Published online May 14, 2013. doi: 10.3748/wjg.v19.i18.2781
Revised: March 27, 2013
Accepted: April 10, 2013
Published online: May 14, 2013
Processing time: 98 Days and 4.6 Hours
AIM: To investigate role of putative mitogen-activated protein kinase activator with WD40 repeats (MAWD)/MAWD binding protein (MAWBP) in gastric cancer (GC).
METHODS: MAWBP and MAWD mRNA expression level was examined by real-time reverse transcriptase-polymerase chain reaction and semi-quantitative polymerase chain reaction in six GC cell lines. Western blotting was used to examine the protein expression levels. We developed GC cells that stably overexpressed MAWBP and MAWD, and downregulated expression by RNA interference assay. Proliferation and migration of these GC cells were analyzed by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide (MTT), soft agar, tumorigenicity, migration and transwell assays. The effect of expression of MAWBP and MAWD on transforming growth factor (TGF)-β1-induced epithelial-mesenchymal transition (EMT) was examined by transfection of MAWBP and MAWD into GC cells. We detected the levels of EMT markers E-cadherin, N-cadherin and Snail in GC cells overexpressing MAWBP and MAWD by Western blotting. The effect of MAWBP and MAWD on TGF-β signal was detected by analysis of phosphorylation level and nuclear translocation of Smad3 using Western blotting and immunofluorescence.
RESULTS: Among the GC cell lines, expression of endogenous MAWBP and MAWD was lowest in SGC7901 cells and highest in BGC823 cells. MAWBP and MAWD were stably overexpressed in SGC7901 cells and knocked down in BGC823 cells. MAWBP and MAWD inhibited GC cell proliferation in vitro and in vivo. MTT assay showed that overexpression of MAWBP and MAWD suppressed growth of SGC7901 cells (P < 0.001), while knockdown of these genes promoted growth of BGC823 cells (P < 0.001). Soft agar colony formation experiments showed that overexpression of MAWBP and MAWD alone or together reduced colony formation compared with vector group in SGC7901 (86.25 ± 8.43, 12.75 ± 4.49, 30 ± 6.41 vs 336.75 ± 22.55, P < 0.001), and knocked-down MAWBP and MAWD demonstrated opposite effects (131.25 ± 16.54, 88.75 ± 11.12, 341.75 ± 22.23 vs 30.25 ± 8.07, P < 0.001). Tumorigenicity experiments revealed that overexpressed MAWBP and MAWD inhibited GC cell proliferation in vivo (P < 0.001). MAWBP and MAWD also inhibited GC cell invasion. Transwell assay showed that the number of traverse cells of MAWBP, MAWD and coexpression group were more than that in vector group (84 ± 16.57, 98.33 ± 9.8, 29 ± 16.39 vs 298 ± 11.86, P < 0.001). Coexpression of MAWBP and MAWD significantly decreased the cells traversing the matrix membrane. Conversely, knocked-down MAWBP and MAWD correspondingly promoted invasion of GC cells (100.67 ± 14.57, 72.66 ± 8.51, 330.67 ± 20.55 vs 27 ± 11.53, P < 0.001). More importantly, coexpression of MAWBP and MAWD promoted EMT. Cells that coexpressed MAWBP and MAWD displayed a pebble-like shape and tight cell-cell adhesion, while vector cells showed a classical mesenchymal phenotype. Western blotting showed that expression of E-cadherin was increased, and expression of N-cadherin and Snail was decreased when cells coexpressed MAWBP and MAWD and were treated with TGF-β1. Nuclear translocation of p-Smad3 was reduced by attenuating its phosphorylation.
CONCLUSION: Coexpression of MAWBP and MAWD inhibited EMT, and EMT-aided malignant cell progression was suppressed.
Core tip: Our previous study revealed that mitogen-activated protein kinase activator with WD40 repeats (MAWD) and MAWD binding protein (MAWBP), acting as a complex, were differentially expressed in gastric cancer (GC) tissues compared with that in normal gastric tissues. The present study provided direct evidence that MAWBP and MAWD inhibited proliferation and migration of GC cells. Importantly, interaction of MAWBP and MAWD influenced expression of epithelial-mesenchymal transition (EMT) markers induced by transforming growth factor (TGF)-β1 in GC cells. It indicated that coexpression of MAWBP and MAWD inhibited TGF-β1-induced EMT, thus suppressing EMT-aided GC malignant progression.
- Citation: Li DM, Zhang J, Li WM, Cui JT, Pan YM, Liu SQ, Xing R, Lu YY. MAWBP and MAWD inhibit proliferation and invasion in gastric cancer. World J Gastroenterol 2013; 19(18): 2781-2792
- URL: https://www.wjgnet.com/1007-9327/full/v19/i18/2781.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i18.2781
Gastric cancer (GC) is the second most common cause of cancer death worldwide and is especially common in China[1]. Multiple factors are involved in the development of GC carcinogenesis. Molecular genetic studies have addressed accumulation of multiple genes and proteins alteration involved in GC development[2,3]. Investigation of GC biomarkers has focused on discovering differential protein signatures and has explored their biology mechanisms. The genes involved include activation of c-myc, erbB-2, c-met, and k-ras[4-7] oncogenes and inactivation of tumor suppressor genes p53, APC, E-cadherin and RUNX3[8-10].
Our laboratory previously found (using 2D gel electrophoresis and mass spectrometry) that expression of mitogen-activated protein kinase activator with WD40 repeats (MAWD) and MAWD binding protein (MAWBP) were differentially expressed in GC tissues. MAWD interacts with MAWBP and forms complexes in GC cell lines[11], which suggests that these proteins are involved in GC carcinogenesis. Combined analysis of MAWBP and MAWD expression would provide useful information in uncovering their roles in GC.
The proteins MAWBP and MAWD were discovered in 2000 and 2001, respectively[12,13]. MAWD is widely expressed in many tissues and sequence analysis has indicated that MAWD contains a WD40 repeat domain. Datta et al[14] have shown that MAWD-homolog protein serine-threonine kinase receptor-associated protein (STRAP) recruits Smad7, forming a complex that increases inhibition of transforming growth factor (TGF)-β signaling. Iriyama et al[13] tried to detect MAWD-related protein using a conventional two-hybrid technique and found MAWBP had an affinity for MAWD. The effects of MAWD in cancer have been reported in breast, colon and lung cancer but views about its role in cancer are divergent[15]. However, there is no current report on the function of MAWD in GC, and little is known about MAWBP other than its affinity for MAWD.
We hypothesize that MAWBP and MAWD interactions have a key role in GC tumorigenesis, and therefore investigated their biological function in GC cell lines. We found that these two proteins inhibited cell proliferation, and coexpression of MAWBP and MAWD obviously suppressed migration as well as invasive behavior of GC cells. Recent evidence implies that epithelial-mesenchymal transition (EMT) contributes to cancer progression, invasion and metastasis in various cancers[16,17]. TGF-β is the main and best-characterized inducer of EMT during embryogenesis and cancer pathogenesis[18]. MAWBP and MAWD are involved in the TGF-β signaling pathway[14]. We further sought to determine whether coexpression of MAWBP and MAWD could inhibit TGF-β1-induced EMT.
The canonical EMT program is characterized by complex proteome changes, leading to loss of epithelial markers such as E-cadherin, and expression of mesenchymal markers such as vimentin and N-cadherin[19]. Transcriptional regulator Snail is also activated in EMT. TGF-β signaling regulates expression of Snail, SOX2 and SOX4[20].
In this study, we analyzed the effect of MAWBP and MAWD on expression of E-cadherin, N-cadherin and Snail. We further demonstrated the relationship of this effect with TGF-β signalling pathway via detection of the phosphorylation level and nuclear translocation of Smad3. Our findings suggest that coexpression of MAWBP and MAWD inhibits TGF-β1-induced EMT and suppresses EMT-aided GC cell invasion.
GC cell lines BGC823, MGC803, SGC7901, AGS, N87 and MKN45 were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco BRL, Gaithersburg, MD, United States), supplemented with 5% fetal bovine serum (FBS). All cell lines were maintained at 37 °C in 5% CO2 as previously described[21,22].
We constructed MAWBP and MAWD expression plasmids using pcDNA3.1B(-). Total RNA was extracted from 19-wk-old fetal liver. MAWBP and MAWD cDNA was produced by reverse-transcriptase polymerase chain reaction (RT-PCR). The reaction was initiated by 5-min incubation at 94 °C; 35 cycles of 94 °C for 45 s, 56 °C for 45 s, 72 °C for 60 s; and terminated after a 10-min extension at 72 °C. Products were purified by gel extraction. Recombinant plasmids were transferred into Escherichia coli DH5α, and identified by restriction enzymes digestion and sequencing analysis. Then, we constructed MAWBP and MAWD short hairpin RNA (shRNA) plasmids. Oligonucleotides were annealed and ligated to pSilencer3.1-H1-Neo. All of the primers are shown on Table 1.
| Target gene | Primer ID | Sequence (5'-3') |
| MAWBP (132 bp) | Forward | GGGTCTGCACACGCTGTTC |
| Reverse | TAATGTCAACCCTTCCGTCT | |
| MAWD (162 bp) | Forward | GGGACAGGATAAACTTTAGC |
| Reverse | AGCATGATCCCAAAGTCGAAC | |
| MAWBP (867 bp) | Forward | AACTTGGTCGACCAGCTTGCAAGGAAAATG |
| Reverse | ATAACTCGAGCTAGGCTGTCAGTGTGCC | |
| MAWD (1053 bp) | Forward | CGCGGATCCATGGCAATGAGACAGACG |
| Reverse | CCCAAGCTTTCAGGCCTTAACATCAGG | |
| β-actin (510 bp) | Forward | CGGGAAATCGTGCGTGACATT |
| Reverse | CTAGAAGCATTTGCGGTGGAC | |
| β-actin (150 bp) | Forward | TTAGTTGCGTTACACCCTTTC |
| Reverse | ACCTTCACCGTTCCAGTTT | |
| MAWD (shRNA) | Ps-F1 | GATCCGCTTATGGACGATCTATTGCTTCAAGAGAGCAATAGATCGTCCATAAGTTTTTTGGAAA |
| Ps-R1 | AGCTTTTCCAAAAAACTTATGGACGATCTATTGCTCTCTTGAAGCAATAGATCGTCCATAAGCG | |
| MAWBP (shRNA) | Ps-F1 | GATCCGTAGCACGCTCACGTTTGTCTTCAAGAGAGACAAACGTGAGCGTGCTATTTTTTGGAAAAGC |
| Ps-R1 | AGCTTGGCGTAATCATGGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCACAATTCCACACA |
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, United States), and subjected (5 μg) to RT-PCR (Table 1). The internal control, β-actin, was processed with all specimens simultaneously. Real-time PCR was performed using Applied Biosystem 7500 Real-Time PCR System (Foster City, CA, United States). Data were analyzed using the relative standard curve method.
Proteins were extracted from cells for western blotting. Proteins (50 μg) were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinyl difluoride membranes (Bio-Rad, Hercules, CA, United States). Immunoreactivity was tested with anti-MAWD (1:500, our laboratory), anti-MAWBP (1:500, our laboratory)[11], E-cadherin (1:500, BD, Franklin Lakes, NJ, United States), N-cadherin (1:500, BD), Snail (1:500, Cell Signaling, Danvers, MA, United States), diluted in blocking buffer at 4 °C overnight. The signal was detected by Super Signal West Dura Extended Duration Substrate (Thermo Scientific, Rockford, IL, United States).
SGC7901 cells were transfected with overexpression plasmids, while BGC823 cells were transfected with shRNA plasmids. Cells were cultured at 60%-70% confluence in 35-mm plates and were transfected using Lipofectamine 2000 (Invitrogen). Except that mono-plasmids and empty vector were transfected into GC cells, overexpressed plasmids of MAWBP and MAWD were cotransfected into SGC7901 cells. shRNA plasmids of MAWBP and MAWD were cotransfected into BGC823 cells. At 48 h post-transfection, cells were seeded for 21 d in selection medium containing 400 μg/mL G418, to screen for stable clones. The efficacy of transfection was identified by RT-PCR and Western blotting.
Stable transfected cells (1 × 103) in 200 μL DMEM supplemented with 5% FBS were seeded in duplicate into each well of 96-well culture plates, and 10 μL 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide (MTT, Gen-View, Jacksonville, FL, United States) (5 mg/mL) was added at 0, 24, 48, 72 and 96 h. The MTT was removed after 4 h incubation, and 100 μL dimethylsulfoxide (Amresco, Solon, OH, United States) was pipetted into each well and incubated for 30 min. Absorbance was measured at 570 nm using an iMark Microplate Reader (Bio-Rad, Hercules, CA, United States).
Cells (3 × 103) were trypsinized and resuspended in 4 mL 0.3% agar in DMEM containing 10% FBS, and overlaid with 0.6% agar in 60-mm culture dishes. The dishes were incubated routinely for 21 d. Colonies were stained with 0.2% p-iodonitrotetrazolium violet, photographed, and counted.
Animal experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Ethics Committee of Peking University. All efforts were made to minimize suffering. Transfected cells were resuspended in 1× Hank’s Buffer at a concentration of 5 × 105 cells/mL. A 100-μL suspension was injected subcutaneously into the left dorsal flank of 15 5-wk-old female nude mice, and the right side was inoculated with GC cells transfected by vector alone as a control. The mice were checked every 3 d for tumor appearance, and the large (a) and small (b) diameters of the palpable tumors were recorded for tumor volume calculation according to a × b2× 0.5.
Cells were cultured at 80%-90% confluence in 60-mm dishes. Cells were scratched with a pipette tip to produce a straight line. The detached cells were washed three times with phosphate buffered solution (PBS) and incubated for a further 24 h. The scratched gap was inspected at 0, 2, 4, 6, 12 and 24 h. Photographs were taken at × 200 magnification, using a TS100 inverted microscope (Nikon, Tokyo, Japan).
The invasion assay was performed using a BD Matrigel Invasion Chamber. Cells (1 × 105) were suspended in serum-free DMEM and seeded on matrix membranes. DMEM supplemented with 10% FBS was used as a chemoattractant. After 48 h incubation, cells were fixed with methanol and stained with crystal violet for 20 min. Cells that penetrated the membrane were counted.
Cells were grown on glass slides, washed with PBS, fixed in methanol for 10 min, and processed for immunofluorescence. Cells were exposed to anti-p-Smad3 overnight at 4 °C, incubated for 1 h with rhodamine-conjugated anti-rabbit secondary antibodies, and nuclei were stained with 4’,6-diamidino-2-phenylindole. Cells were studied with a confocal fluorescence imaging microscope (TCS-SP5; Leica, Mannheim, Germany).
Cells were starved for 24 h, and then incubated in 5% FBS–DMEM containing 2 or 4 ng/mL TGF-β for 24 or 48 h. Plasminogen activator inhibitor (PAI)-1 promoter assays were used to select the optimum TGF-β conditions. Transfected cells were cultured in 5% FBS-DMEM containing 4 ng/mL TGF-β for 24 h. PhosphoSafe Extraction Reagent (Merck, San Diego, CA, United States) was used to extract phosphoprotein. P-Smad3 (1:1000, Abcam, Cambridge, United Kingdom) and p-Smad2 (1:500, Millipore, Temecula, CA, United States) were analyzed by western blotting as described above. Smad2 (1:500, Bioworld, Boston, MA, United States) and Smad3 (1:500, Bioworld) were detected at the same time. We then separated the cytosolic and nuclear fractions according to the protocol of the Nuclear-Cytosol Extraction Kit (Applygen Technologies Inc., Beijing, China) and detected p-Smad3 levels. Nuclear translocation ability of p-Smad3 (1:50) was analyzed by confocal microscopy as described above.
The study has been examined and approved ethically by the Ethics Committee of Beijing Cancer Hospital.
Statistical analysis used SPSS version 16.0. Student’s t test and analysis of variance were used for data measurement. Quantitative values were presented as mean ± SD. Differences with P < 0.05 were considered statistically significant.
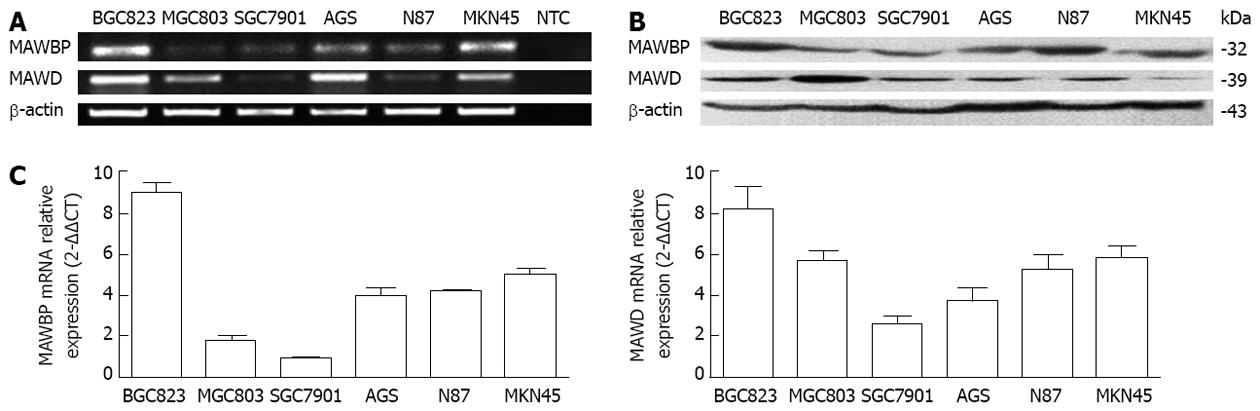
BGC823, MGC803, SGC7901, AGS, N87 and MKN45 GC cell lines were used to detect MAWBP and MAWD expression, which was found to differ between the cell types. RT-PCR and real-time PCR revealed low levels of endogenous MAWBP and MAWD mRNA in SGC7901 cells but high levels in BGC823 cells, and Western blotting confirmed these results (Figure 1). These two cell lines were selected for the following experiments.
To investigate further biological function of MAWBP and MAWD in GC cells, MAWBP-pcDNA3.1 and MAWD-pcDNA3.1, alone or in combination, and empty vector were transfected into SGC7901 cells, in which these two proteins were expressed at a lower level compared with other GC cells. G418-resistant clones were isolated, which were stably transfected cells. These cells were termed as MAWBP, MAWD, MAWBP/D (MAWBP and MAWD cotransfected cells) and vector, respectively. shRNA plasmid MAWBP-pSilencer3.1 and MAWD-pSilencer3.1, alone or in combination, and empty vector were transfected into BGC823 cells, in which they were expressed at a higher level compared with other GC cells. The stably transfected cells were termed as sh-MAWBP, sh-MAWD, sh-MAWBP/D (MAWBP and MAWD co-downregulated groups) and sh-vector, respectively. We analyzed MAWBP and MAWD expression at the mRNA and protein levels by semi-quantitative RT-PCR and Western blotting, respectively.
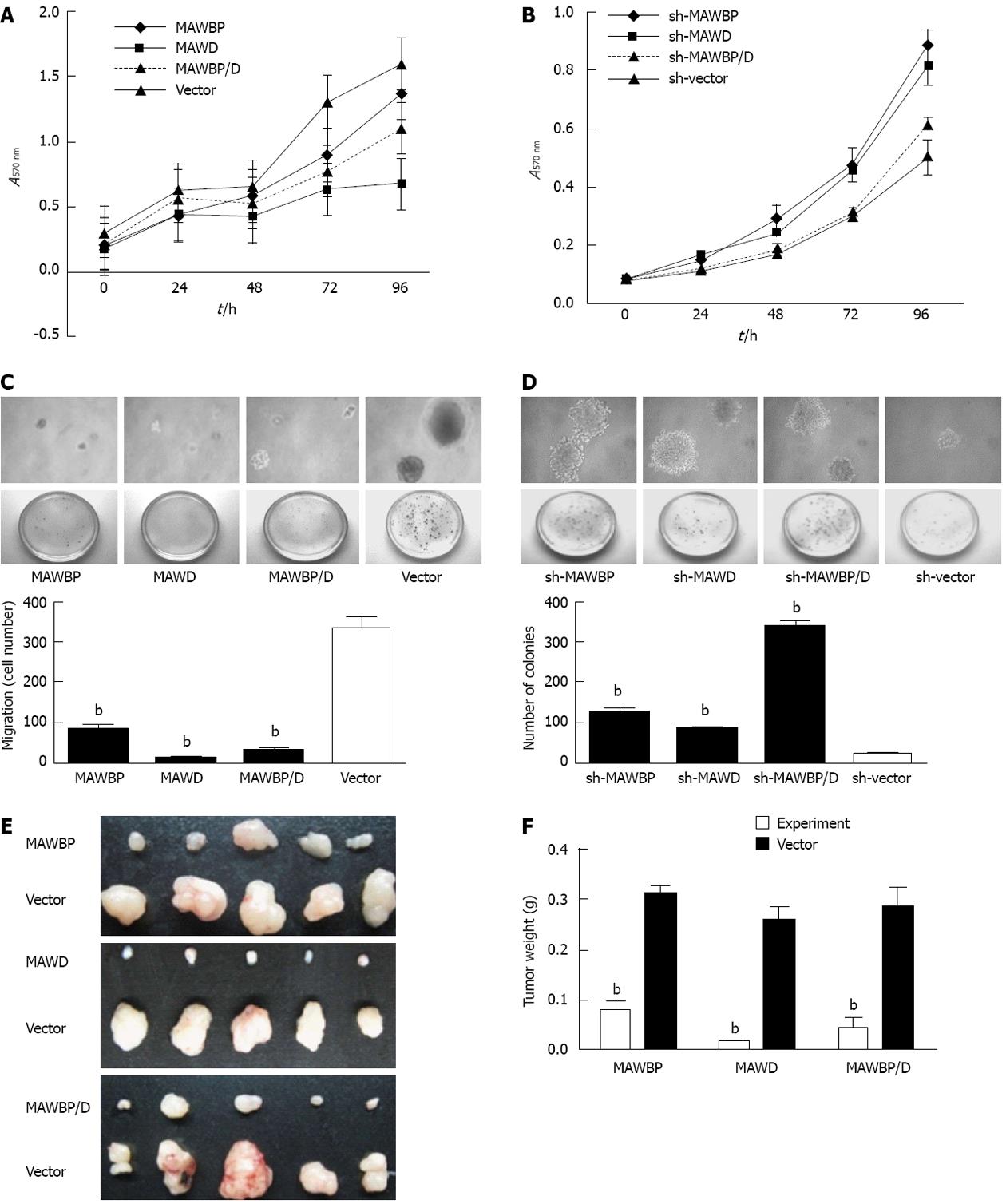
In the MTT assay, cell growth in the overexpressed group was suppressed in the MAWBP, MAWD and MAWBP/D groups, compared with the vector group (Figure 2A, P < 0.001). Knockdown of MAWBP and MAWD in BGC823 cells using RNA interference (RNAi) increased cell growth (Figure 2B, P < 0.001). The soft agar assay in overexpressed cells showed reduced colony formation for the MAWBP, MAWD, and MAWBP/D groups for cell number and size compared with the vector group (Figure 2C, clones number: 86.25 ± 8.43, 12.75 ± 4.49, 30 ± 6.41 vs 336.75 ± 22.55, P < 0.001). The corresponding knockdown group demonstrated the opposite effects (Figure 2D, 131.25 ± 16.54, 88.75 ± 11.12, 341.75 ± 22.23 vs 30.25 ± 8.07, P < 0.001). These results suggested that expression of MAWBP and MAWD play a role in inhibiting proliferation of GC cells.
In vivo experiments, tumor growth appeared to be slow in nude mice injected with MAWBP, MAWD, and MAWBP/D compared with the control group. Tumors from MAWD-transfected cells were smaller than those from the other groups (Figure 2E and F, P < 0.001).
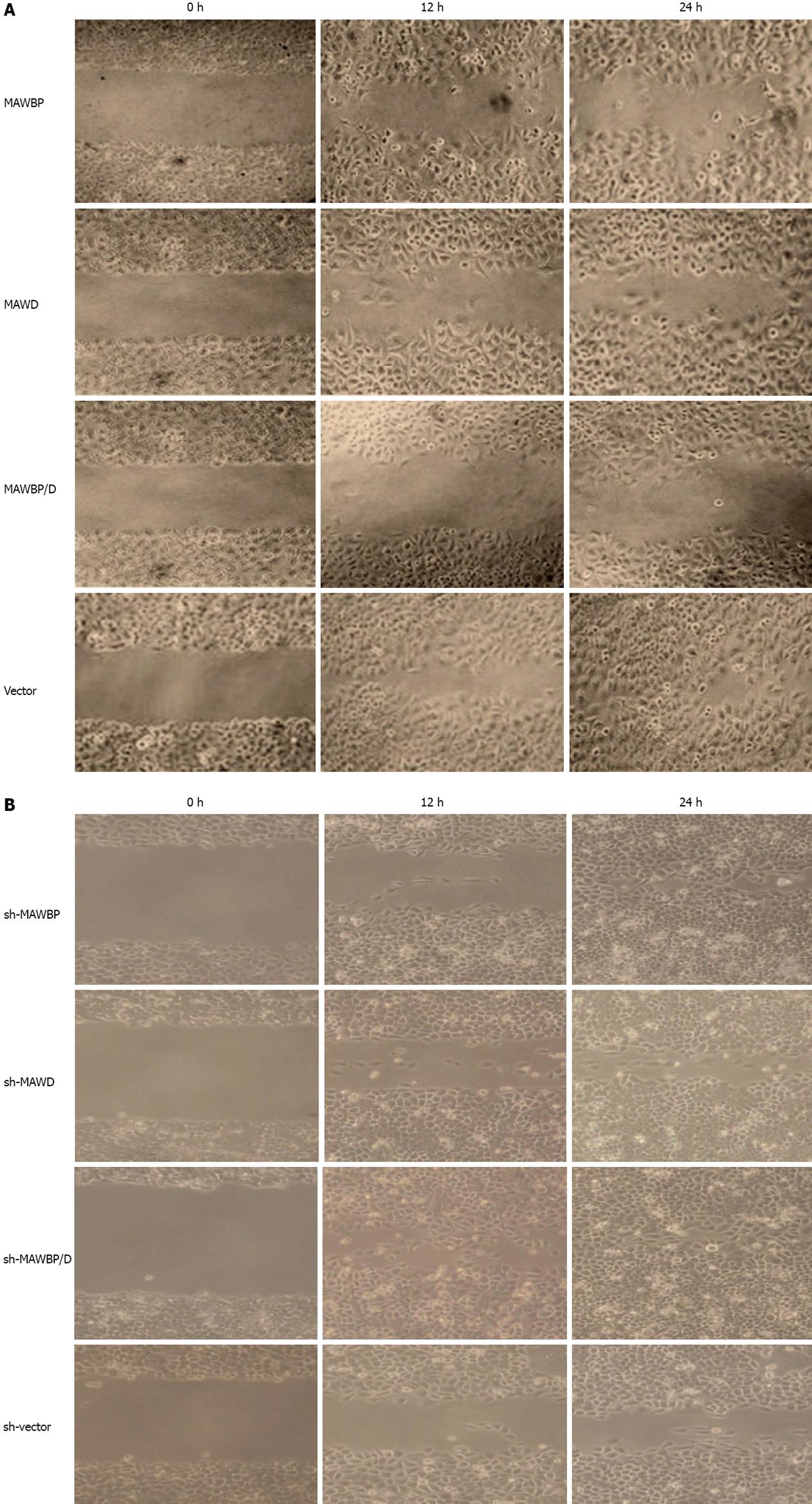
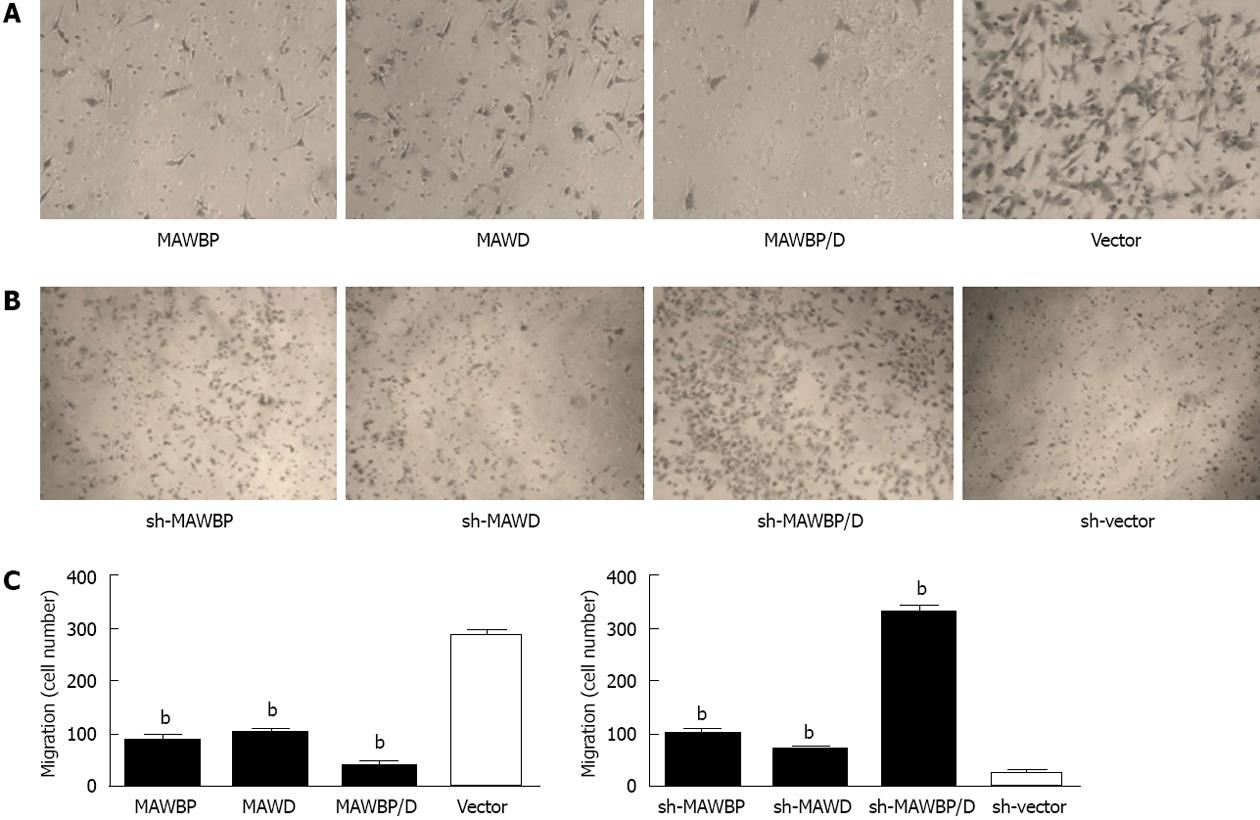
To explore the potential role of MAWBP and MAWD in GC metastasis and invasion, we evaluated the effects of the stably transfected cells on migration and invasion using a wound healing assay and transwell invasive activity assay, respectively. In the wound healing assay, the scratch gap of vector transfected cells was almost closed at 24 h in the overexpressed group. Cells with co-overexpression of MAWBP and MAWD showed the slowest rate of migration (Figure 3A). In the knockdown group, the cells with combined downregulation of MAWBP and MAWD expression migrated faster than the other cells. Migration of sh-vector cells was slowest (Figure 3B). In the transwell assay, there was significant difference in the number of the cells traversing the matrix membrane between the vector and other groups. Combined overexpression of MAWBP and MAWD decreased the invasive ability of GC cells (Figure 4A). The number of traverse cells of MAWBP, MAWD and co-expression group was higher than that in the vector group (Figure 4C, 84 ± 16.57, 98.33 ± 9.8, 29 ± 16.39 vs 298 ± 11.86, P < 0.001). Knockdown of expression of MAWBP and MAWD increased the number of cells that traversed the matrix membrane (Figure 4B). Cells with combined downregulation of MAWBP and MAWD expression migrated faster than the other cells (Figure 4B). Invasion of sh-vector cells was slowest (Figure 4C, 100.67 ± 14.57, 72.66 ± 8.51, 330.67 ± 20.55 vs 27 ± 11.53, P < 0.001). These data showed that MAWBP and MAWD inhibited migration and invasion of GC cells.
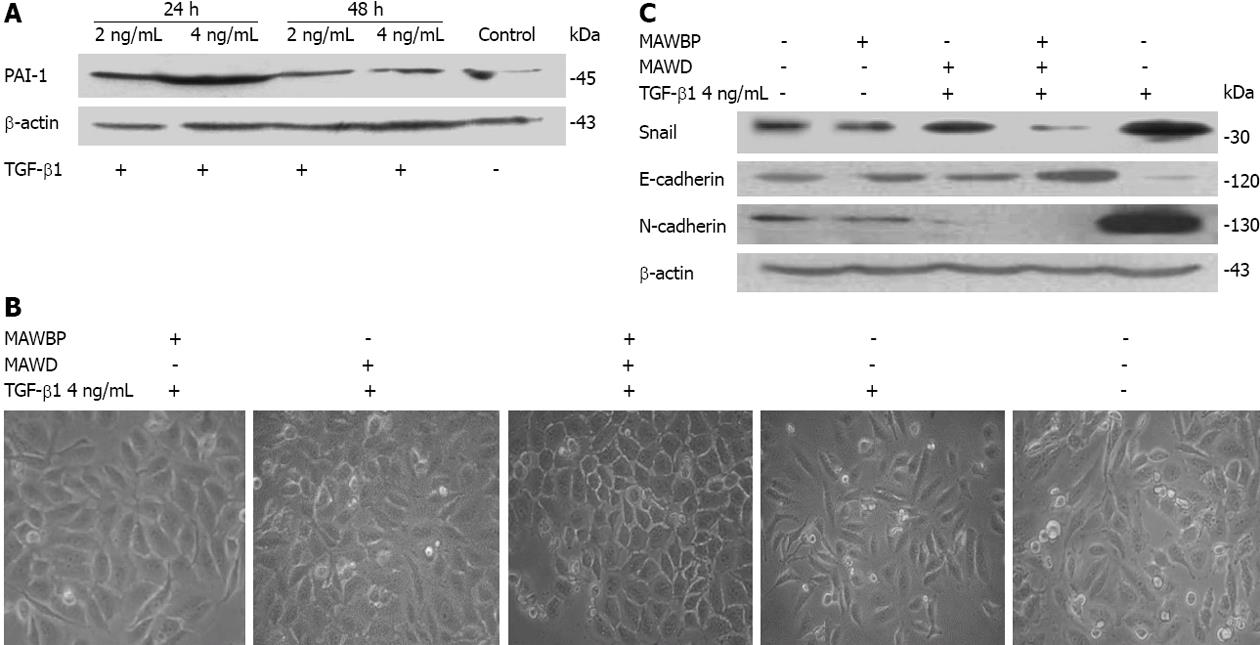
EMT contributes to cancer progression and metastasis. TGF-β is the main and best-characterized inducer of EMT. We sought to determine whether co-expression of MAWBP and MAWD inhibited TGF-β1-induced EMT, thus suppressing migration and transwell behavior of GC cells. We detected a relationship between the expression of MAWBP and MAWD and the EMT markers induced by TGF-β1, such as E-cadherin, N-cadherin, and transcription factor Snail. We established the optimum TGF-β1 concentration and treatment time to stimulate cells. We used the expression of TGF-β reporter gene PAI-1 to indicate that the optimum TGF-β1 concentration and treatment time was 4 ng/mL for 24 h (Figure 5A). We stimulated GC cells that overexpressed MAWBP and MAWD with TGF-β1, and observed their morphology. We found that cells that overexpressed both MAWBP and MAWD displayed a pebble-like shape and tight cell-cell adhesion, while vector-treated cells showed a classical mesenchymal phenotype (Figure 5B). That means that co-expression of MAWBP and MAWD inhibited morphological changes of TGF-β1-induced EMT. We next detected expression of E-cadherin, N-cadherin and Snail in cells overexpressing MAWBP and MAWD. Expression of E-cadherin was strongest in the MAWBP/D group and weakest in the vector group. N-cadherin and Snail expression was inversely associated with E-cadherin expression (Figure 5C). Collectively, these data demonstrated that MAWBP and MAWD were involved in TGF-β1-induced EMT through upregulating E-cadherin and downregulating N-cadherin and Snail in GC cells.
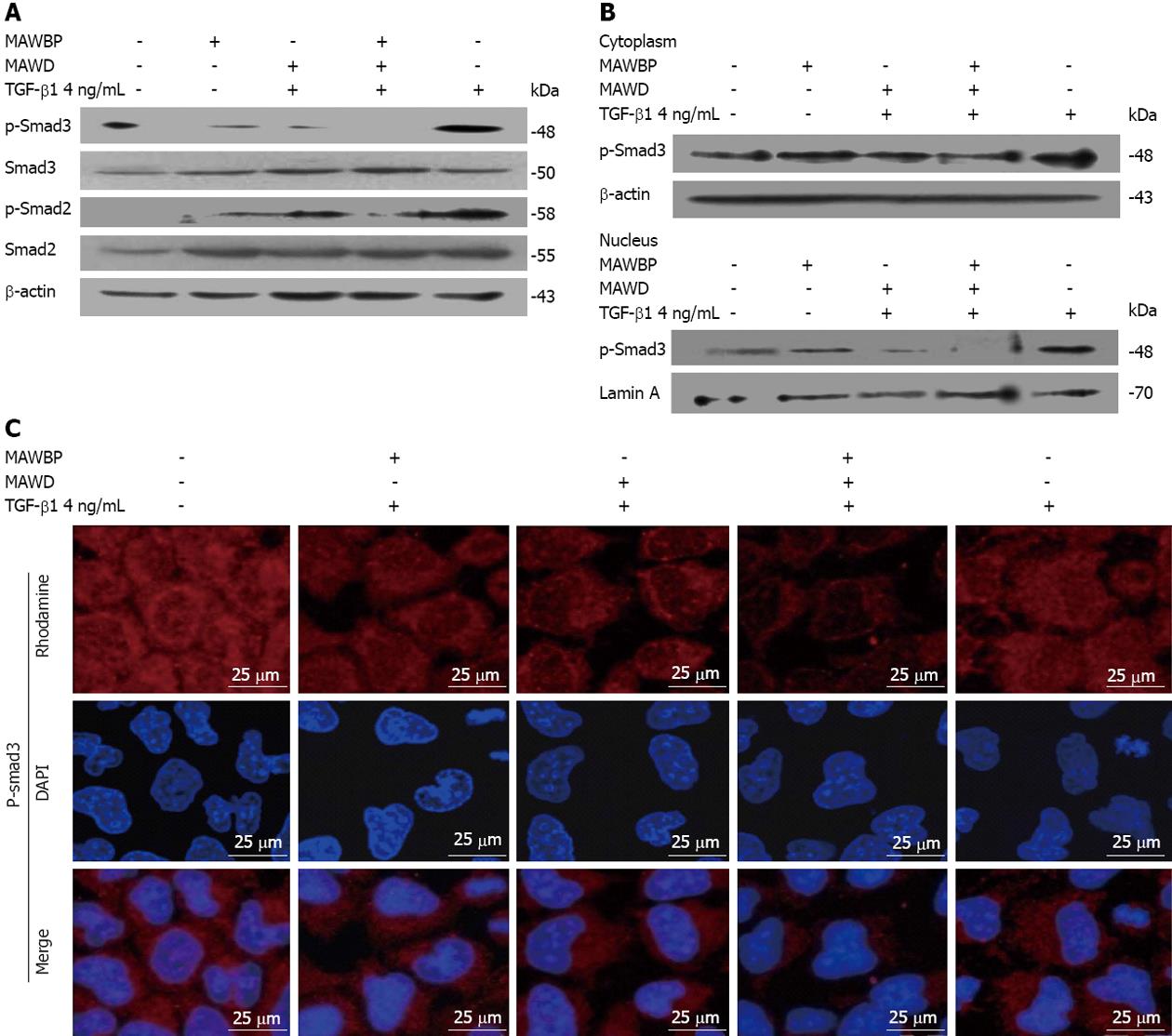
Following MAWBP and MAWD overexpression in cells stimulated with TGF-β1, the level of p-Smad3 was lowest in the MAWBP/D group and highest in the vector group (Figure 6A). The level of p-Smad2 was also lower in the MAWBP/D group. Furthermore, we separated the proteins in the cytoplasm and nucleus and found that p-Smad3 in the nucleus had the lowest level, as shown by Western blotting (Figure 6B) and confocal microscopy (Figure 6C). That means that the nuclear translocation capability of p-Smad3 in cotransfected cells was weakest. These results imply that the MAWBP/D complex suppressed TGF-β signaling by inhibiting downstream phosphorylation.
From the outset of this study, we analyzed the biological function of MAWBP and MAWD in GC cell lines. We found that MAWBP and MAWD inhibited proliferation and migration of GC cells. Importantly, combined overexpression of MAWBP and MAWD in GC cells suppressed TGF-β1-induced EMT by attenuating phosphorylation of Smad3 and reducing its nuclear translocation.
In a previous study, we reported proteomic data acquired from screening protein profiles from GC tissues, including MAWBP and MAWD, and showed that they formed a complex in GC cells by co-immunoprecipitation[11]. MAWD has been reported to have divergent effects in cancer. Some researchers have suggested that MAWD promotes cancer development. Matsuda et al[12] found that MAWD was overexpressed in 45.6% (21/46) of human breast tumor tissues, and promoted anchorage-independent cell growth. Kim et al[15] reported MAWD upregulation in 50.8% (30/59) of adenomas and 70.7% (87/123) of colorectal cancers. Halder et al[23] found that STRAP was upregulated in 60% (12/20) of colon and 78% (11/14) of lung carcinomas. However, other researchers have found that MAWD suppressed development of malignant cells. Buess et al[24] reported complete or partial allelic loss of MAWD in 45.2% (75/166) of colorectal cancer patients. Jung et al[25] found that MAWD was a binding partner of NM23-H1, creating a complex that interacted with and potentiated p53. Dong et al[26] detected chromosomal deletions in prostate cancer that overlapped MAWD gene locations. Zhao et al[27] reported that MAWBP was downregulated in ulcerative colitis. The function of MAWBP and MAWD in GC has not been reported.
In the present study, we investigated the biological role of MAWBP and MAWD in GC. We first investigated expression levels of MAWBP and MAWD in six GC cell lines at the RNA and protein levels. We found that expression was lowest in SGC7901 cells and highest in BGC823 cells. Thus, we selected SGC7901 for overexpression of MAWBP and MAWD and BGC823 for RNAi assay, and constructed the eukaryotic expression plasmid and RNAi plasmid for these two proteins for cell transfection. We generated MAWBP/D-cotransfected cells to establish whether one complements the function of the other. We found that MAWBP and MAWD acted as tumor suppressors. Our results showed that overexpression of MAWBP and MAWD suppressed growth of SGC7901 cells. Knockdown of their expression enhanced proliferation of BGC823 cells. The suppressive ability of MAWD was more pronounced than that of MAWBP. Interestingly, the results from the migration and transwell assays indicated that combined overexpression of these two proteins more obviously limited migration and invasive behavior of GC cells. The cotransfected cells showed mixed characteristics for proliferation and migration, meaning that MAWBP and MAWD had a synergetic role in regulating migration and invasion of GC cells.
EMT is thought to be a key step in the progression of tumors toward invasion and metastasis[28]. EMT is a cellular process during which epithelial polarized cells become motile mesenchymal-appearing cells. This process can lead to loss of epithelial markers - especially E-cadherin - and expression of mesenchymal markers such as vimentin and N-cadherin[29]. E-cadherin is a cell-adhesion protein that is regulated by transcription factors including Snail and Slug. Snail act as a repressor and blocks E-cadherin transcription, and has emerged as an essential regulator of physiological and pathological EMT processes[30]. It has been shown that TGF-β induces changes in cell morphology that are consistent with the acquisition of the EMT phenotype[31].
In this study, we sought to determine whether co-expression of MAWBP and MAWD inhibited TGF-β1-induced EMT, thus suppressing migration and transwell behavior of GC cells. We stimulated GC cells with overexpression of MAWBP and MAWD with TGF-β1 and detected the expression level of epithelial and mesenchymal markers and transcription factors. We found that E-cadherin was upregulated in the co-expression group and N-cadherin and Snail expression was inversely associated with E-cadherin expression. This revealed that MAWBP and MAWD had a synergetic function in inhibiting TGF-β1-induced EMT.
TGF-β1 stimulation induces upregulation of Snail and induces EMT in Smad-dependent signaling[30]. MAWD was found to recruit Smad7 and form a complex that inhibited TGF-β signaling. To confirm whether the MAWBP and MAWD complex further suppressed TGF-β1 and decreased Snail expression, we evaluated TGF-β activity in MAWBP and MAWD overexpressed cells. Phosphorylation of effector molecules is often essential for downstream receptor kinase signaling[31]. Thus, we detected the phosphorylation level and nuclear translocation of p-Smad2 and p-Smad3 to indicate TGF-β activity. We found that the level of p-Smad3 was lowest in the combined overexpression group and highest in the vector group. Nuclear translocation of p-Smad3 was weakest in cells with combined overexpression. These results imply that the MAWBP/D complex suppresses TGF-β signaling, and therefore downregulates Snail level and inhibits EMT.
All together, the present study demonstrates that MAWBP and MAWD have a suppressive role in progression of tumor growth and invasion of GC. Co-expression of MAWBP and MAWD inhibits TGF-β1-induced EMT, which suppresses EMT-assisted GC cell malignant progression. In future research, we should attempt to find the mechanisms mediating MAWBP and MAWD expression in GC. MAWBP and MAWD interaction domains will be predicted by biological information analysis and tested in cell assays.
We thank the Tissue Bank of Beijing Cancer Hospital/Institute for providing gastric specimens.
Gastric cancer (GC) is the second most common cause of cancer death worldwide. GC incidence in Asian countries, particularly in East Asia, is significantly higher than that in other parts of the world. GC creates a serious public health problem. Early diagnosis is important for therapy and prognosis of patients. Therefore, investigation of sensitive biomarkers and analysis of their function are important.
During the past decade, a great effort has been made to define better the biological profile of GC. Molecular genetic studies have investigated the accumulation of mutations and alterations in proteins involved in GC. These include activation of c-myc, erbB-2, c-met, and k-ras oncogenes and inactivation of tumor suppressor genes p53, APC, E-cadherin and RUNX3. Their previous study found that mitogen-activated protein kinase activator with WD40 repeats (MAWD) and MAWD binding protein (MAWBP) were differentially expressed and interacted in GC.
The present study provided direct evidence that MAWBP and MAWD inhibited proliferation and migration of GC cells. Importantly, interaction of MAWBP and MAWD influenced expression of epithelial-mesenchymal transition (EMT) markers induced by transforming growth factor (TGF)-β1 in GC cells. It also reduced nuclear translocation of p-Smad3. This means that co-expression of MAWBP and MAWD inhibits TGF-β1-induced EMT and suppresses EMT-aided GC malignant progression.
The authors found that interaction between MAWBP and MAWD could shed new light on the carcinogenic mechanisms of GC. MAWBP and MAWD as biomarkers might be diagnostic and therapeutic targets for GC.
MAWD is a protein that is evolutionarily conserved and is widely expressed in many tissues. Sequence analysis indicates that the protein structure of MAWD contains a WD40 repeat domain. WD repeat proteins help to assemble macromolecular complexes, such as shown for the β-subunit of G proteins. The homologous protein of MAWD, serine-threonine kinase receptor-associated protein recruits Smad7 to the activated typeIreceptor and forms a complex. MAWBP is a MAWD binding protein. EMT is the morphological and molecular changes that occur when epithelial cells lose their characteristics, gain mesenchymal properties and become motile, which is a key event in tumor invasion and metastasis.
The authors analyzed MAWD and MAWBP in a series of GC cell lines. They found that co-expression of both genes is potentially involved in the suppression of migration and invasion in their selected cell lines. This study was a straightforward continuation of the authors’ own work and they have recently reported differential expression of both genes in GC tissues. Now, they have analyzed the functional consequences of suppression or overexpression of both genes in an in vitro setting.
P- Reviewer Becker KF S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 2. | Becker K, Langer R, Reim D, Novotny A, Meyer zum Buschenfelde C, Engel J, Friess H, Hofler H. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011;253:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 271] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 3. | Hass HG, Smith U, Jäger C, Schäffer M, Wellhäuber U, Hehr T, Markmann HU, Nehls O, Denzlinger C. Signet ring cell carcinoma of the stomach is significantly associated with poor prognosis and diffuse gastric cancer (Lauren’s): single-center experience of 160 cases. Onkologie. 2011;34:682-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Leal MF, Calcagno DQ, Borges da Costa Jde F, Silva TC, Khayat AS, Chen ES, Assumpção PP, de Arruda Cardoso Smith M, Burbano RR. MYC, TP53, and chromosome 17 copy-number alterations in multiple gastric cancer cell lines and in their parental primary tumors. J Biomed Biotechnol. 2011;2011:631268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Cidon EU, Centeno RG, Lagarto EG, Peral JI. HER-2 Evaluation in a Specific Gastric Cancer Population with the Highest Rate of Mortality in Spain. J Oncol. 2011;2011:391564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Zhao J, Zhang X, Xin Y. Up-regulated expression of Ezrin and c-Met proteins are related to the metastasis and prognosis of gastric carcinomas. Histol Histopathol. 2011;26:1111-1120. [PubMed] |
| 7. | Matkar SS, Durham A, Brice A, Wang TC, Rustgi AK, Hua X. Systemic activation of K-ras rapidly induces gastric hyperplasia and metaplasia in mice. Am J Cancer Res. 2011;1:432-445. [PubMed] |
| 8. | Mimata A, Fukamachi H, Eishi Y, Yuasa Y. Loss of E-cadherin in mouse gastric epithelial cells induces signet ring-like cells, a possible precursor lesion of diffuse gastric cancer. Cancer Sci. 2011;102:942-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Li QL, Ito K, Sakakura C, Fukamachi H, Inoue Ki, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113-124. [PubMed] |
| 10. | Lu M, Zhang L, Maul RS, Sartippour MR, Norris A, Whitelegge J, Rao JY, Brooks MN. The novel gene EG-1 stimulates cellular proliferation. Cancer Res. 2005;65:6159-6166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Zhang J, Kang B, Tan X, Bai Z, Liang Y, Xing R, Shao J, Xu N, Wang R, Liu S. Comparative analysis of the protein profiles from primary gastric tumors and their adjacent regions: MAWBP could be a new protein candidate involved in gastric cancer. J Proteome Res. 2007;6:4423-4432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Matsuda S, Katsumata R, Okuda T, Yamamoto T, Miyazaki K, Senga T, Machida K, Thant AA, Nakatsugawa S, Hamaguchi M. Molecular cloning and characterization of human MAWD, a novel protein containing WD-40 repeats frequently overexpressed in breast cancer. Cancer Res. 2000;60:13-17. [PubMed] |
| 13. | Iriyama C, Matsuda S, Katsumata R, Hamaguchi M. Cloning and sequencing of a novel human gene which encodes a putative hydroxylase. J Hum Genet. 2001;46:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Datta PK, Moses HL. STRAP and Smad7 synergize in the inhibition of transforming growth factor beta signaling. Mol Cell Biol. 2000;20:3157-3167. [PubMed] |
| 15. | Kim CJ, Choi BJ, Song JH, Park YK, Cho YG, Nam SW, Yoo NJ, Lee JY, Park WS. Overexpression of serine-threonine receptor kinase-associated protein in colorectal cancers. Pathol Int. 2007;57:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Suh Y, Yoon CH, Kim RK, Lim EJ, Oh YS, Hwang SG, An S, Yoon G, Gye MC, Yi JM. Claudin-1 induces epithelial-mesenchymal transition through activation of the c-Abl-ERK signaling pathway in human liver cells. Oncogene. 2012;Nov 19; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol. 2011;13:317-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 611] [Cited by in RCA: 603] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 18. | Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 654] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 19. | Tran MN, Choi W, Wszolek MF, Navai N, Lee IL, Nitti G, Wen S, Flores ER, Siefker-Radtke A, Czerniak B. The p63 protein isoform ΔNp63α inhibits epithelial-mesenchymal transition in human bladder cancer cells: role of MIR-205. J Biol Chem. 2013;288:3275-3288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Merikallio H, Turpeenniemi-Hujanen T, Pääkkö P, Mäkitaro R, Riitta K, Salo S, Salo T, Harju T, Soini Y. Snail promotes an invasive phenotype in lung carcinoma. Respir Res. 2012;13:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Deng GR, Lu YY, Chen SM, Miao J, Lu GR, Li H, Cai H, Xu XL, E Z, Liu PN. Activated c-Ha-ras oncogene with a guanine to thymine transversion at the twelfth codon in a human stomach cancer cell line. Cancer Res. 1987;47:3195-3198. [PubMed] |
| 22. | Li Y, Lu YY. Isolation of diallyl trisulfide inducible differentially expressed genes in human gastric cancer cells by modified cDNA representational difference analysis. DNA Cell Biol. 2002;21:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Halder SK, Anumanthan G, Maddula R, Mann J, Chytil A, Gonzalez AL, Washington MK, Moses HL, Beauchamp RD, Datta PK. Oncogenic function of a novel WD-domain protein, STRAP, in human carcinogenesis. Cancer Res. 2006;66:6156-6166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Buess M, Terracciano L, Reuter J, Ballabeni P, Boulay JL, Laffer U, Metzger U, Herrmann R, Rochlitz C. STRAP is a strong predictive marker of adjuvant chemotherapy benefit in colorectal cancer. Neoplasia. 2004;6:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Jung H, Seong HA, Ha H. NM23-H1 tumor suppressor and its interacting partner STRAP activate p53 function. J Biol Chem. 2007;282:35293-35307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Dong JT. Chromosomal deletions and tumor suppressor genes in prostate cancer. Cancer Metastasis Rev. 2001;20:173-193. [PubMed] |
| 27. | Zhao X, Kang B, Lu C, Liu S, Wang H, Yang X, Chen Y, Jiang B, Zhang J, Lu Y. Evaluation of p38 MAPK pathway as a molecular signature in ulcerative colitis. J Proteome Res. 2011;10:2216-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Sehrawat A, Kim SH, Vogt A, Singh SV. Suppression of FOXQ1 in benzyl isothiocyanate-mediated inhibition of epithelial-mesenchymal transition in human breast cancer cells. Carcinogenesis. 2013;34:864-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Scanlon CS, Van Tubergen EA, Inglehart RC, D’Silva NJ. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J Dent Res. 2013;92:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 30. | Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11:943-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 529] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 31. | Achyut BR, Yang L. Transforming growth factor-β in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology. 2011;141:1167-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |