipitate of the supernatant and was dissolved in 3.0 mL of distilled water for analyses using a MUC2 enzyme-linked immunosorbent assay kit (USCN life science, China). Absorption at 450 nm was measured using a plate reader (ARVOsx).
After rinsing the ileal tissues with saline, the mucosa was scraped, weighed and homogenised in 1 mL of sterile PBS per 100 mg of wet tissue. Aliquots of the homogenate were placed on blood agar and Gifu anaerobic medium (GAM) agar (Nissui, Osaka, Japan). Blood agar plates were incubated at 37 °C for 24 h under aerobic conditions, and GAM agar plates were incubated at 37 °C for 24 h under anaerobic conditions (BBL GasPack Pouch Anaerobic System, BectonDickinson, MD). Both types of plates, containing between 10 and 200 colony-forming units (CFU), were analysed and summed to determine the total number of enterobacteria, and the number of enterobacteria that were present the small intestine was expressed as log CFU/g tissue.
Stomach and ileal tissues were immediately fixed in 10% neutral buffered formalin. After fixation, the materials were dehydrated with ethanol, cleared using xylene, and embedded in paraffin. From these specimens, 3 μm paraffin sections were stained with hematoxylin and eosin (HE) and periodic acid-Schiff (PAS) for immunohistochemical analyses. The sections were cut, dewaxed, rehydrated, and immersed in methanol containing 0.3% (w/v) H2O2 for 30 min to inactivate endogenous peroxidases. Sections were incubated overnight at 4 °C with monoclonal mouse anti-proliferating cell nuclear antigen (PCNA; Dako, Denmark). After washing with PBS, the slides were incubated for 30 min with biotinylated horse anti-mouse serum (Vector, Burlingame, United States) followed by avidin-conjugated horseradish peroxidase (Vector, Burlingame, United States). The enzyme activity was detected using DAB (3,3’-diaminobenzidine).
Blood samples were collected 1 h after administration of indomethacin for evaluation of its absorption. The concentration of indomethacin in plasma was measured using high-performance liquid chromatography (HPLC). The HPLC system consisted of an AS-2055 injector, a UV-2070 detector, and a PU-2080 chromatographic pump (Nihonbunkoh, Japan). Separation was achieved on a reversed-phase column (4.6 mm × 250 mm, 3 μm, ODS, Waters, United States). The mobile phase was acetonitrile-phosphoric acid (60:40 v/v), and the flow-rate was 0.8 mL/min. The chromatogram was monitored at a wavelength of 254 nm throughout the experiments. Blood samples were centrifuged at 500 g for 20 min and subsequently at 1000 g for 20 min. The resulting plasma was mixed with 0.15 mL of acetonitrile containing 5 μg/mL of the internal standard, mefenamic acid. Denatured protein precipitates were separated using a solid-phase extraction cartridge (Waters). The extract was evaporated at 40 °C under N2 gas, and 100 μL of the supernatant was redistilled into the mobile phase and injected into the HPLC column.
All data are presented as the mean ± SE. Statistical analyses were performed using one-way analysis of variance (ANOVA) with Dunnett’s test or Student’s unpaired t test. Significant differences were indicated by P values less than 0.05.
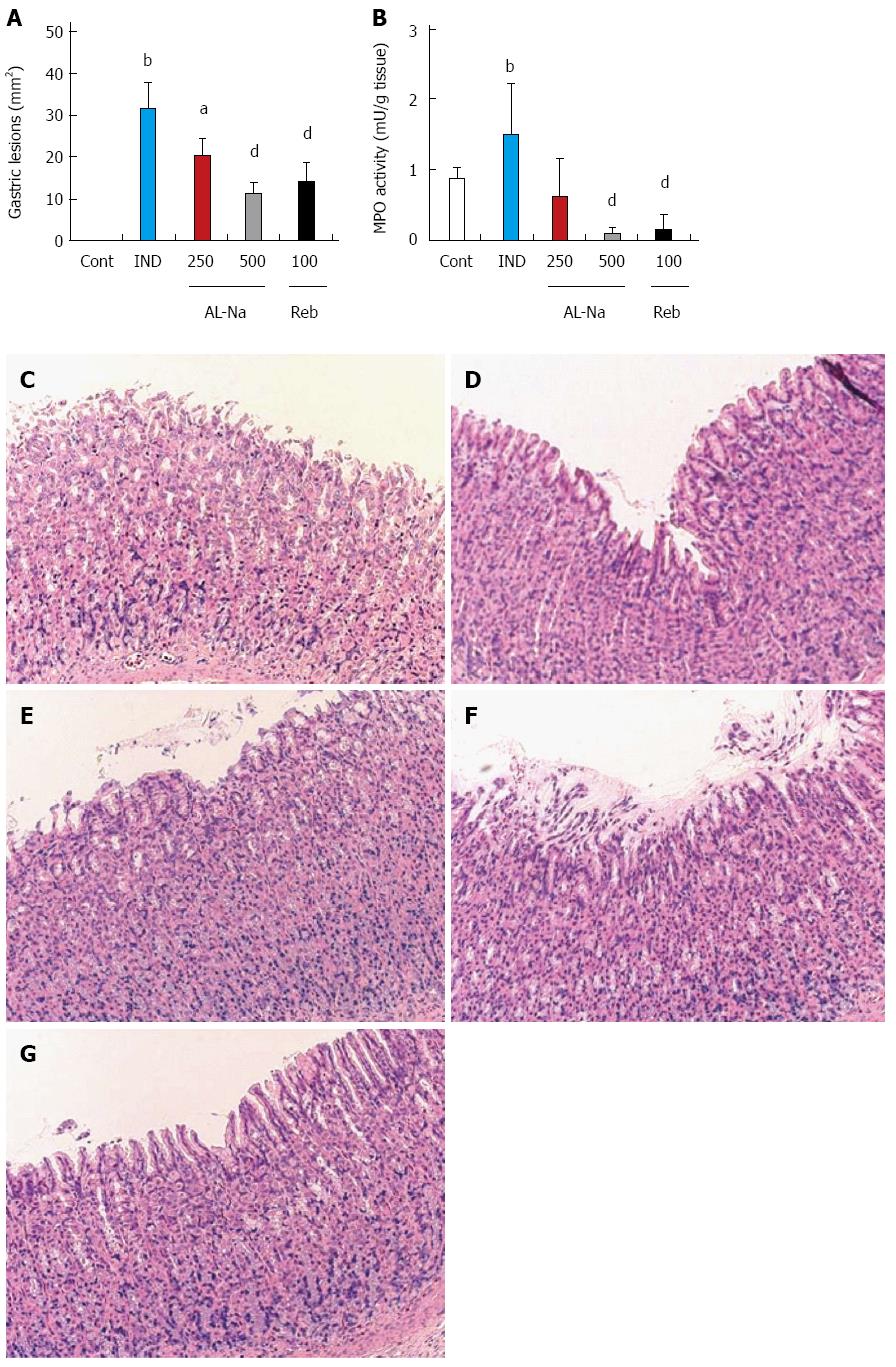
Administration of indomethacin at 25 mg/kg caused severe hemorrhagic lesions covering the entire glandular area of the stomach (Figure 1A). Oral treatment with AL-Na at 250 and 500 mg/kg significantly reduced the areas of indomethacin-related ulcers relative to those of indomethacin-treated control animals. Rebamipide at 100 mg/kg also reduced indomethacin-induced gastric lesions. Figure 1B shows the effect of AL-Na on gastric MPO induction by indomethacin. A single oral dose of 500 mg/kg AL-Na significantly inhibited indomethacin-mediated increases in MPO activity. Moreover, 100 mg/kg rebamipide also reduced indomethacin-induced MPO activity. Histological comparisons of treated and untreated tissues indicated that indomethacin caused exfoliation of gastric epithelial cells and disrupted the mucosal layer of the stomach (Figure 1D), compared with the controls (Figure 1C). Administration of AL-Na alleviated the ulceration induced by indomethacin (Figure 1E and F). Rebamipide also ameliorated indomethacin-induced gastric injury (Figure 1G).
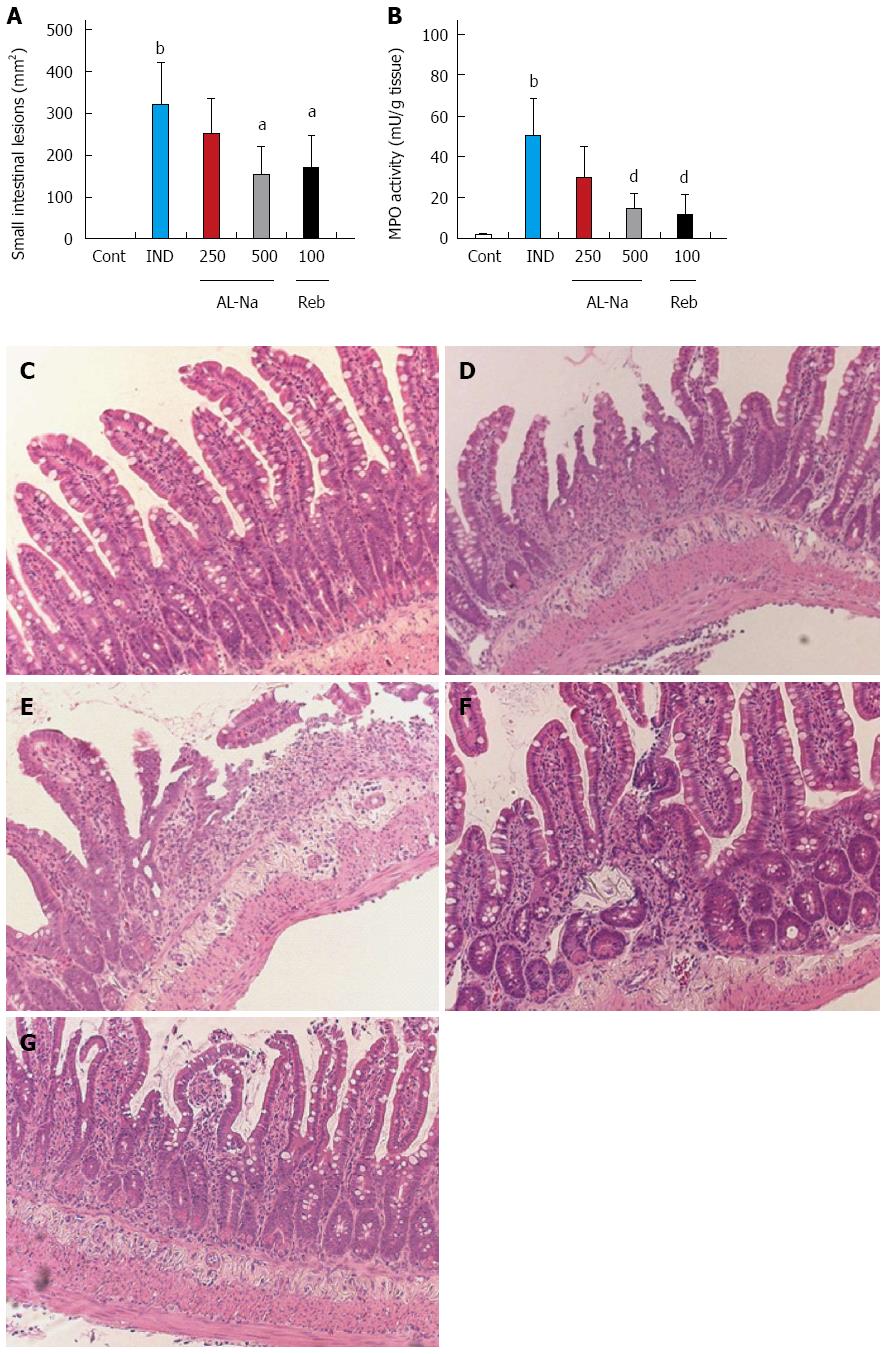
Administration of indomethacin at 10 mg/kg caused severe hemorrhagic lesions in the small intestine, primarily the jejunum and ileum (Figure 2A). Oral treatment with AL-Na at 500 mg/kg significantly reduced the areas of indomethacin-related ulcers compared to those of the indomethacin-treated control animals. Rebamipide at 100 mg/kg also reduced indomethacin-induced small intestinal lesions. Figure 2B shows the effect of AL-Na on intestinal MPO induction by indomethacin. A single oral dose of 500 mg/kg AL-Na significantly inhibited indomethacin-mediated increases in MPO activity. Moreover, 100 mg/kg rebamipide also reduced indomethacin-induced MPO activity. Histological comparisons of treated and untreated tissues indicated that indomethacin caused an inflammatory reaction that was characterised by epithelial losses; ulcers; inflammatory infiltration into the lamina propria, submucosa, and serosa; and shortening of crypts (Figure 2D) compared with indomethacin-untreated groups (Figure 2C). The severity of these inflammatory reactions was reduced in animals treated with AL-Na (Figure 2E and F). Rebamipide also ameliorated indomethacin-induced small intestinal injury (Figure 2G).
Indomethacin caused decreases in body weight, daily food intake, and feces weight. These changes were almost completely ameliorated by treatment with AL-Na (Table 1), with significant differences observed in the decreases of body weight, food intake, and feces weight between indomethacin-treated control and AL-Na-treated (500 mg/kg) animals. In contrast, rebamipide did not prevent decreases in body weight, food intake, or feces weight.
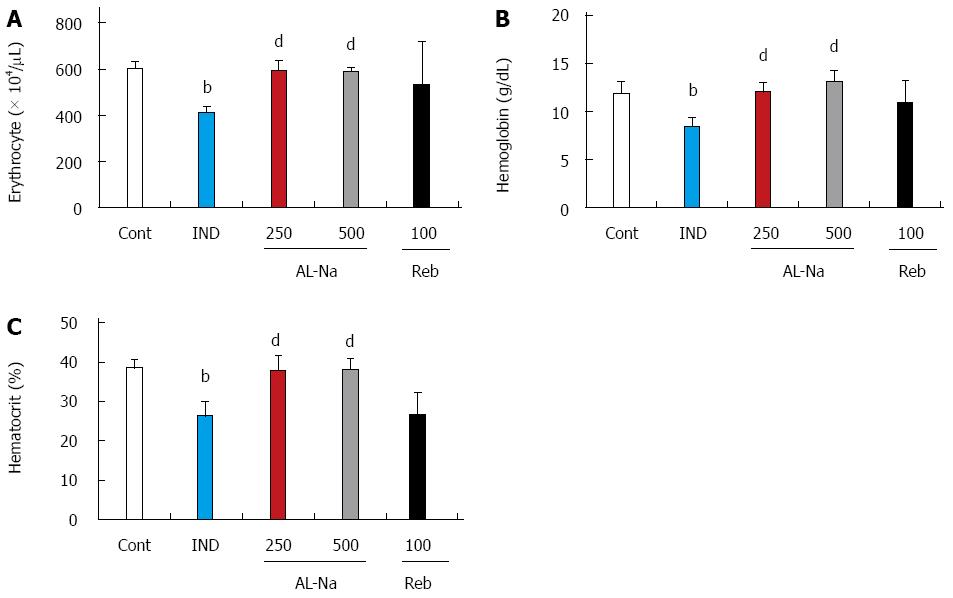
Figure 3 shows the effects of AL-Na on indomethacin-induced anemia. The administration of 10 mg/kg indomethacin lead to decreased blood cell numbers. The number of erythrocytes, hemoglobin levels, and hematocrit were significantly reduced compared with those in untreated rats (Figure 3A-C). Oral administration of AL-Na at 250 or 500 mg/kg significantly preserved the erythrocyte numbers, hemoglobin levels, and hematocrit. In contrast, rebamipide at 100 mg/kg had no significant effect on these parameters.
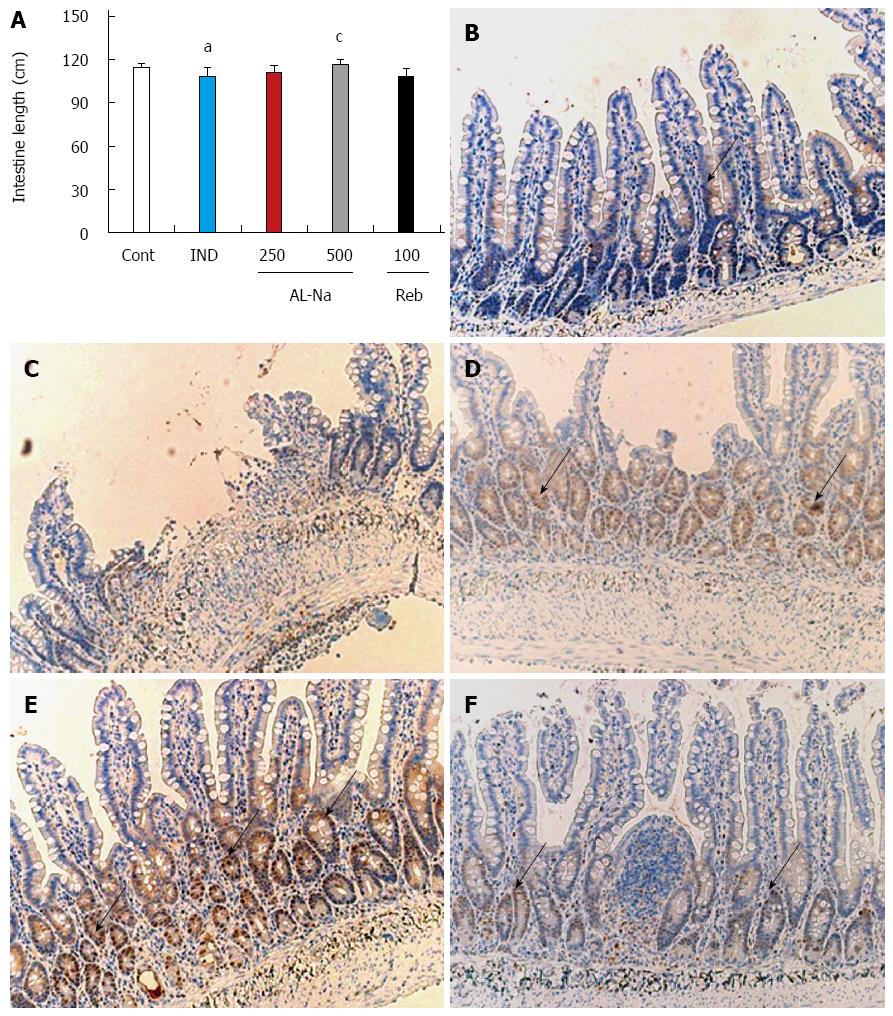
Figure 4A shows the effects of AL-Na on indomethacin-induced atrophy of the small intestine. The administration of indomethacin reduced the length of the small intestines compared with those of the untreated animals. A dose of 500 mg/kg AL-Na significantly ameliorated the losses in intestine length compared with those of indomethacin-treated control rats. However, a dose of 100 mg/kg rebamipide showed no significant effect. To confirm these data, we performed immunostaining for PCNA. As shown in Figure 4B, only a few crypt cells were positive for PCNA in the animals treated with indomethacin alone, and disintegration of the crypt structures was observed (Figure 4C). In contrast, strong PCNA staining was detected in ileal crypts of animal tissues treated with AL-Na (500 mg/kg) (Figure 4D). However, rebamipide at 100 mg/kg had no effect on PCNA staining in indomethacin-treated control animals (Figure 4F).
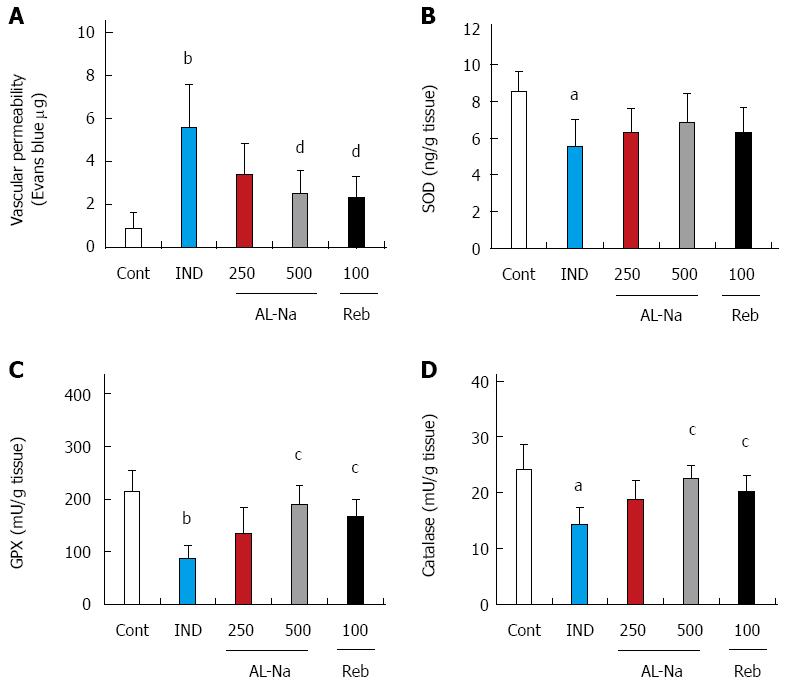
Figure 5 shows the effects of AL-Na on vascular permeability and oxidative stress induced by indomethacin. Indomethacin significantly increased ileal vascular permeability (Figure 5A). Oral doses of 500 mg/kg AL-Na or 100 mg/kg rebamipide significantly inhibited indomethacin-induced vascular permeability. At a dose of 10 mg/kg, indomethacin significantly reduced the SOD, GPx, and catalase activities in ileal tissues (Figure 5B-D). At 500 mg/kg, AL-Na significantly restored the GPx and catalase activities in indomethacin-treated control rats. At 100 mg/kg, rebamipide also inhibited indomethacin-mediated decreases in the GPx and catalase activities.
Plasma indomethacin concentrations were measured using HPLC. In plasma from indomethacin-treated control animals, indomethacin was detected at 30.7 ± 7.3 mg/mL. In animals that were treated with AL-Na at 250 mg/kg or 500 mg/kg, the indomethacin concentrations were not different from those in indomethacin-treated control animals, with 32.5 ± 7.0 mg/mL and 31.5 ± 5.8 mg/mL detected, respectively. Rebamipide treatment also had no effect, with 31.5 ± 5.7 mg/mL indomethacin detected in the plasma of these animals.
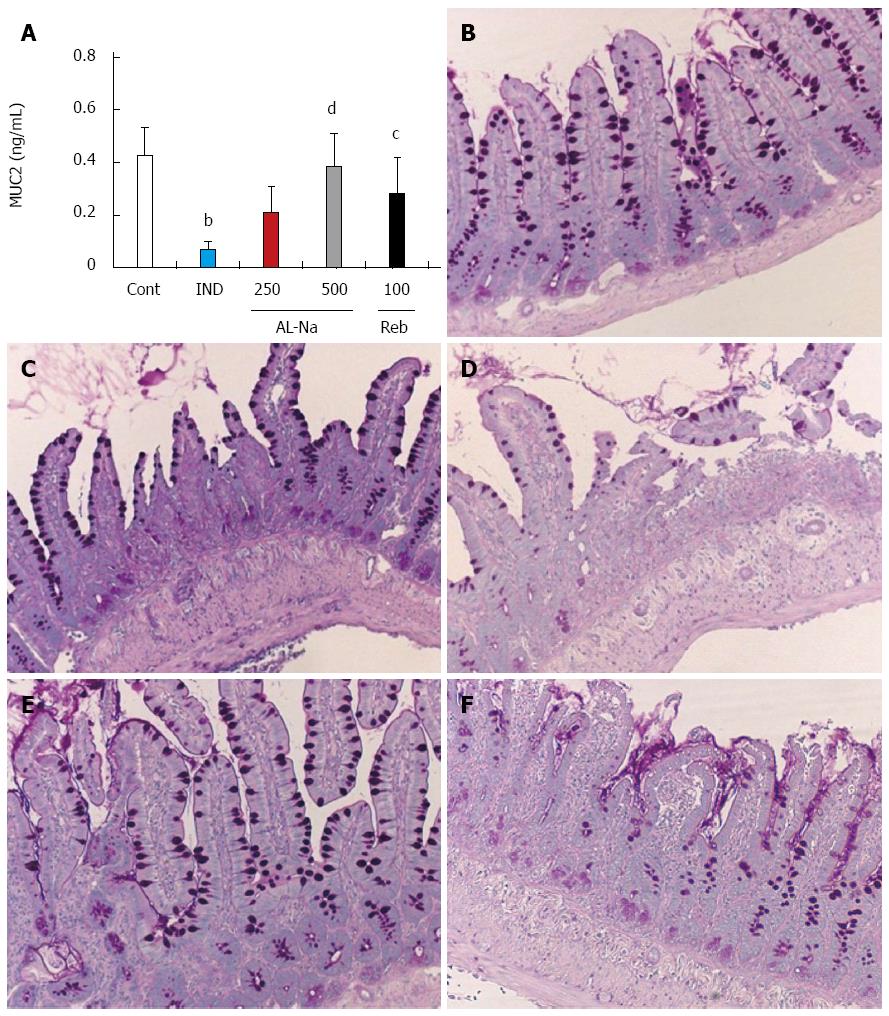
Figure 6A shows the effects of AL-Na on indomethacin-induced mucin depletion. Administration of 10-mg/kg indomethacin decreased the MUC2 protein levels. Oral doses of 500 mg/kg AL-Na significantly inhibited this decrease in MUC2 protein. Rebamipide at 100 mg/kg also reduced the indomethacin-induced decreases in MUC2 protein levels. Hence, we examined goblet cells, which are the major source of mucin, through the PAS staining of ileal tissues. Indomethacin-treated control animals showed depleted goblet cell numbers (Figure 6C) compared to untreated animals (Figure 6B). Treatment with AL-Na or rebamipide ameliorated this indomethacin-induced deficiency (Figure 6D-F).
Indomethacin caused a marked increase in the mucosal invasion of enterobacteria (Table 2). Administration of AL-Na (500 mg/kg) significantly decreased the number of enterobacteria compared with the indomethacin-treated control group.
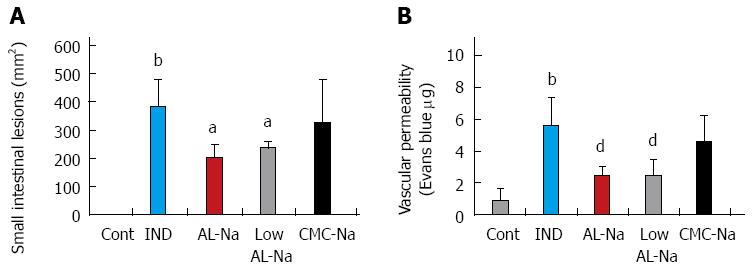
Oral administration of 5% low molecular weight (7.26 ± 0.6 mPas) or original AL-Na solutions (360 ± 4.4 mPas) at a dose of 500 mg/kg significantly reduced indomethacin-induced small intestinal lesions and vascular permeability compared with those of indomethacin-treated control rats (Figure 7). However, treatments with 2.5% CMC-Na solutions, which show similar viscosity to original AL-Na (CMC-Na, 376 ± 5.6 mPas), did not affect indomethacin-induced small intestinal lesions or vascular permeability.
Previous studies have demonstrated that AL-Na layers cover lesions, inhibit the lytic actions of pepsin and hydrochloric acid, and protect the mucosal surface of the upper gastrointestinal tract[17]. These studies also show that AL-Na enhances the production of gastric hexosamine in hydrochloric acid-induced gastric ulcers in rats[30]. Presently, we confirmed the effects of AL-Na on indomethacin-induced gastric ulcers, and additional protection from indomethacin-induced small intestinal injury, symptoms of anemia, reduction of intestinal length, increases in inflammatory response, and oxidative stress in small intestine has been observed. has been observed. Next, we tested the influence of AL-Na on indomethacin plasma concentration, and results in AL-Na had no influence. Therefore, the effect of AL-Na on indomethacin-induced gastrointestinal injury is not thought to be involved in the inhibition of indomethacin absorption. Several studies have reported intestinal bleeding and chronic anemia associated with indomethacin[2,31]. Moreover, decreased haemoglobin levels have been reported as a consequence of indomethacin treatment in rodents[32]. In the present study, AL-Na also inhibited indomethacin-induced decreases in haemoglobin levels, whereas rebamipide showed no significant effect. Daigo et al[19] reported that AL-Na precipitated fibrinogen and increased fibrin polymerisation. They also demonstrated enhanced aggregation of platelets following AL-Na treatment[20]. Hence, we suggest that AL-Na elicits mucoprotective effects and a hemostatic effect in the lower gastrointestinal tract in addition to protective effect on stomach.
Subsequently, we focused on the effects of AL-Na in small intestinal mucosa. The importance of mucus to the physiological defence mechanisms of the gastrointestinal tract is well documented[33]. Mucin comprises highly glycosylated large glycoproteins with protein backbone structures that are rich in serine and threonine and are linked to a wide variety of O-linked oligosaccharides[34]. It has been reported that indomethacin decreases the mucus content of the small intestine in rats[12]. In addition, higher magnification of the surface mucus gel layer of the small intestine has been reported during the healing process of NSAIDs-induced enteritis in rats[35,36]. Therefore, it is clear that mucin plays an important role in NSAID-induced gastrointestinal disease. Barcelo et al[37] reported that AL-Na induced mucin secretion in rat colon. Therefore, we hypothesised that mucin induction by AL-Na is the main mechanism involved in the healing of NSAIDs-induced enteritis. Mucin genes are broadly classified as secretory or membrane-associated. MUC2, MUC5AC, MUC5B, and MUC6 have been identified as gel-forming secretory mucin proteins. MUC2 is the major mucin produced by the goblet cells of the intestinal mucosa[38]. In stomach, AL-Na increased hexosamine levels, which are glycoprotein constituting gastric mucus[30]. Next, we examined MUC2 production in rat small intestines and showed that AL-Na inhibited indomethacin-induced MUC2 reduction in goblet cell.
Mucin plays an important role in intestinal barrier function[36] and in protection from bacterial translocation[39]. Bacterial translocation is the one of main sources of pathogenicity arising from NSAIDs-induced enteritis[7]. In these studies, the mucin inducer AL-Na affected bacterial infiltration into the small intestine, and administration of AL-Na prevented an increase in the number of enterobacteria in ileal tissue. These data suggest that AL-Na-induced MUC2 production in intestinal goblet cells defended against the infiltration of enterobacteria from small intestinal injuries caused by NSAIDs, in addition to protective effect on stomach.
Satoh et al[40] reported that foods containing soluble, but not insoluble, dietary fibers ameliorated the induction of intestinal lesions by indomethacin. Subsequently, they reported a strong correlation between the viscosity and the muco-protective actions of soluble dietary fibers. Hence, we also tested the effects of low molecular AL-Na and CMC-Na, which have low viscosities, on indomethacin-induced small intestinal injury. Low molecular AL-Na also prevented small intestinal injury, but CMC-Na did not. Hence, we suggest that the protective effects of AL-Na are independent of viscosity. Barcelo et al[37] also reported that AL-Na increased the secretion of mucin, but not cellulose, in control rat colons. They reported that glucuronic acid and galacturonic acid also increased mucin content[37] and indicated that uronic acid, which is a major constituent of AL-Na, may play an important role in mucus secretion.
In conclusion, AL-Na prevented indomethacin-induced lesions in the stomach and small intestines of rats via inhibiting bacterial translocation. In addition, the results suggest that the therapeutic effects of AL-Na are independent of its viscosity. Therefore, AL-Na may be an effective treatment for NSAID-induced gut and small intestinal mucosal injury.
We would like to thank Koji Takeuchi (Professor of Kyoto Pharmaceutical University, Kyoto, Japan) and Kikuko Amagase (Assistant professor of Kyoto Pharmaceutical University) for technical support and important advice.
Non-steroidal anti-inflammatory drugs (NSAIDs) damage the stomach. Small bowel injuries from these drugs have also been reported. Patients who are on long-term NSAIDs treatment develop mucosal injuries of the small intestine, including bleeding, erosion, and ulcers. Therefore, the development of a better therapeutic agent is needed. It was reported that rebamipide, one of mucosal protective agent, suppressed NSAIDs-induced intestinal injury. But, the development of more therapeutic agent is demanded.
Sodium alginate (AL-Na) elicits a muco-protective effect by covering the surface of the gastrointestinal tract. Therefore, it may be useful for the treatment of gastric and esophageal ulcers and bleeding. In this study, the authors demonstrate that the effect of AL-Na on NSAIDs-induced enteropathy in rats.
AL-Na is a useful in the treatment of upper gastrointestinal disease. Therefore, AL-Na may be effective in both the upper and lower gastrointestinal tracts. AL-Na is reportedly an effective treatment for experimental colitis and for radiation-induced colon damage. Therefore, the authors hypothesised that AL-Na may ameliorate small intestinal damage and evaluated its effects on indomethacin-induced small intestinal injuries in rats. This is the first study to report that the effect of AL-Na on small intestinal injury.
The study results suggest that the AL-Na is a potentially therapeutic agenta that could be used for preventing NSAIDs-induced small intestinal enteritis via bacterial translocation.
AL-Na is a polysaccharide with homopolymeric blocks of (1-4)-linked β-D-mannuronate, and its C-5 epimer α-L-glucuronate residues are widely distributed in the cell walls of brown algae.
This is a good descriptive study in which the authors analyzed the preventive effect of AL-Na on enteropathy induced by NSAIDs in rats. The results are interesting and suggest that AL-Na is a potential therapeutic agent that could be used for small intestinal injury induced by NSAIDs.
P- Reviewers: Nakayama Y, Xia SH, Yamamoto H, Zhang XC S- Editor: Wen LL L- Editor: A E- Editor: Liu XM
| 1. | Hatazawa R, Ohno R, Tanigami M, Tanaka A, Takeuchi K. Roles of endogenous prostaglandins and cyclooxygenase isozymes in healing of indomethacin-induced small intestinal lesions in rats. J Pharmacol Exp Ther. 2006;318:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 390] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 3. | Rimbaş M, Marinescu M, Voiosu MR, Băicuş CR, Caraiola S, Nicolau A, Niţescu D, Badea GC, Pârvu MI. NSAID-induced deleterious effects on the proximal and mid small bowel in seronegative spondyloarthropathy patients. World J Gastroenterol. 2011;17:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Higuchi K, Umegaki E, Watanabe T, Yoda Y, Morita E, Murano M, Tokioka S, Arakawa T. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44:879-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Anthony A, Pounder RE, Dhillon AP, Wakefield AJ. Vascular anatomy defines sites of indomethacin induced jejunal ulceration along the mesenteric margin. Gut. 1997;41:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Takeuchi K, Miyazawa T, Tanaka A, Kato S, Kunikata T. Pathogenic importance of intestinal hypermotility in NSAID-induced small intestinal damage in rats. Digestion. 2002;66:30-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Porras M, Martín MT, Yang PC, Jury J, Perdue MH, Vergara P. Correlation between cyclical epithelial barrier dysfunction and bacterial translocation in the relapses of intestinal inflammation. Inflamm Bowel Dis. 2006;12:843-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Okayama T, Yoshida N, Uchiyama K, Takagi T, Ichikawa H, Yoshikawa T. Mast cells are involved in the pathogenesis of indomethacin-induced rat enteritis. J Gastroenterol. 2009;44 Suppl 19:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Omatsu T, Naito Y, Handa O, Mizushima K, Hayashi N, Qin Y, Harusato A, Hirata I, Kishimoto E, Okada H. Reactive oxygen species-quenching and anti-apoptotic effect of polaprezinc on indomethacin-induced small intestinal epithelial cell injury. J Gastroenterol. 2010;45:692-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Kunikata T, Tanaka A, Miyazawa T, Kato S, Takeuchi K. 16,16-Dimethyl prostaglandin E2 inhibits indomethacin-induced small intestinal lesions through EP3 and EP4 receptors. Dig Dis Sci. 2002;47:894-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Scheiman JM, Yeomans ND, Talley NJ, Vakil N, Chan FK, Tulassay Z, Rainoldi JL, Szczepanski L, Ung KA, Kleczkowski D. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101:701-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 347] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 13. | Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 437] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 14. | Wallace JL, Syer S, Denou E, de Palma G, Vong L, McKnight W, Jury J, Bolla M, Bercik P, Collins SM. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314-1322, 1322.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 340] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 15. | Mizukami K, Murakami K, Abe T, Inoue K, Uchida M, Okimoto T, Kodama M, Fujioka T. Aspirin-induced small bowel injuries and the preventive effect of rebamipide. World J Gastroenterol. 2011;17:5117-5122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Diao L, Mei Q, Xu JM, Liu XC, Hu J, Jin J, Yao Q, Chen ML. Rebamipide suppresses diclofenac-induced intestinal permeability via mitochondrial protection in mice. World J Gastroenterol. 2012;18:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Daigo K, Wada Y, Yamada C, Yamaji M, Okuda S, Okada M, Miyazato T. [Pharmacological studies of sodium alginate. I. Protective effect of sodium alginate on mucous membranes of upper-gastrointestinal tract]. Yakugaku Zasshi. 1981;101:452-457. [PubMed] |
| 18. | Daigo K, Yamada C, Wada Y, Yamaji M, Okuda S, Okada M, Miyazato T. [Pharmacological studies of sodium alginate. II. Hemostatic effect of sodium alginate on gastrointestinal bleeding]. Yakugaku Zasshi. 1981;101:458-463. [PubMed] |
| 19. | Daigo K, Yamaji M, Yamada C, Wada Y, Okuda S, Okada M, Miyazato T. Pharmacological studies of sodium alginate. III. Acceleration of fibrin formation by sodium alginate. Yakugaku Zasshi. 1981;101:464-469. [PubMed] |
| 20. | Diago K, Yamada C, Yamaji M, Nakagiri N, Okada M, Komiya H, Horiuchi Y, Miyazato T. [Pharmacological studies of sodium alginate. V. Effect of sodium alginate on platelet aggregation]. Yakugaku Zasshi. 1985;105:171-182. [PubMed] |
| 21. | Poynard T, Vernisse B, Agostini H. Randomized, multicentre comparison of sodium alginate and cisapride in the symptomatic treatment of uncomplicated gastro-oesophageal reflux. Aliment Pharmacol Ther. 1998;12:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Dettmar PW, Sykes J, Little SL, Bryan J. Rapid onset of effect of sodium alginate on gastro-oesophageal reflux compared with ranitidine and omeprazole, and relationship between symptoms and reflux episodes. Int J Clin Pract. 2006;60:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Manabe N, Haruma K, Ito M, Takahashi N, Takasugi H, Wada Y, Nakata H, Katoh T, Miyamoto M, Tanaka S. Efficacy of adding sodium alginate to omeprazole in patients with nonerosive reflux disease: a randomized clinical trial. Dis Esophagus. 2012;25:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Humphreys ER, Triffitt JT. Absorption by the rat of alginate labelled with carbon-14. Nature. 1968;219:1172-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Mirshafiey A, Khodadadi A, Rehm BH, Khorramizadeh MR, Eslami MB, Razavi A, Saadat F. Sodium alginate as a novel therapeutic option in experimental colitis. Scand J Immunol. 2005;61:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Razavi A, Khodadadi A, Eslami MB, Eshraghi S, Mirshafiey A. Therapeutic effect of sodium alginate in experimental chronic ulcerative colitis. Iran J Allergy Asthma Immunol. 2008;7:13-18. [PubMed] |
| 27. | Nakatsugawa S, Yukawa Y, Abe M. [Radioprotective effects of sodium alginate on radiation induced intestinal damage]. Nihon Gan Chiryo Gakkai Shi. 1988;23:1025-1030. [PubMed] |
| 28. | Oshitani T, Okada K, Kushima T, Suematsu T, Obayashi K, Hirata Y, Takada Y, Ishida T, Yoshida M, Narabayashi I. [Clinical evaluation of sodium alginate on oral mucositis associated with radiotherapy]. Nihon Gan Chiryo Gakkai Shi. 1990;25:1129-1137. [PubMed] |
| 29. | Mizoguchi H, Ogawa Y, Kanatsu K, Tanaka A, Kato S, Takeuchi K. Protective effect of rebamipide on indomethacin-induced intestinal damage in rats. J Gastroenterol Hepatol. 2001;16:1112-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Daigo K, Yamaji M, Yamada C, Matsutani K, Yagi M, Nakajima Y. Curative effect of sodium alginate on experimental hydrochloric acid and tetra-gastrin induced ulcer. Japanese Pharmacol Ther. 1982;10:281-289. |
| 31. | Davies NM, Saleh JY, Skjodt NM. Detection and prevention of NSAID-induced enteropathy. J Pharm Pharm Sci. 2000;3:137-155. [PubMed] |
| 32. | Tugendreich S, Pearson CI, Sagartz J, Jarnagin K, Kolaja K. NSAID-induced acute phase response is due to increased intestinal permeability and characterized by early and consistent alterations in hepatic gene expression. Toxicol Pathol. 2006;34:168-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Allen A, Flemström G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol. 2005;288:C1-19. [PubMed] |
| 34. | Allen A. Structure of gastrointestinal mucus glycoproteins and the viscous and gel-forming properties of mucus. Br Med Bull. 1978;34:28-33. [PubMed] |
| 35. | Iwai T, Ichikawa T, Goso Y, Ikezawa T, Saegusa Y, Okayasu I, Saigenji K, Ishihara K. Effects of indomethacin on the rat small intestinal mucosa: immunohistochemical and biochemical studies using anti-mucin monoclonal antibodies. J Gastroenterol. 2009;44:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Iwai T, Ichikawa T, Kida M, Goso Y, Saegusa Y, Okayasu I, Saigenji K, Ishihara K. Vulnerable sites and changes in mucin in the rat small intestine after non-steroidal anti-inflammatory drugs administration. Dig Dis Sci. 2010;55:3369-3376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Barcelo A, Claustre J, Moro F, Chayvialle JA, Cuber JC, Plaisancié P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 267] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 38. | Sharma R, Young C, Neu J. Molecular modulation of intestinal epithelial barrier: contribution of microbiota. J Biomed Biotechnol. 2010;2010:305879. [PubMed] |
| 39. | Frankel W, Zhang W, Singh A, Bain A, Satchithanandam S, Klurfeld D, Rombeau J. Fiber: effect on bacterial translocation and intestinal mucin content. World J Surg. 1995;19:144-148; discussion 148-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Satoh H. Role of dietary fiber in formation and prevention of small intestinal ulcers induced by nonsteroidal anti-inflammatory drug. Curr Pharm Des. 2010;16:1209-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |