Published online Mar 14, 2013. doi: 10.3748/wjg.v19.i10.1563
Revised: January 31, 2013
Accepted: February 5, 2013
Published online: March 14, 2013
AIM: To evaluate the role of multi-detector row computed tomography (MDCT) angiography for assessing the therapeutic effects of percutaneous transhepatic variceal embolization (PTVE) for esophageal varices (EVs).
METHODS: The subjects of this prospective study were 156 patients who underwent PTVE with cyanoacrylate for EVs. Patients were divided into three groups according to the filling range of cyanoacrylate in EVs and their feeding vessels: (1) group A, complete obliteration, with at least 3 cm of the lower EVs and peri-/EVs, as well as the adventitial plexus of the gastric cardia and fundus filled with cyanoacrylate; (2) group B, partial obliteration of varices surrounding the gastric cardia and fundus, with their feeding vessels being obliterated with cyanoacrylate, but without reaching lower EVs; and (3) group C, trunk obliteration, with the main branch of the left gastric vein being filled with cyanoacrylate, but without reaching varices surrounding the gastric cardia or fundus. We performed chart reviews and a prospective follow-up using MDCT images, angiography, and gastrointestinal endoscopy.
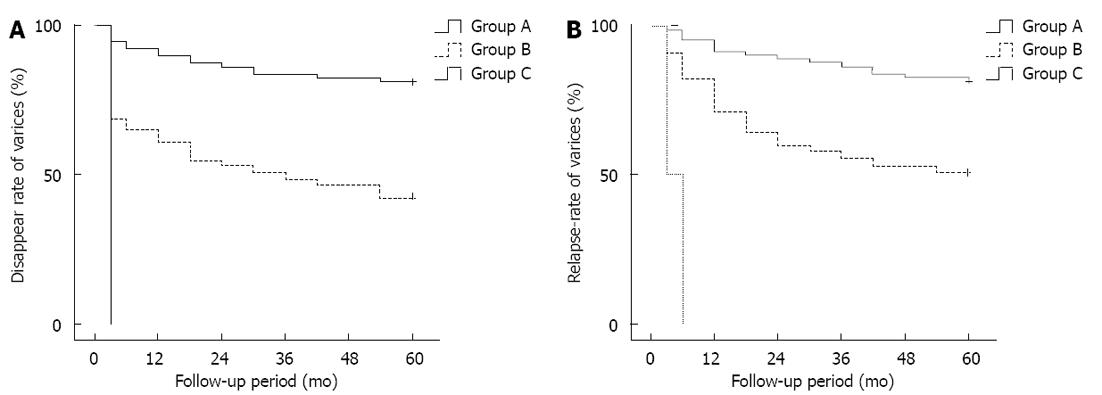
RESULTS: The median follow-up period was 34 mo. The rate of eradication of varices for all patients was 56.4% (88/156) and the rate of relapse was 31.3% (41/131). The rates of variceal eradication at 1, 3, and 5 years after PTVE were 90.2%, 84.1% and 81.7%, respectively, for the complete group; 61.2%, 49% and 42.9%, respectively, for the partial group; with no varices disappearing in the trunk group. The relapse-free rates at 1, 3 and 5 years after PTVE were 91.5%, 86.6% and 81.7%, respectively, for the complete group; 71.1%, 55.6% and 51.1%, respectively, for the partial group; and all EVs recurred in the trunk group. Kaplan-Meier analysis showed P values of 0.000 and 0.000, and odds ratios of 3.824 and 3.603 for the rates of variceal eradication and relapse free rates, respectively. Cyanoacrylate in EVs disappeared with time, but those in the EVs and other feeding vessels remained permanently in the vessels without a decrease with time, which is important for the continued obliteration of the feeding vessels and prevention of EV relapse.
CONCLUSION: MDCT provides excellent visualization of cyanoacrylate obliteration in EV and their feeding veins after PTVE. It confirms that PTVE is effective for treating EVs.
- Citation: Sun A, Shi YJ, Xu ZD, Tian XG, Hu JH, Wang GC, Zhang CQ. MDCT angiography to evaluate the therapeutic effect of PTVE for esophageal varices. World J Gastroenterol 2013; 19(10): 1563-1571
- URL: https://www.wjgnet.com/1007-9327/full/v19/i10/1563.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i10.1563
Percutaneous transhepatic variceal embolization (PTVE) was introduced in the 1970s to manage esophageal varices (EVs)[1,2], but this approach has not attained widespread clinical acceptance because of the associated high rebleeding rate[3-5]. PTVE with cyanoacrylate is a modified procedure for conventional percutaneous transhepatic obliteration[6-12]. Since both the varices and their feeding vessels are sufficiently and permanently obliterated by cyanoacrylate, this modified PTVE technique has been confirmed as an effective and safe method for preventing EV rebleeding[11,12]. Multi-detector row computed tomography (MDCT) angiography has significantly improved our understanding of the complex vascular structural changes that occur during portal hypertension[13-18], along with their clinical and prognostic significance[19-24]. This technique enables the noninvasive study of the hemodynamic changes in the portal venous system[14] and plays an important role in the treatment and follow-up of esophageal and gastric varices[19,24].
The purpose of this study was to assess the relationship between hemodynamic changes in portosystemic collaterals and the long-term results of modified PTVE with cyanoacrylate for patients with EVs using multiplanar reformatted image MDCT imaging.
This was a retrospective-prospective follow-up study to evaluate the long-term results of modified PTVE with cyanoacrylate for treating variceal bleeding. Patients with cirrhosis who had a history of EVs bleeding within 6 mo between January 2005 and December 2011 and who underwent PTVE were selected. A chart review of all patients was performed. We recorded demographic and clinical information, including type of underlying liver disease, liver function tests, and renal function prior to and after PTVE. Liver function was classified according to the Child-Pugh classification. PTVE was performed as a rescue maneuver in all patients, including patients who had uncontrolled severe bleeding during endoscopic therapy or those who had recurrent bleeding episodes from EVs during or after band ligation. All patients underwent diagnostic endoscopy before the intervention to confirm the presence of EV as the bleeding source. This study was approved by the local ethics committee and informed written consent was obtained from each patient.
The inclusion criteria were as follows: (1) cirrhosis confirmed by laboratory and imaging examinations; (2) preoperative gastroscopy showing mild to severe EVs and gastric fundal varices; (3) acute bleeding because of ruptured varices or a history of rupture and bleeding within the last 3 mo; (4) surgery not indicated or preferred; and (5) the patient and their family agreeing to complete preoperative examinations and postoperative follow-ups.
The exclusion criteria were as follows: (1) concomitant liver cancer or other cancers; (2) concomitant widespread portal vein embolism or severe portal vein cavernous transformation; (3) phase II or higher hepatic encephalopathy; (4) obvious jaundice with total bilirubin levels three times higher than normal; (5) obvious bleeding tendency with a prothrombin time > 25 s; (6) severe hypertension, coronary heart disease, or cardiopulmonary insufficiency; and (7) concomitant hepatorenal syndrome or chronic renal insufficiency.
MDCT images were used to evaluate EVs and their feeding vessels before and after the procedures. Computed tomography (CT) was performed via a 64-MDCT scanner (Sensation, Siemens Healthcare, Erlangen, Germany) during the unenhanced, arterial, and portal venous phases. A bolus-tracking technique was applied with a trigger threshold at the upper abdominal aorta of 150 Hounsfield units, with a delay time of 10 s for the arterial phase and an additional 30 s after the first acquisition for the portal venous phase. Iohexol 350 (100-150 mL) was administered at a dose calculated according to patient weight at a rate of 3 mL/s. MDCT was performed with Virtual Place Advance (AZE Ltd., Irvine CA, United States). Three reformatting techniques were used for image reconstruction in this study.
Imaging studies were independently reviewed by two abdominal imaging specialists with fellowship-level training and 5 years of experience as attending radiologists. Said reviewers were blinded to all other clinical and imaging information. EVs and peri-/para-EVs, as well as the adventitial plexus of the gastric cardia and fundus, could be detected by cross-sectional CT scanning during the unenhanced and portal venous phases. After PTVE, the varices adequately embolized with cyanoacrylate to show hyperdensity without enhancement on post-procedural images, whereas isodensity was observed on pre-contrast images in patients with inadequate eradication of the vessels, and with no enhancement being shown on post-contrast images. Portosystemic collaterals were independently assessed on MDCT images before and after PTVE with cyanoacrylate. The supplying vessels, including the left gastric vein (LGV), posterior gastric vein (PGV), and paraesophageal vein were identified on the three-dimensional CT portogram.
According to the findings of CT images, patients were divided into three groups on the basis of the filling range of cyanoacrylate in EVs and their feeding vessels: (1) group A, complete obliteration, with at least 3 cm of the lower EVs and peri-/para-EVs, as well as the adventitial plexus of the gastric cardia and fundus filled with cyanoacrylate; (2) group B, partial obliteration, with the varices surrounding the gastric cardia, fundus, and their feeding vessels being obliterated with cyanoacrylate, but without reaching the lower EVs; and (3) group C, trunk obliteration, with the main branch of the LGV being filled with cyanoacrylate, but without reaching the varices surrounding the gastric cardia or fundus.
All subjects included in this study were followed up at regular intervals of 1, 3 and 6 mo after the procedures, and then every 6-12 mo in a specialized clinic under coordination by a research nurse. Patients underwent a brief recording of medical history at each visit, including possible gastrointestinal bleeding, alcohol intake, concurrent medications, and patient complaints. A brief physical examination, including estimation of ascites and HE by routine neurological examination and laboratory profiling, was performed. Gastrointestinal endoscopy and CT portal venography were performed every 6-12 mo to evaluate EVs and their feeding veins.
Relapse after PTVE was assessed by endoscopy. Follow-up endoscopy was performed at 6 mo intervals after treatment. EVs were evaluated independently during endoscopy by two experienced endoscopists. The endoscopic EVs findings were evaluated according to the classification system of the Japanese Society for Portal Hypertension and EVs[25]. A final decision regarding the endoscopic findings was reached by consensus. The appearance of the localized red color sign F1 or bleeding on follow-up endoscopy was regarded as a relapse of EVs. In this prospective study, we defined relapse of EVs as the primary endpoint, and survival as the secondary endpoint. The relationship between the cyanoacrylate filling range in the vessels and prognosis of PTVE for EVs was determined by the MDCT imaging results.
The PTVE procedure was performed under radiological guidance as described previously. In brief, after transhepatic puncture of the intrahepatic portal vein branch under ultrasonographic or fluoroscopic guidance, a 5F Cobra catheter was introduced into the splenic vein, and splenoportography was performed to evaluate the index varices as well as the feeding vessels and draining veins. The main feeding vessel (e.g., left, short or PGV) was selected with the 5F cobra catheter, and cyanoacrylate was injected into all varices in the lower esophagus and gastric fundus, as well as into all feeding vessels. Next, splenoportography was repeated to assess the extent of varix obliteration. If other feeding vessels were detected, the procedure was repeated until blood flow in the varices completely ceased. The lower EVs, peri-EVs, and/or the gastric cardiac submucosal and perforating vessels were sufficiently obliterated with cyanoacrylate. Finally, the 5F sheath system was withdrawn, and the puncture tract was embolized with microcoils.
All data are expressed as mean ± SD or median values. The cumulative relapse-free rate and cumulative survival rate among the groups based on the reduction rate after treatment were determined using the Kaplan-Meier method and JMP version 5 statistical software (SAS Institute, Tokyo, Japan). Significance was tested using the generalized Wilcoxon signed-rank test and Student’s t-test. A P value < 0.05 was regarded as significant.
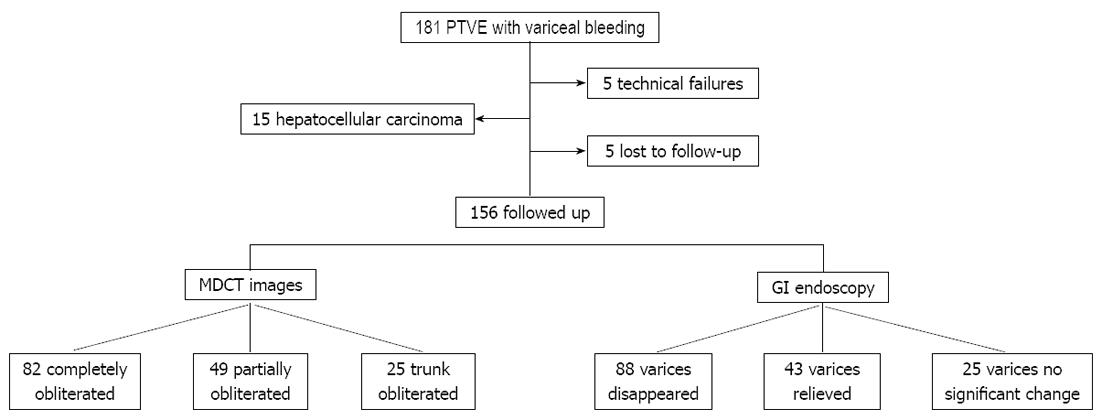
A total of 181 patients underwent PTVE during the study period. Fifteen patients with hepatocellular carcinoma and five cases of technical failures were excluded. Five cases were lost in follow-up. Thus, the final study population consisted of 156 patients (Figure 1). The clinical characteristics of the patients in the groups, including age, gender, etiology of cirrhosis, severity of liver disease, and size of EVs, are shown in Table 1.
| Characteristics | Value (n = 156) |
| Gender: Male/female | 91/65 |
| Age range, yr (mean ± SD) | 35-74 (53 ± 13.6) |
| Cause: Hepatitis B/hepatitis C/alcoholic liver disease/other | 109/6/25/16 |
| Child-Pugh classification: A/B/C | 41/79/36 |
| Variceal size: F2/F3 | 31/125 |
| Portal hypertensive gastropathy (yes/no) | 45/111 |
| Encephalopathy: (II stage/I stage/no) | 23/56/77 |
| Ascites (yes/no) | 62/94 |
| Laboratory findings | |
| Leukocytes (× 109/L) | 4.6 ± 2.1 |
| Hemoglobin (g/L) | 83.7 ± 18.8 |
| Platelets (× 109/L) | 94.9 ± 53.2 |
| ALT (U/L) | 88.2 ± 34.5 |
| Albumin (g/L) | 32.1 ± 12.4 |
| Bilirubin (μmol/L) | 29.3 ± 18.1 |
| Prothrombin time (s) | 16.4 ± 3.8 |
| Creatinine (μmol/L) | 72.7 ± 23.8 |
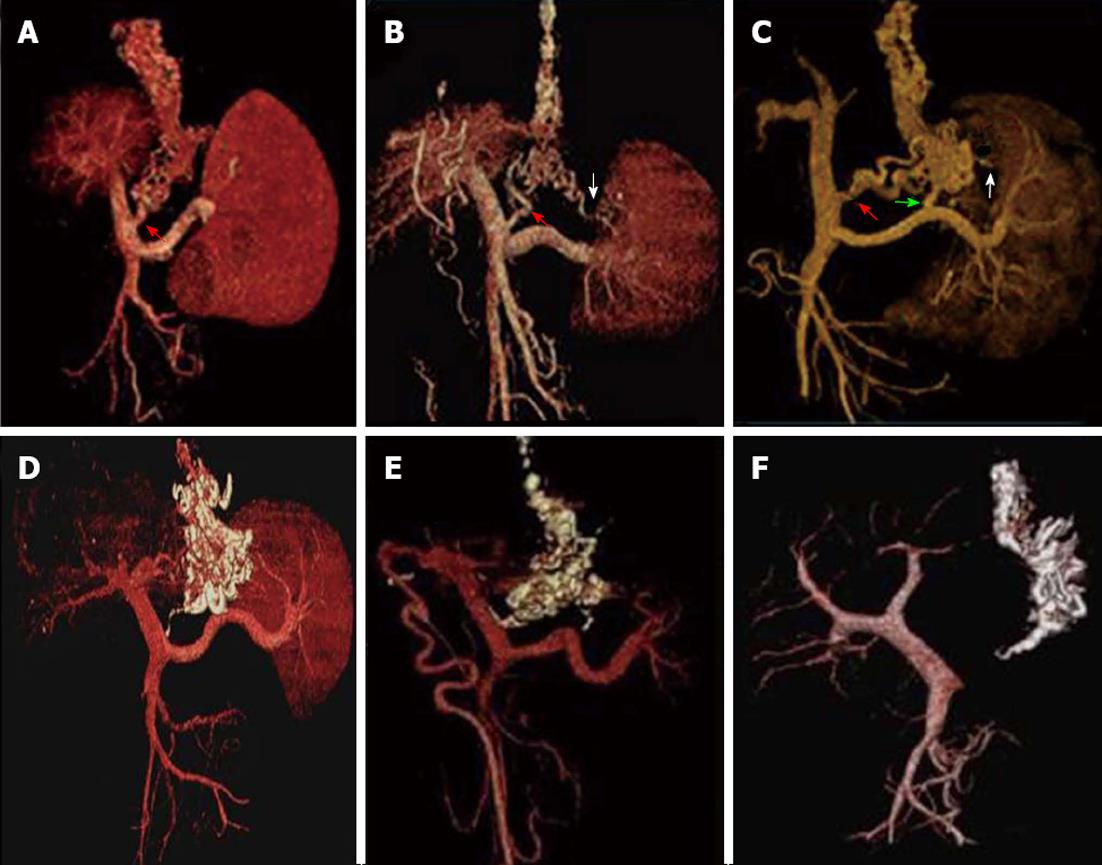
(1) All EVs were detected in all patients by MDCT angiography during the portal venous phase, and ninety-seven of the 156 patients had paraesophageal varices. According to the MDCT angiography findings before PTVE, 86 of the patients had a LGV alone (Figure 2A), 46 had a LGV plus a PGV (Figure 2B), and 37 patients had a LGV plus a PGV and a short gastric vein (SGV) (Figure 2C). The supplying portosystemic collaterals were further confirmed according to the findings on DSA following the PTVE procedures; and (2) MDCT scanning and angiography during the portal venous phase were used to evaluate EVs and their collaterals after the procedures. Figures 2 and 3 show the three typical groups of obliteration with cyanoacrylate according to coronal thin slap maximum intensity projection of MDCT angiography follow-up. Group A: complete obliteration, the lower EVs and peri-/para-EVs, as well as the adventitial plexus of the gastric cardia and fundus, were completely filled with cyanoacrylate; Group B: partial obliteration, the varices surrounding the gastric cardia and fundus and their feeding vessels were obliterated with cyanoacrylate, but cyanoacrylate did not obliterate the lower EVs; Group C: trunk obliteration, the main branch of the LGV was filled with cyanoacrylate, but it did not reach the varices surrounding the gastric cardia or fundus. Volume rendering of the MDCT angiography data set during the portal venous phase demonstrated that the esophageal-gastric varices and afferent vessels were completely filled with cyanoacrylate, but without flow signal in the varices (Figure 2D-F). Of the 156 patients, 82 achieved complete obliteration, 49 achieved partial obliteration, and 25 achieved trunk obliteration.
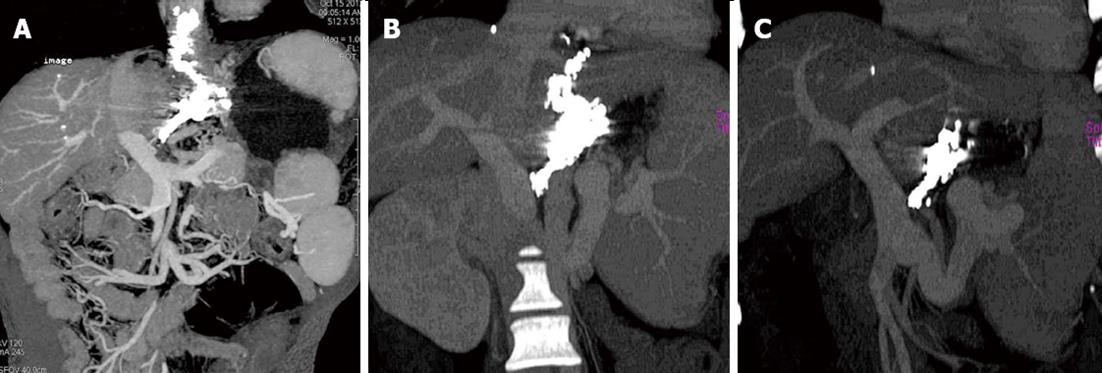
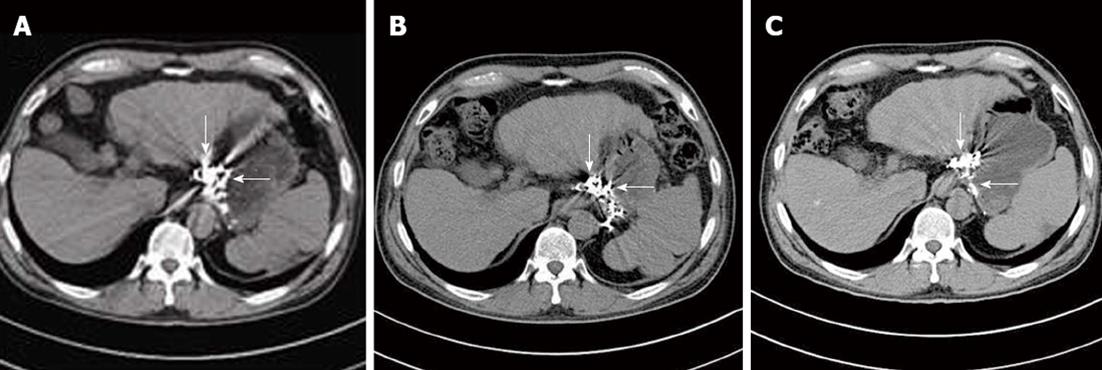
During the follow-up, CT images revealed dynamic changes and outcome of cyanoacrylate injection in different veins. Cyanoacrylate in esophageal and gastric varices gradually reduced with time, but that in the para-and peri-varices was retained permanently in the vessels without a time-dependent decrease, which is important to continue the obliteration of the feeding vessels and prevent the relapse of esophageal-gastric varices (Figure 4).
The median follow-up period was 34 mo, ranged from 6 mo to 71 mo. The rate of eradication of varices for all patients was 56.4% (88/156), and the rate of relapse was 31.3% (41/131). The rates of variceal eradication at 1, 3, and 5 years after PTVE were 90.2%, 84.1% and 81.7%, respectively, for the complete group; 61.2%, 49% and 42.9%, respectively, for the partial group; and with no varices disappearing in the trunk group (Figure 5A). The relapse free rates at 1, 3 and 5 years after PTVE were 91.5%, 86.6% and 81.7%, respectively, for the complete group; 71.1%, 55.6% and 51.1%, respectively, for the partial group; and all EVs recurring in the trunk group (Figure 5B). Kaplan-Meier analysis showed a P = 0.000 and 0.000 and odds ratios (OR) = 3.824 and 3.603 for rates of variceal eradication and relapse free rates, respectively (Tables 2 and 3).
| Variable | Univariate | Multivariate | |
| P value | Hazard ratio (95%CI) | P value | |
| Gender (male vs female) | 0.591 | - | NS |
| Age, yr (≥ 53 vs < 53) | 0.966 | - | NS |
| Child-Pugh classification C vs (A + B) | 0.031 | 1.730 (1.1394-2.6279) | 0.010 |
| Cause: Hepatitis B + hepatitis C vs alcoholic liver disease vs other | 0.557 | - | NS |
| Obliteration type | 0.000 | 3.279 (2.132-5.042) | 0.000 |
| Variceal grade, severe | 0.909 | - | NS |
| Portal hypertensive gastropathy (yes vs no) | 0.036 | 2.525 (0.898-7.097) | 0.075 |
| Encephalopathy (yes vs no) | 0.065 | - | NS |
| Ascites (yes vs no) | 0.024 | 0.702 (0.252-1.958) | 0.217 |
| Laboratory findings | |||
| Leukocytes (× 109/L) (≥ 4.6 vs < 4.6) | 0.726 | - | NS |
| Hemoglobin (g/L) (< 83.7 vs≥ 83.7) | 0.103 | - | NS |
| Platelets (× 109/L) (< 94.9 vs≥ 94.9) | 0.437 | - | NS |
| ALT (U/L) (< 88.2 vs≥ 88.2) | 0.649 | - | NS |
| Albumin (g/L) (< 32.1 vs≥ 32.1) | 0.138 | - | NS |
| Bilirubin (μmol/L) (< 29.3 vs≥ 29.3) | 0.240 | - | NS |
| Prothrombin time(s) (≥ 16.4 vs < 16.4) | 0.176 | - | NS |
| Creatinine (μmol/L) (≥ 72.7 vs < 72.7) | 0.385 | - | NS |
| Variable | Univariate | Multivariate | |
| P value | Hazard ratio (95%CI) | P value | |
| Gender (male vs female) | 0.256 | - | NS |
| Age, yr (≥ 53 vs < 53) | 0.715 | - | NS |
| Child-Pugh classification C vs (A + B) | 0.015 | 1.827 (1.184-2.819) | 0.006 |
| Cause: Hepatitis B + hepatitis C vs alcoholic liver disease vs other | 0.654 | - | NS |
| Obliteration type | 0.020 | 2.535 (1.779-3.613) | 0.000 |
| Variceal grade, severe | 0.721 | - | NS |
| Portal hypertensive gastropathy (yes vs no) | 0.249 | - | NS |
| Encephalopathy (yes vs no) | 0.041 | 3.240 (0.725-6.874) | 0.082 |
| Ascites (yes vs no) | 0.087 | - | NS |
| Laboratory findings | - | NS | |
| Leukocytes (× 109/L) (≥ 4.6 vs < 4.6) | 0.759 | - | NS |
| Hemoglobin (g/L) (< 83.7 vs≥ 83.7) | 0.073 | - | NS |
| Platelets (× 109/L) (< 94.9 vs≥ 94.9) | 0.641 | - | NS |
| ALT (U/L) (< 88.2 vs≥ 88.2) | 0.528 | - | NS |
| Albumin (g/L) (< 32.1 vs≥ 32.1) | 0.193 | - | NS |
| Bilirubin (µmol/L) (< 29.3 vs≥ 29.3) | 0.282 | - | NS |
| Prothrombin time (s) (≥ 16.4 vs < 16.4) | 0.149 | - | NS |
| Creatinine (μmol/L) (≥ 72.7 vs < 2.7) | 0.591 | - | NS |
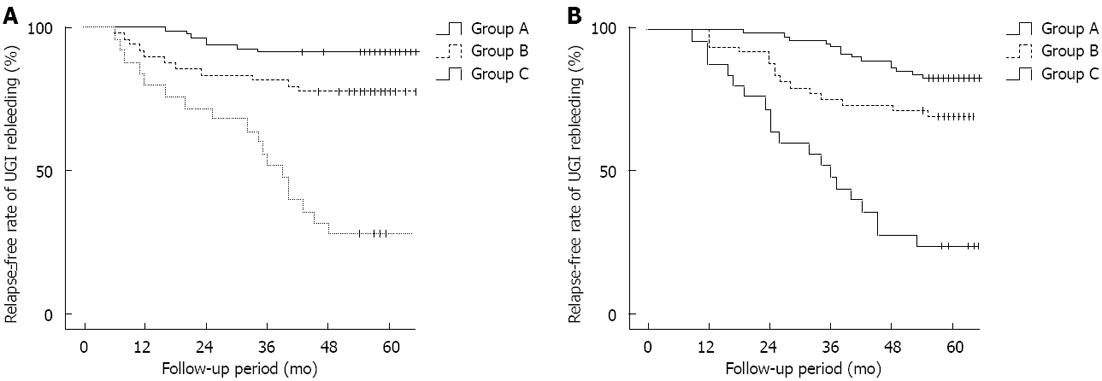
Thirty-six of the 156 patients experienced upper gastrointestinal rebleeding during the follow-up period. The overall bleeding rate was 23.1% (36/156). Seven patients (8.5%, 7/82) in the complete obliteration group, 11 patients (22.4%, 11/49) in the partial obliteration group, and 18 patients (72%, 18/25) in the trunk obliteration group experienced rebleeding. The different rebleeding rates among the groups was statistically significant (Kaplan-Meier values: P = 0.000; OR, 3.560; Figure 5A). Portal hypertensive gastropathy was the main cause of bleeding in the complete obliteration group (six cases, the remaining one was bleeding from a peptic ulcer). Esophagogastric variceal rupture was the main cause of bleeding in the trunk obliteration group (12 cases, the others were five cases of peptic ulcer bleeding and one case of portal hypertensive gastropathy); four cases of variceal rupture occurred in the partial obliteration group (two due to portal hypertensive gastropathy, and three from a peptic ulcer).
Thirty-eight patients died during the follow-up period; 14 patients (17%, 14/82) in the complete obliteration group died. Of these, three died of gastrointestinal tract bleeding, eight of hepatic failure, and three of primary liver cancer. Fifteen patients (30.6%, 15/49) in the partial obliteration group died. Of these, four died of gastrointestinal tract bleeding, six from hepatic failure, and two from primary liver cancer. Nineteen patients (76%, 19/25) in the trunk obliteration group died. Of these, 10 died of gastrointestinal tract bleeding, five from hepatic failure, and four from primary liver cancer. The overall 5-year mortality rate after the operation was 30.8% (48/156). The differences in the mortality rates among the groups were statistically significant (Kaplan-Meier values, P = 0.02; OR, 2.822; Figure 6B). A Cox regression analysis was performed for factors that might influence the rebleeding and survival rates after the operation (type of obliteration, gender, age, Child-Pugh classification, cause of cirrhosis, severity of varices, hepatic encephalopathy, ascites, and laboratory data) and showed that the range of obliteration and Child-Pugh grade were independent predictors of rebleeding and survival rates after the embolism operation (Tables 2 and 3).
MDCT has recently become more useful for examining EVs and the entire portosystemic shunt[14,26]. MDCT allows for visualization of abnormal vessels of the intrinsic circulation, such as peri-EVs that are attached to the muscularis externa of the esophagus and para-EVs localized to the surrounding tissue[14,27]. MDCT also allows for the detection of perigastric and paragastric varices[23,28].
EVs are located within the wall of the lower esophagus, whereas para-esophageal veins are situated outside the wall of the lower esophagus. These vessels are supplied primarily by the LGV, which divides into anterior and posterior branches. The anterior branch supplies EVs, and the posterior branch forms the paraesophageal veins. EVs are usually connected with para-EVs in the distal esophagus[29-31]. Previous studies have shown that large paraesophageal collateral veins (> 5 mm) are associated with high rates of variceal recurrence and rebleeding after banding[32-34], and the increased risk of bleeding also seems to be associated with large perforating veins[29,35].
With regard to the vascular anatomy of the lower esophagus and upper stomach in patients with EVs, it is important to achieve not only obliteration of EVs, but also embolization of the feeding vessels supplied by the portal venous system. We have a long-lasting interest in applying PTVE with cyanoacrylate to sufficiently obliterate the entire lower esophageal and peri-/para-EVs, and the adventitial plexus of the gastric cardia and fundus, to achieve this purpose[11,12].
In this study we used multi-slice helical CT and three-dimensional portography to evaluate the hemodynamic changes in EVs and their collaterals after PTVE with cyanoacrylate. Patients were divided into three groups on the basis of the filling range of cyanoacrylate filling in EVs and their feeding vessels: (1) Group A, complete obliteration: at least 3 cm lower than EVs and peri-/para-EVs, with the adventitial plexus of the gastric cardia and fundus being filled with cyanoacrylate; (2) Group B, partial obliteration: the varices surrounding the gastric cardia and fundus and their feeding vessels being obliterated with cyanoacrylate, but not reaching the lower EVs; and (3) Group C, trunk obliteration: the tissue adhesive filling the main branch of the LGV, but not reaching the varices surrounding the gastric cardia or fundus.
Our results demonstrated the relationship between MDCT image-based embolization range and the prognosis of patients with EVs after PTVE. Of the 156 patients who underwent PTVE with cyanoacrylate for EVs, 82 had their varices completely obliterated; relapse and rebleeding of varices were very low in this group [13.4% (11/82) and 8.5% (7/82), respectively]. However, the relapse rate and rebleeding rate of varices was significantly higher in patients with inadequate embolization of the varices and the feeding vessels than in those with adequate embolization. Multivariate analyses showed that the cyanoacrylate embolization range was an independent risk factor for rebleeding and the relapse of EVs. Thus, a close relationship was observed between the range of EV obstruction, their feeding veins, and the recurrence of EVs. These results demonstrate that it is important to achieve not only obliteration of the feeding vessels, but also embolization of EVs. This result may also explain why conventional PTVE does not achieve long-term prevention of variceal rebleeding, as it focuses mainly on the embolization of feeding veins using a coil or gelatin sponge.
In this study, we first reported the different changes and regression of cyanoacrylate in different veins. Cyanoacrylate in esophageal and gastric varices (vessels within the wall) gradually reduced with time, but the cyanoacrylate in the para- and peri- varices (vessels outside the wall) was permanently retained in the vessels without a time-dependent decrease. This is important in order to continue obliterating the feeding vessels and prevent the relapse of esophageal-gastric varices, as the para-and peri- varices (vessels outside the wall) are the feeding vessels for EVs.
In summary, standard liver MDCT has significantly improved our understanding of the complex vascular structural changes that occur during portal hypertension, and MDCT provides excellent visualization of glue obliteration in EVs and their feeding veins. The results of our study emphasize the importance of sufficient eradication of EVs and their feeding vessels.
Thanks to Dr. Edward C Mignot of Shandong University for his linguistic advice.
Bleeding from esophageal varices (EVs) is a serious complication of portal hypertension and a leading cause of death in patients with liver cirrhosis. Recurrent variceal bleeding is a challenging problem in patients with advanced cirrhosis.
Percutaneous transhepatic variceal embolization (PTVE) with cyanoacrylate, a modified procedure for conventional percutaneous transhepatic obliteration, has been confirmed as an effective and safe method for preventing EVs rebleeding. Multi-detector row computed tomography (MDCT) has recently become more useful for examining EVs and the entire portosystemic shunt.
PTVE is effective for treating EVs, and the sufficient eradication of EVs and their feeding vessels is important in treating varices. MDCT has significantly improved our understanding of the complex vascular structural changes that occur during portal hypertension, and provides excellent visualization of cyanoacrylate obliteration in EVs and their feeding veins.
With the extensive and permanent obliteration of both EVs and their feeding veins, PTVE with cyanoacrylate is a prospective modality for the treatment of esophageal varices. MDCT is effective in evaluating the range of cyanoacrylate obliteration in EV and their feeding veins after PTVE.
In the present study, instead of using EV embolization, PTVE for the eradication of varices feeding veins was instead attempted. The outcomes are encouraging; with clear data showing that PTVE could be an effective method. The MDCT images verified the importance of sufficient eradication of the feeding vessels for the long-term effect of PTVE with cyanoacrylate. The application of this modified technique in the presented cohort with EVs provides sufficient evidence for drawing conclusions. These findings were interesting, and contributed new data to the various effective EV treatment modalities.
P- Reviewers Lee DW, Croffie J, Said S S- Editor Wen LL L- Editor Rutherford A E- Editor Xiong L
| 1. | Lunderquist A, Vang J. Sclerosing injection of esophageal varices through transhepatic selective catheterization of the gastric coronary vein. A preliminary report. Acta Radiol Diagn (Stockh). 1974;15:546-550. [PubMed] [Cited in This Article: ] |
| 2. | Scott J, Dick R, Long RG, Sherlock S. Percutaneous transhepatic obliteration of gastro-oesophageal varices. Lancet. 1976;2:53-55. [PubMed] [Cited in This Article: ] |
| 3. | L’Herminé C, Chastanet P, Delemazure O, Bonnière PL, Durieu JP, Paris JC. Percutaneous transhepatic embolization of gastroesophageal varices: results in 400 patients. AJR Am J Roentgenol. 1989;152:755-760. [PubMed] [Cited in This Article: ] |
| 4. | Benner KG, Keeffe EB, Keller FS, Rösch J. Clinical outcome after percutaneous transhepatic obliteration of esophageal varices. Gastroenterology. 1983;85:146-153. [PubMed] [Cited in This Article: ] |
| 5. | Smith-Laing G, Scott J, Long RG, Dick R, Sherlock S. Role of percutaneous transhepatic obliteration of varices in the management of hemorrhage from gastroesophageal varices. Gastroenterology. 1981;80:1031-1036. [PubMed] [Cited in This Article: ] |
| 6. | Lunderquist A, Vang J. Transhepatic catheterization and obliteration of the coronary vein in patients with portal hypertension and esophageal varices. N Engl J Med. 1974;291:646-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 230] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Kwak HS, Han YM. Percutaneous transportal sclerotherapy with N-butyl-2-cyanoacrylate for gastric varices: technique and clinical efficacy. Korean J Radiol. 2008;9:526-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Kiyosue H, Matsumoto S, Yamada Y, Hori Y, Okino Y, Okahara M, Mori H. Transportal intravariceal sclerotherapy with N-butyl-2-cyanoacrylate for gastric varices. J Vasc Interv Radiol. 2004;15:505-509. [PubMed] [Cited in This Article: ] |
| 9. | Uflacker R. Percutaneous transhepatic obliteration of gastroesophageal varices using absolute alcohol. Radiology. 1983;146:621-625. [PubMed] [Cited in This Article: ] |
| 10. | Tian X, Wang Q, Zhang C, Liu F, Cui Y, Liu F, Liu J. Modified percutaneous transhepatic variceal embolization with 2-octylcyanoacrylate for bleeding gastric varices: long-term follow-up outcomes. AJR Am J Roentgenol. 2011;197:502-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Zhang CQ, Liu FL, Liang B, Sun ZQ, Xu HW, Xu L, Feng K, Liu ZC. A modified percutaneous transhepatic variceal embolization with 2-octyl cyanoacrylate versus endoscopic ligation in esophageal variceal bleeding management: randomized controlled trial. Dig Dis Sci. 2008;53:2258-2267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Zhang CQ, Liu FL, Liang B, Xu HW, Xu L, Feng K, Liu ZC. A modified percutaneous transhepatic varices embolization with 2-octyl cyanoacrylate in the treatment of bleeding esophageal varices. J Clin Gastroenterol. 2009;43:463-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Kim HC, Yang DM, Jin W, Ryu CW, Ryu JK, Park SI, Park SJ, Shin HC, Kim IY. Multiplanar reformations and minimum intensity projections using multi-detector row CT for assessing anomalies and disorders of the pancreaticobiliary tree. World J Gastroenterol. 2007;13:4177-4184. [PubMed] [Cited in This Article: ] |
| 14. | Kang HK, Jeong YY, Choi JH, Choi S, Chung TW, Seo JJ, Kim JK, Yoon W, Park JG. Three-dimensional multi-detector row CT portal venography in the evaluation of portosystemic collateral vessels in liver cirrhosis. Radiographics. 2002;22:1053-1061. [PubMed] [Cited in This Article: ] |
| 15. | Nakayama Y, Imuta M, Funama Y, Kadota M, Utsunomiya D, Shiraishi S, Hayashida Y, Yamashita Y. CT portography by multidetector helical CT: comparison of three rendering models. Radiat Med. 2002;20:273-279. [PubMed] [Cited in This Article: ] |
| 16. | Kim H, Choi D, Gwak GY, Lee JH, Park MK, Lee HIe, Kim SH, Nam S, Yoo EY, Do YS. Evaluation of esophageal varices on liver computed tomography: receiver operating characteristic analyses of the performance of radiologists and endoscopists. J Gastroenterol Hepatol. 2009;24:1534-1540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Kim H, Choi D, Gwak GY, Lee JH, Lee SJ, Kim SH, Lee JY, Park Y, Chang I, Lim HK. High-risk esophageal varices in patients treated with locoregional therapies for hepatocellular carcinoma: evaluation with regular follow-up liver CT. Dig Dis Sci. 2009;54:2247-2252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Campbell WJ, Irwin ST, Biggart JD. Benign lymphangioma of the jejunal mesentery: an unusual cause of small bowel obstruction. Gut. 1991;32:1568. [PubMed] [Cited in This Article: ] |
| 19. | Shimizu T, Namba R, Matsuoka T, Tabuchi K, Yamamoto K, Uesugi Y, Matsui R, Sueyoshi K, Narabayashi I. Esophageal varices before and after endoscopic variceal ligation: evaluation using helical CT. Eur Radiol. 1999;9:1546-1549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Ishikawa T, Ushiki T, Mizuno K, Togashi T, Watanabe K, Seki K, Ohta H, Yoshida T, Takeda K, Kamimura T. CT-maximum intensity projection is a clinically useful modality for the detection of gastric varices. World J Gastroenterol. 2005;11:7515-7519. [PubMed] [Cited in This Article: ] |
| 21. | Kim HC, Kim TK, Sung KB, Yoon HK, Kim PN, Ha HK, Kim AY, Kim HJ, Lee MG. CT during hepatic arteriography and portography: an illustrative review. Radiographics. 2002;22:1041-1051. [PubMed] [Cited in This Article: ] |
| 22. | Rydberg J, Buckwalter KA, Caldemeyer KS, Phillips MD, Conces DJ, Aisen AM, Persohn SA, Kopecky KK. Multisection CT: scanning techniques and clinical applications. Radiographics. 2000;20:1787-1806. [PubMed] [Cited in This Article: ] |
| 23. | Willmann JK, Weishaupt D, Böhm T, Pfammatter T, Seifert B, Marincek B, Bauerfeind P. Detection of submucosal gastric fundal varices with multi-detector row CT angiography. Gut. 2003;52:886-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Chen TW, Yang ZG, Wang QL, Li X, Yu JQ, Qian LL, Wang RR. Evaluation of gastric fundic and oesophageal varices by 64-row multidetector computed tomography before and after transjugular intrahepatic portosystemic shunt with concurrent left gastric vein embolization. Eur J Gastroenterol Hepatol. 2010;22:289-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Idezuki Y. Present status of sclerotherapy and surgical treatment for esophageal varices in Japan. Japanese Research Society for Portal Hypertension and Japanese Research Society for Sclerotherapy of Esophageal Varices. World J Surg. 1992;16:1193-1200; discussion 1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Agarwal A, Jain M. Multidetector CT portal venography in evaluation of portosystemic collateral vessels. J Med Imaging Radiat Oncol. 2008;52:4-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Zhao LQ, He W, Chen G. Characteristics of paraesophageal varices: a study with 64-row multidetector computed tomography portal venography. World J Gastroenterol. 2008;14:5331-5335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Zhu KS, Meng XC, Zhang JS, Guan SH, Li ZR, He KK, Pang PF, Jiang ZB, Huang MS, Shan H. [The role of multi-detector row CT in the diagnosis and hemodynamic studies of gastric varices in portal hypertension]. Zhonghua Yi Xue Za Zhi. 2007;87:3251-3255. [PubMed] [Cited in This Article: ] |
| 29. | Hino S, Kakutani H, Ikeda K, Uchiyama Y, Sumiyama K, Kuramochi A, Kitamura Y, Matsuda K, Arakawa H, Kawamura M. Hemodynamic assessment of the left gastric vein in patients with esophageal varices with color Doppler EUS: factors affecting development of esophageal varices. Gastrointest Endosc. 2002;55:512-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Yamamoto K, Tsuda T, Mochizuki T, Ikezoe J. Intravenous three-dimensional CT portography using multi-detector row CT in patients with hepatic cirrhosis: evaluation of scan timing and image quality. Radiat Med. 2002;20:83-87. [PubMed] [Cited in This Article: ] |
| 31. | Balfe DM, Mauro MA, Koehler RE, Lee JK, Weyman PJ, Picus D, Peterson RR. Gastrohepatic ligament: normal and pathologic CT anatomy. Radiology. 1984;150:485-490. [PubMed] [Cited in This Article: ] |
| 32. | Leung VK, Sung JJ, Ahuja AT, Tumala IE, Lee YT, Lau JY, Chung SC. Large paraesophageal varices on endosonography predict recurrence of esophageal varices and rebleeding. Gastroenterology. 1997;112:1811-1816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Dhiman RK, Choudhuri G, Saraswat VA, Agarwal DK, Naik SR. Role of paraoesophageal collaterals and perforating veins on outcome of endoscopic sclerotherapy for oesophageal varices: an endosonographic study. Gut. 1996;38:759-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Lin CY, Lin PW, Tsai HM, Lin XZ, Chang TT, Shin JS. Influence of paraesophageal venous collaterals on efficacy of endoscopic sclerotherapy for esophageal varices. Hepatology. 1994;19:602-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Kume K, Yamasaki M, Watanabe T, Yoshikawa I, Otsuki M, Harada M. Mild collateral varices and a fundic plexus without perforating veins on EUS predict endoscopic non-recurrence of esophageal varices after EVL. Hepatogastroenterology. 2011;58:798-801. [PubMed] [Cited in This Article: ] |