Published online Dec 28, 2012. doi: 10.3748/wjg.v18.i48.7184
Revised: November 2, 2012
Accepted: November 6, 2012
Published online: December 28, 2012
Processing time: 179 Days and 0.1 Hours
AIM: To investigate the effect of sulfated cholecystokinin-8 (CCK-8S) on calcium mobilization in cultured murine gastric antral interstitial cells of Cajal (ICC) and its possible mechanisms.
METHODS: ICC were isolated from the gastric antrum of mice and cultured. Immunofluorescence staining with a monoclonal antibody for c-Kit was used to identify ICC. The responsiveness of ICC to CCK-8S was measured using Fluo-3/AM based digital microfluorimetric measurement of intracellular Ca2+ concentration ([Ca2+]i). A confocal laser scanning microscope was used to monitor [Ca2+]i changes. The selective CCK1 receptor antagonist lorglumide, the intracellular Ca2+-ATPase inhibitor thapsigargin, the type III inositol 1,4,5-triphosphate (InsP3) receptor blocker xestospongin C and the L-type voltage-operated Ca2+ channel inhibitor nifedipine were used to examine the mechanisms of [Ca2+]i elevation caused by CCK-8S. Immunoprecipitation and Western blotting were used to determine the regulatory effect of PKC on phosphorylation of type III InsP3 receptor (InsP3R3) in ICC. Protein kinase C (PKC) activator phorbol 12-myristate 13-acetate (PMA) and inhibitor chelerythrine were used to assess the role of PKC in the CCK-8S-evoked [Ca2+]i increment of ICC.
RESULTS: ICC were successfully isolated from the gastric antrum of mice and cultured. Cultured ICC were identified by immunofluorescence staining. When given 80 nmol/L or more than 80 nmol/L CCK-8S, the [Ca2+]i in ICC increased and 100 nmol/L CCK-8S significantly increased the mean [Ca2+]i by 59.30% ± 4.85% (P < 0.01). Pretreatment of ICC with 5 μmol/L lorglumide inhibited 100 nmol/L CCK-8S-induced [Ca2+]i increment from 59.30% ± 4.85% to 14.97% ± 9.05% (P < 0.01), suggesting a CCK1R-mediated event. Emptying of intracellular calcium stores by thapsigargin (5 μmol/L) prevented CCK-8S (100 nmol/L) from inducing a [Ca2+]i increase. Moreover, pretreatment with xestospongin C (1 μmol/L) could also abolish the CCK-8S-induced effect, indicating that Ca2+ release from InsP3R-operated stores appeared to be a major mechanism responsible for CCK-8S-induced calcium mobilization in ICC. On the other hand, by removing extracellular calcium or blocking the L-type voltage-operated calcium channel with nifedipine, a smaller but significant rise in the [Ca2+]i could be still elicited by CCK-8S. These data suggest that the [Ca2+]i release is not stimulated or activated by the influx of extracellular Ca2+ in ICC, but the influx of extracellular Ca2+ can facilitate the [Ca2+]i increase evoked by CCK-8S. CCK-8S increased the phosphorylation of InsP3R3, which could be prevented by chelerythrine. Pretreatment with lorglumide (5 μmol/L) could significantly reduce the CCK-8S intensified phosphorylation of InsP3R3. In the positive control group, treatment of cells with PMA also resulted in an enhanced phosphorylation of InsP3R3. Pretreatment with various concentrations of PMA (10 nmol/L-10 μmol/L) apparently inhibited the effect of CCK-8S and the effect of 100 nmol/L PMA was most obvious. Likewise, the effect of CCK-8S was augmented by the pretreatment with chelerythrine (10 nmol/L-10 μmol/L) and 100 nmol/L chelerythrine exhibited the maximum effect.
CONCLUSION: CCK-8S increases [Ca2+]i in ICC via the CCK1 receptor. This effect depends on the release of InsP3R-operated Ca2+ stores, which is negatively regulated by PKC-mediated phosphorylation of InsP3R3.
- Citation: Gong YY, Si XM, Lin L, Lu J. Mechanisms of cholecystokinin-induced calcium mobilization in gastric antral interstitial cells of Cajal. World J Gastroenterol 2012; 18(48): 7184-7193
- URL: https://www.wjgnet.com/1007-9327/full/v18/i48/7184.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i48.7184
In the gastrointestinal (GI) tract, phasic contractions are caused by electrical activity termed slow waves, which are generated and propagated by the interstitial cells of Cajal (ICC)[1,2]. The initiation of pacemaker activity in the ICC is caused by rhythmic cytoplasmic Ca2+ oscillation[3,4]. The ICC can regulate slow-wave-driven peristaltic activity as well as mediate motor inputs from the GI nervous system[5,6]. ICC abnormalities are associated with many GI motility disorders, such as achalasia of cardia, slow transit constipation and irritable bowel syndrome[7,8]. Therefore, understanding the mechanisms underlying pacemaker activity and excitability of ICC is of crucial importance[9]. Although neurohumoral regulation of GI function has been studied in some detail, less is known about the mechanisms of how neurohumoral factors regulate pacemaker activities in the ICC.
Cholecystokinin (CCK) is a bioactive peptide that regulates a variety of physiological functions, acting as both hormone and neurotransmitter in the GI tract[10,11]. Sulfated CCK-8 (CCK-8S), which has frequently been used in research studies, is one of the main biologically active forms of CCK[12]. The regulatory actions of CCK are mediated by two receptor subtypes, CCK1 and CCK2 receptors. CCK receptors are distributed in enteric nerves and smooth muscles[13]. In addition, CCK1 receptors are also found in the ICC, suggesting a role for the ICC in the mediation of CCK effects[14].
CCK inhibits gastric emptying by relaxing the proximal part of the stomach and increasing pyloric pressure[10,12]. However, the mechanisms of the regulatory effects of CCK on gastrointestinal motility are not clear. Previous studies have shown that CCK can activate phospholipase C (PLC) through binding to its distinct receptors[15]. This activation leads to the production of diacylglycerol (DAG) and inositol 1,4,5- triphosphate (InsP3), which in turn activates protein kinase C (PKC) and mobilizes intracellular calcium ([Ca2+]i)[16]. Through this signaling pathway, CCK may participate in various physiological responses, such as secretion, neurotransmission and muscle contraction[17]. However, the cross-talk of the CCK-8S triggered PKC and Ca2+ signaling pathways is not been well understood. The aims of this study were to investigate the effect of CCK-8S on [Ca2+]i in the ICC and the respective contributions of InsP3R-sensitive intracellular Ca2+ stores and extracellular Ca2+ sources in those responses. Additionally, the role of PKC in regulation of CCK-8S-triggered calcium signaling pathway was also studied.
All experiments were performed according to the guiding principles for the care and use of animals approved by Institutional Animal Use and Care Committee of the First Affiliated Hospital of Nanjing Medical University. Every effort was made to minimize both the number of animals used and their suffering.
Balb/c mice (5-6 wk) of either sex were purchased from the laboratory of the First Affiliated Hospital of Nanjing Medical University. The animals were anesthetized by chloroform inhalation and killed by cervical dislocation. The stomach was excised and the contents were washed away with ice-cold Krebs-Ringer bicarbonate (KRB). The mucosa was removed by peeling. In Sylgard dishes filled with Krebs solution, the tissues were washed three times and then cut into about 0.5 cm segments. The segments were transferred into a centrifuge tube and dispersed with an enzyme solution containing collagenase 1.3 mg/mL, trypsin inhibitor 2 mg/mL and ATP 0.27 mg/mL. The centrifuge tube was incubated at 37 °C for 30 min and the tissue segments were blown for 30 s with pipette every 5 min during incubation. An equal volume Medium 199 containing 10% fetal bovine serum was added to stop digestion. The tube was centrifuged at 1000 rpm for 3 min and then the supernatant was removed. The sediment was suspended with Medium 199 and cells were collected by pouring the suspension through a 200-mesh sieve. After centrifugation at 1000 rpm for 3 min, the cells were dispersed with Medium 199 and then plated onto 35 mm glass-bottom culture dishes (NEST Biotechnology Co., Ltd, China) coated with rat-tail tendon collagen (5 mg/mL). The cells were then cultured at 37 °C in a 95% O2-5% CO2 incubator in Medium 199 supplemented with 2% Penicillin-Streptomycin liquid and murine stem cell factor (5 ng/mL).
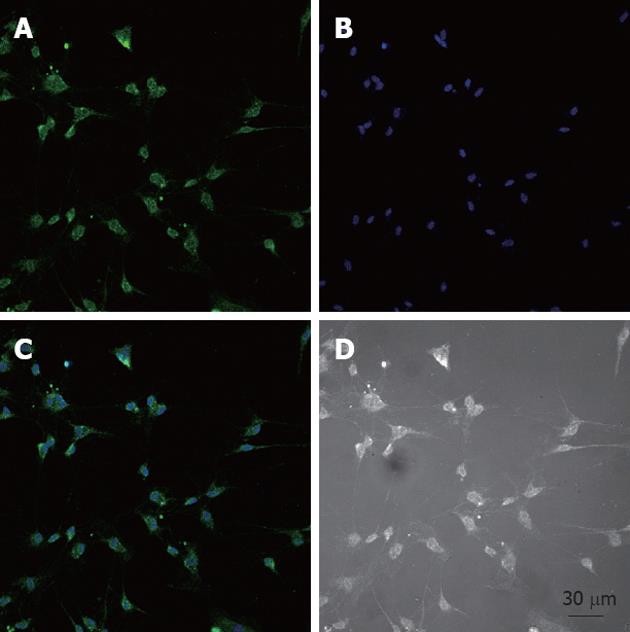
Cultured ICC were fixed in acetone (4 °C, 8 min). Following fixation, preparations were washed for 40 min in phosphate buffered saline (PBS; 0.01 mol/L, pH 7.4) and then incubated in 10% goat serum for 1 h to reduce nonspecific antibody binding. To examine the ICC, we incubated cultured ICC overnight at 4 °C with a rabbit anti-mouse monoclonal c-Kit antibody (1:300 in PBS). Immunoreactivity was detected using Alexa Fluor 488 (1:1000 in PBS, 60 min, room temperature), and nuclei were stained with Hoechst 33 258. Cells were examined under a confocal laser scanning microscope (LSM710, Zeiss, Germany) at an excitation wavelength appropriate for Alexa Fluor 488 (488 nm).
Changes in [Ca2+]i were monitored using Fluo-3/AM, which was initially dissolved in dimethyl sulfoxide (DMSO) and stored at -20 °C. The cultured ICC grown on glass-bottom dishes were rinsed twice with PBS and then incubated in Medium 199 containing 5 μmol/L Fluo-3/AM in the 95% O2-5% CO2 incubator for 40 min. Following rinsing for two more times, the dishes were scanned every 2 s with a confocal laser scanning microscope. Fluorescence was excited at a wavelength of 488 nm and emitted light was observed at 515 nm. The variations of [Ca2+]i fluorescence emission intensity were expressed as F/F0, where F0 is the intensity of the first imaging.
Prepared from gastric ICC using equal volumes of a cell suspension, isolated cells were assayed for protein concentration. Following appropriate treatments, cells were pelleted and resuspended in 0.5 mL of ice-cold lysis buffer. Cell samples were sonicated and left on ice for 30 min to solubilize. Immunoprecipitation of InsP3R was performed using a 1:100 dilution of an InsP3R3-specific monoclonal antibody. Following 2 h of incubation with the InsP3R3 antibody at 4 °C, immobilized protein A beads were added to each sample for 1 h at 4 °C. As a control, samples were also prepared without immunoprecipitating antibody. Following immunoprecipitation of InsP3R3, proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose. Membranes were probed with a 1:1000 dilution of phospho-(Ser/Thr) substrate antibody that specifically detected phosphorylated Ser/Thr residues with Arg at the -2 or -3 position within the PKC substrate sequence. Immunoreactivity was visualized using a 1:1000 dilution of peroxidase-conjugated secondary antibody. Where indicated, the nitrocellulose membrane was stripped of primary and secondary antibodies at 50 °C for 30 min.
CCK-8S, collagenase, trypsin inhibitor, ATP, thapsigargin, chelerythrine, phorbol 12-myristate 13-acetate (PMA), nifedipine, Hoechst 33258, DMSO, HEPES were purchased from Sigma (United States). SCF was purchased from Peprotech (United States). Rat-tail tendon collagen was purchased from Shengyou Biotechnology Co., Ltd (Hangzhou, China). C-Kit monoclonal antibody and phospho-(Ser/Thr) substrate antibody were from Cell Signaling Technology (United States). Type III InsP3R-specific monoclonal antibody, protein A beads were from BD Biosciences Transduction Laboratories (United States). Alexa Fluor 488 (goat anti-rabbit secondary antibody) and Fluo-3/AM were from Invitrogen (United States). Lorglumide was purchased from Santa Cruz (United States). Xestospongin C was from Calbiochem (Germany). Medium 199, fetal bovine serum and Penicillin-Streptomycin liquid were purchased from Gibco (United States).
The KRB solution contains (mmol/L): NaCl 117, KCl 4.7, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, Glucose 11 and CaCl2 2.6; pH was 7.4. Lysis buffer contains (mmol/L): NaCl 150, NaF 100, Tris 50, EDTA 10, Triton X-100 1%, and 1 complete EDTA-free protease inhibitor mixture tablet; pH was 7.4.
Data were expressed as mean ± SE. Differences in the data were evaluated by ANOVA or by Student’s t test. Zeiss Zen 9.0 was used to analyze the calcium intensity data and GraphPad Prism 5.0 for charting. Differences between control and test values were considered significant when P < 0.05.
After the cells were isolated and plated onto culture dishes, it was initially difficult to identify the ICC. After prolonged culture (4-7 d), the cultured ICC, were identified by c-Kit immunofluorescence and showed distinctive shapes, such as spindle, triangular or stellar-like with two to five long processes (Figure 1).
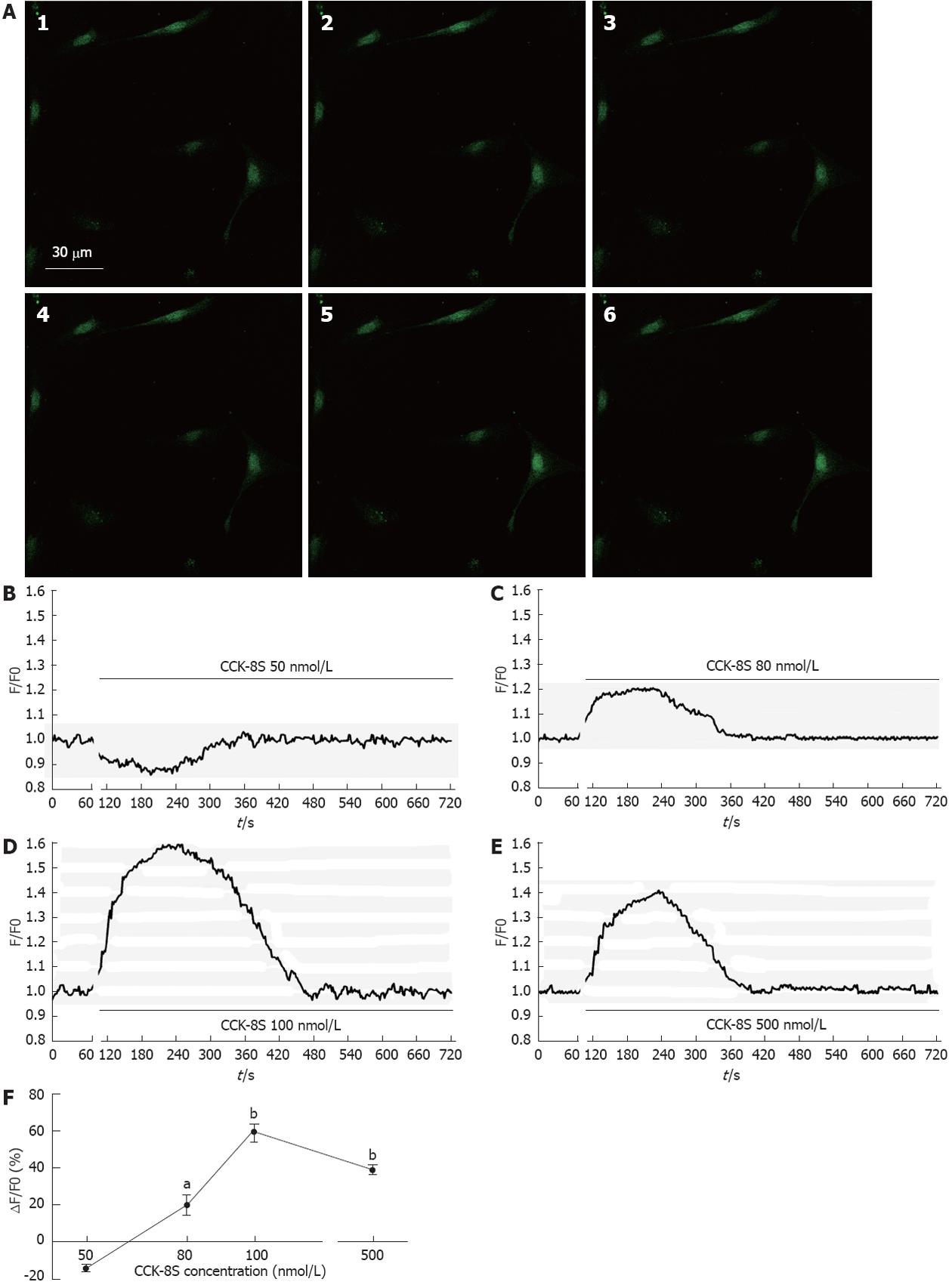
Addition of CCK-8S produced substantial, dose-dependent elevations of Fluo-3/AM fluorescence in cytoplasm an nucleus of the ICC, indicating that free calcium level had increased compared with the control (Figure 2A). When given ≤ 50 nmol/L CCK-8S, the [Ca2+]i did not increase (Figure 2B). As shown in Figure 2D, CCK-8S (100 nmol/L) significantly increased the mean [Ca2+]i by 59.30% ± 4.85% (P < 0.01, n = 6) and CCK-8S (80 nmol/L and 500 nmol/L) also evoked [Ca2+]i increases in the percentage of cells responding (20.22% ± 5.48% and 39.32% ± 2.51%, respectively, Figure 2C, E and F). Group data for the [Ca2+]i changes in response to CCK-8S at different concentrations are shown in Figure 2F.
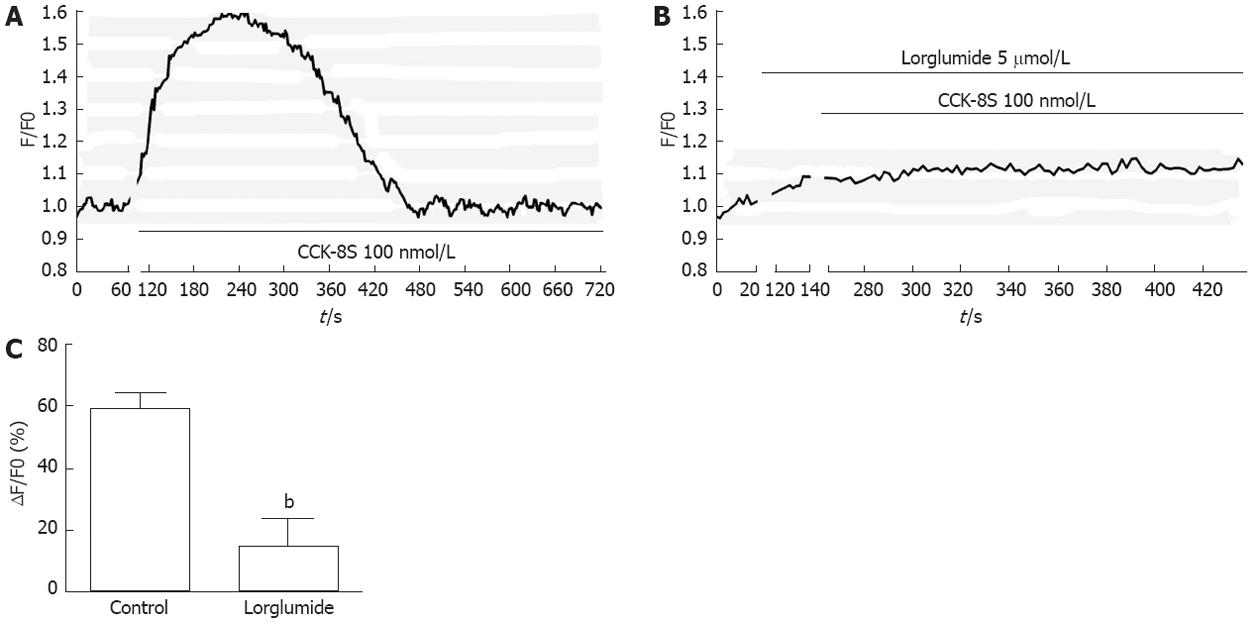
The CCK1 receptor (CCK1R) has been reported to be expressed in GI ICC[14]. To identify the subtype of CCK receptor involved in the CCK-8S-induced increase in [Ca2+]i, the CCK1R selective antagonist lorglumide was employed. Pretreatment of ICC with 5 μmol/L lorglumide for 2 min inhibited 100 nmol/L CCK-8S-induced [Ca2+]i increment from 59.30% ± 4.85% to 14.97% ± 9.05% (P < 0.01, n = 6) (Figure 3), suggesting a CCK1R-mediated event.
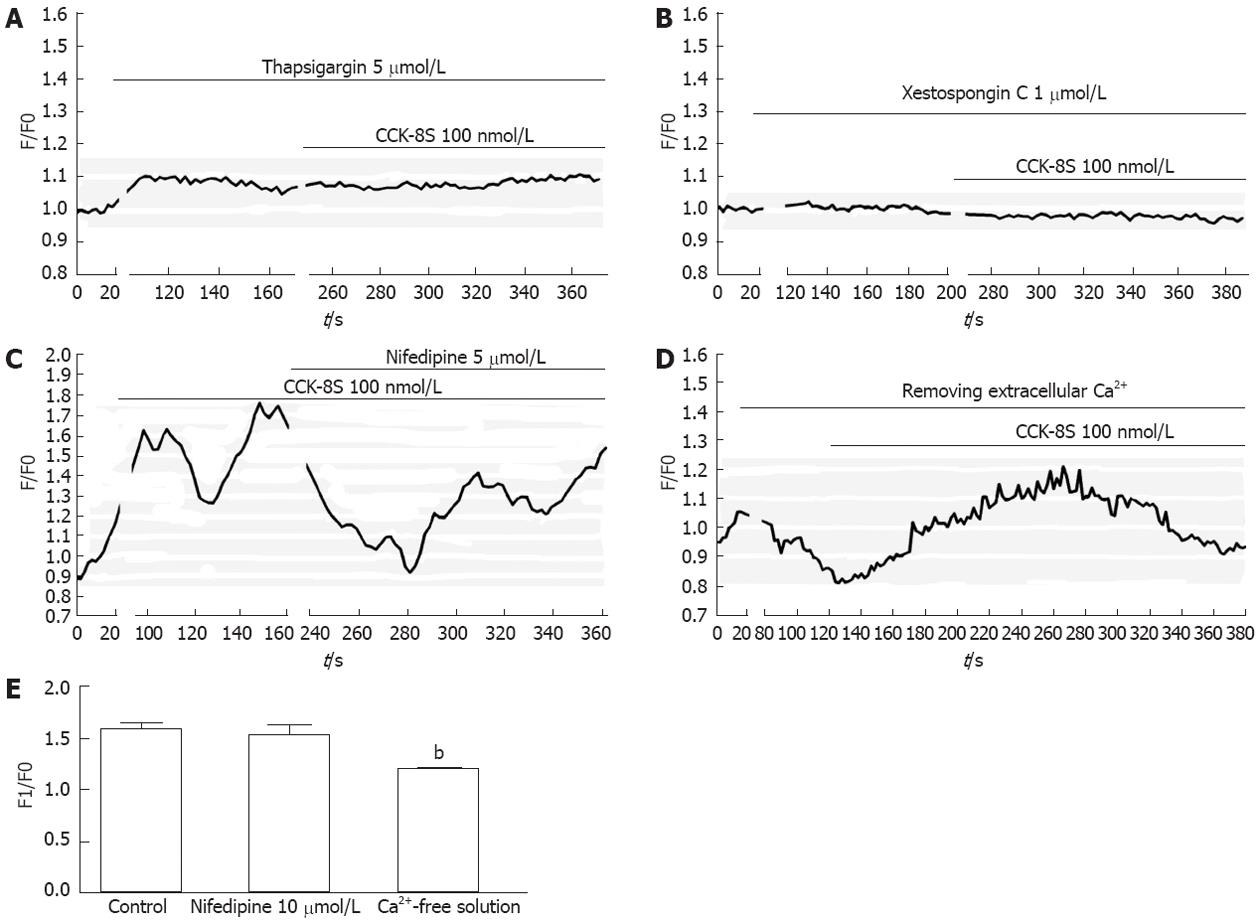
To determine the source of CCK-8S-induced calcium mobilization, intracellular calcium release and extracellular calcium influx were investigated. Firstly, the ICC were exposed to CCK-8S in a medium without extracellular calcium and subsequently to specific intracellular Ca2+-ATPase inhibitor thapsigargin. Compared with control, emptying of intracellular calcium stores by thapsigargin (5 μmol/L) prevented CCK-8S (100 nmol/L) to induce a [Ca2+]i increase (Figure 4A), indicating that Ca2+ release from intracellular stores appeared to be a major mechanism responsible for CCK-8S-induced calcium mobilization in the ICC. This result was similar to the effect of CCK-8S in murine gastric smooth muscle cells[18], but unlike that in murine myenteric neurons[19]. To further understand the mechanisms of CCK-8S-induced intracellular calcium release, the specific InsP3R inhibitor xestospongin C was used. Xestospongin C (1 μmol/L) completely abolished [Ca2+]i increases triggered by CCK-8S (Figure 4B).
Removing extracellular Ca2+ or blocking L-type voltage-operated calcium channel by nifedipine partly decreased the effect of CCK-8S (Figure 4C-E). These data indicate that the [Ca2+]i release is not stimulated or activated by the influx of extracellular Ca2+ in gastric antrum ICC, while the influx of extracellular Ca2+ can facilitate the [Ca2+]i increase evoked by CCK-8S.
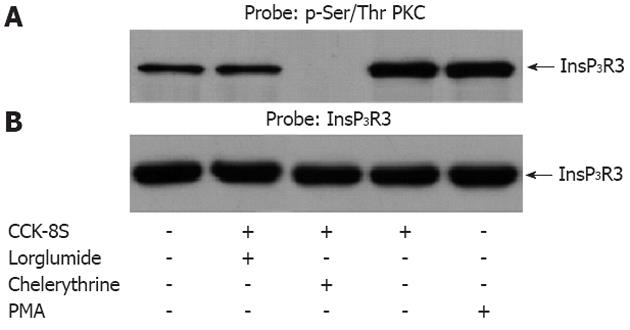
Experiments were undertaken to determine whether CCK-8S could evoke PKC to increase phosphorylation of InsP3R3. Samples containing equal amounts of protein were stimulated with CCK-8S (100 nmol/L) before or following administration of chelerythrine. PMA was used as a positive control. All drug/agonist treatments were 5 min in duration. CCK-8S resulted in a markedly increased phosphorylation of InsP3R3 in the ICC as compared with unstimulated ICC. When pretreated with chelerythrine for 5 min, CCK-8S-induced InsP3R3 phosphorylation was completely inhibited. Pretreatment with CCK1R lorglumide (5 μmol/L) significantly reduced the CCK-8S intensified phosphorylation of InsP3R3. As a positive control, PMA also enhanced phosphorylation of InsP3R3 in ICC (Figure 5).
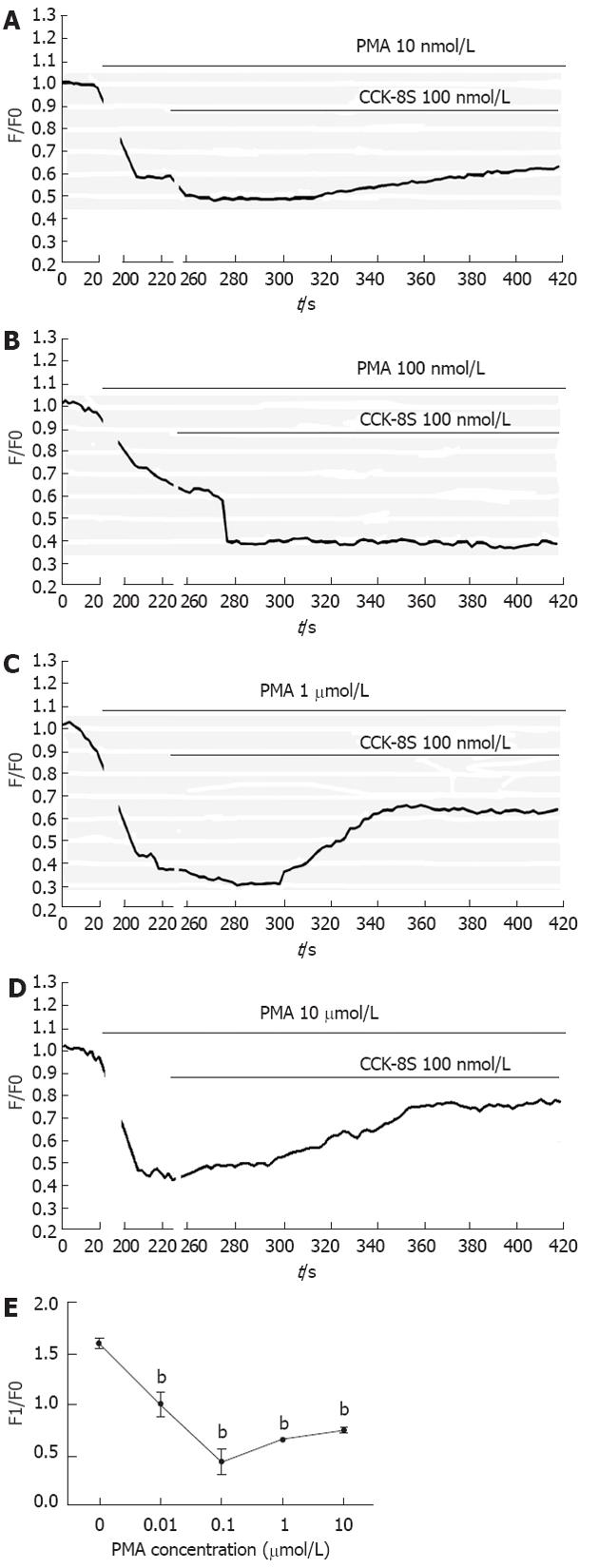
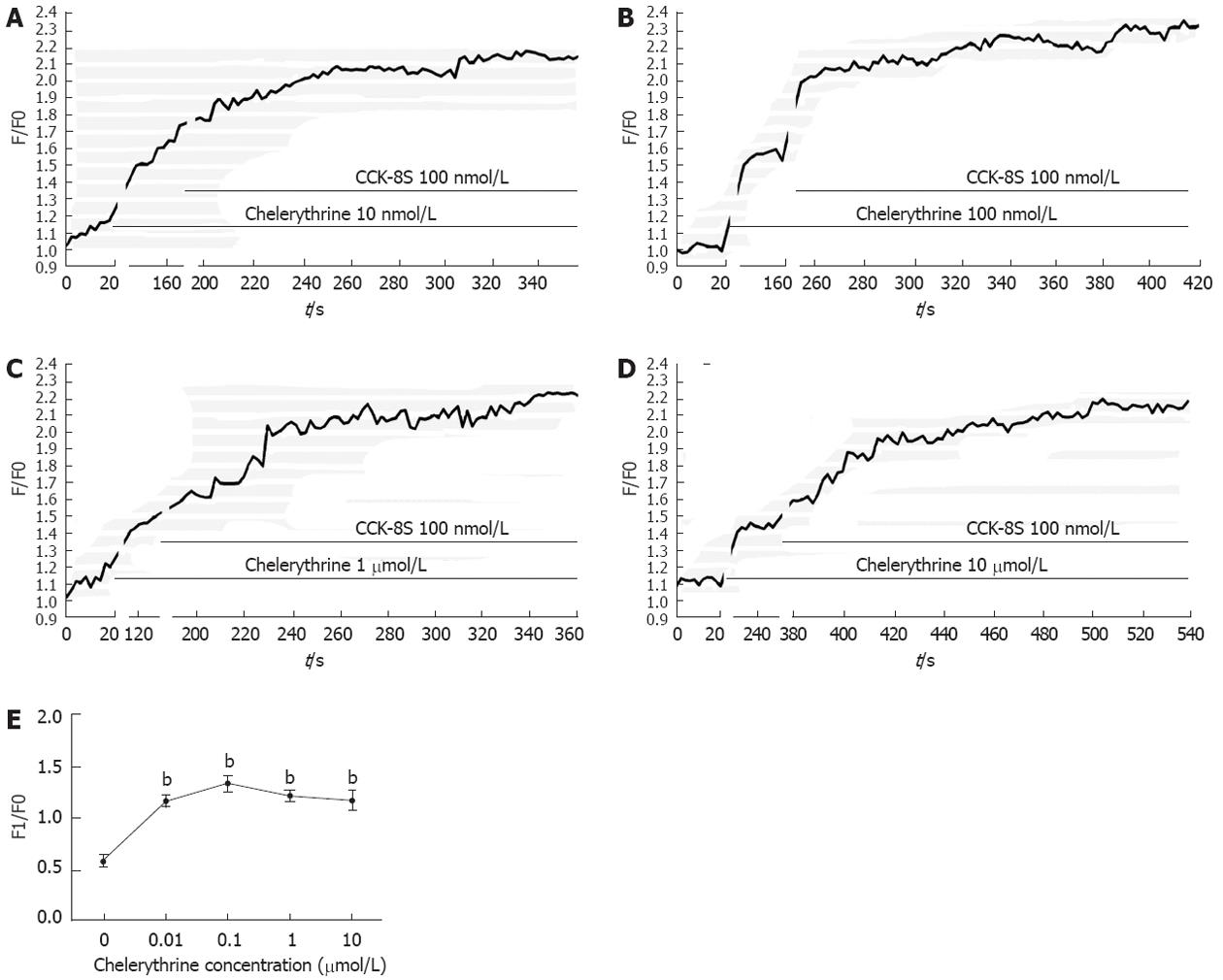
To investigate functional consequence of InsP3R3 phosphorylation by PKC on [Ca2+]i changes of the ICC, the following experiments were performed. Followed by CCK-8S (100 nmol/L), PMA at various concentrations could significantly reduce the CCK-8S-evoked [Ca2+]i response (P < 0.01, n = 6, Figure 6), and the effect of 100 nmol/L PMA was most obvious. Under the same conditions, chelerythrine showed the opposite effect to PMA (P < 0.01, n = 6, Figure 7), and 100 nmol/L chelerythrine exhibited the maximum effect.
In recent years, many studies have focused on the effects of CCK receptor ligands on GI motor functions, as the pharmacological characterization of these agents in humans is of potential therapeutic value[20,21]. CCK and its related peptides have been implicated in the pathophysiology of functional digestive diseases, such as functional dyspepsia, achalasia of cardia and irritable bowel syndrome[22,23]. Previous studies suggested that either an altered release of CCK or abnormal responses to this peptide could contribute to symptoms of GI dysmotility[24,25]. However, the mechanisms of the effects of CCK on GI motility are unclear. Previous studies revealed that CCK could evoke calcium signaling in cultured myenteric neurons and activate them[19,26]. The GI ICC also express CCK1R and play an important role in regulating the GI motility; therefore, it is meaningful and necessary to understand the effect of CCK on the ICC in controlling GI motility. Our study indicated that CCK-evoked gastric contraction is probably mediated through direct action on CCK1R located on the ICC. However, with respect to GI motility, both the ICC network and the gastrointestinal nervous system play essential roles in producing highly coordinated peristalsis[27,28]. Additional studies are needed to investigate the role of interactions between the enteric neurons and the ICC in CCK-evoked effect.
In the GI ICC, the cytoplasmic Ca2+ oscillation is responsible for the pacemaker activity. The pacemaker activity is generated in the ICC and then transferred to smooth muscle cells through the gap junctions[1,29]. In this study, we proved that CCK-8S markedly increased [Ca2+]i in cultured gastric antrum ICC, and the biological effects of CCK-8S were mainly mediated via CCK1R located on the ICC. Two major mechanisms are involved in [Ca2+]i increment during the contraction: calcium release of endoplasmic reticulum Ca2+ store, and/or calcium influx from the extracellular space through activation of calcium channels[29]. We have shown that emptying of the intracellular calcium stores by thapsigargin completely blocked the enhancement effect of CCK-8S on the [Ca2+]i level of ICC. Removing extracellular calcium could also inhibit the effect of CCK-8S, but not abolish it. Therefore, both mechanisms mediate the CCK-8S action and Ca2+ release from intracellular stores appears to be the major mechanism. The CCK-8S-induced [Ca2+]i increment could be abolished by blockage of InsP3R, suggesting a predominant role of InsP3R-operated stores in CCK-8S-induced intracellular Ca2+ release. However, the CCK-8S-evoked [Ca2+]i increment was persistent in the presence of L-type calcium channel blocker nifedipine, indicating that the [Ca2+]i release is not activated by the influx of extracellular calcium, while the influx of extracellular calcium can facilitate the [Ca2+]i increase evoked by CCK-8S.
InsP3Rs are a family of Ca2+ channels of the endoplasmic reticulum (ER) that are widely distributed in different tissues[30,31]. InsP3 triggers opening of the InsP3R, which can rapidly release Ca2+ stored in the ER into the cytosol, generating a transient increase of [Ca2+]i[32]. The kinetics of [Ca2+]i response depends on the amount of InsP3, but many other signaling pathways participate in modulating the response. Among them, phosphorylation of InsP3R by a series of kinases has been reported[16,33,34]. In the present study, increased phosphorylation of InsP3R3 in gastric ICC induced by CCK-8S was significantly prevented by pretreatment with the PKC specific inhibitor chelerythrine, while treatment of cells with the PKC activator PMA alone resulted in an enhanced phosphorylation of InsP3R3, indicating an important regulatory role of PKC activation in this event. In addition, PMA potently reduced the peak of CCK-8S-induced calcium oscillation, while chelerythrine exhibited an opposite effect. Thus, we might conclude that the activation of PKC negatively regulates CCK-8S-evoked calcium mobilization by phosphorylation of InsP3R3. It is consistent with our previous study in other cells[18]. The inhibition of Ca2+ release by PKC may be very useful to avoid full ER-Ca2+ emptying after agonist stimulation, which could have deleterious effects for the cell, first because of the waste of energy that would result from having a full ER-Ca2+ emptying after each agonist stimulation and second because of the effects of ER-Ca2+ depletion in terms of triggering the activation of stress signaling pathways[35,36].
In summary, data obtained in our study suggest that: (1) CCK-8S could evoke calcium mobilization in cultured ICC; (2) the biological effects of CCK-8S are probably via CCK1R located on the ICC; (3) this process is mainly mediated by the release of InsP3-dependent intracellular Ca2+ from ER; and (4) CCK-8S could activate PKC simultaneously, which increases the phosphorylation of InsP3R3 to negatively regulate the intracellular Ca2+ release in the ICC.
The interstitial cells of Cajal (ICC) are pacemaker cells in the gastrointestinal (GI) tract; their pacemaker activity is mediated by rhythmic intracellular Ca2+ oscillation. Cholecystokinin (CCK) contributes to the regulation of GI motility. To date, the mechanisms of the regulatory effects of CCK remain unclear. The finding that ICC express CCK1 receptor (also known as CCK-AR) suggests a role for ICC in the mediation of CCK effects. It is worth investigating the direct effect of CCK on intracellular Ca2+ concentration ([Ca2+]i) in the ICC.
Many neurotransmitters, including circulating hormones, modulate the ICC pacemaker activity. However, the effect of CCK on GI ICC remains unclear.
In the present study, the authors found that CCK evoked the calcium signaling in the ICC mainly via the Ca2+ release of InsP3R-operated stores and CCK activated protein kinase C simultaneously to negatively regulate the release of Ca2+ stores. The findings provide a novel insight into the mechanisms of the regulatory effects of CCK on GI motility.
The findings may provide clues to the mechanisms of the effects of CCK and its analogs in the treatment of GI motility disorders to some extent.
The authors investigated the effect of sulfated cholecystokinin octapeptide on calcium mobilization in cultured murine gastric antral interstitial cells of Cajal and its possible mechanisms. They found that cholecystokinin octapeptide could evoke the [Ca2+]i signaling in interstitial cells of Cajal through the Ca2+ release of InsP3R-operated stores. Additionally, CCK-8S activated PKC simultaneously to negatively regulate the release of [Ca2+] stores. The manuscript is highly interesting.
Peer reviewers: Guanglin Cui, MD, PhD, Laboratory of Gastroenterology, Institute of Clinical Medicine, University of Tromso, N-9037 Tromso, Norway; Fabio Grizzi, PhD, Senior Scientist, Laboratory of Molecular Gastroenterology, Istituto Clinico Humanitas IRCCS, Viale Monza 325, 20126 Milan, Italy
S- Editor Song XX L- Editor Stewart GJ E- Editor Li JY
| 1. | Boddy G, Bong A, Cho W, Daniel EE. ICC pacing mechanisms in intact mouse intestine differ from those in cultured or dissected intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G653-G662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 2. | Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 424] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 3. | Ordög T, Ward SM, Sanders KM. Interstitial cells of cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518:257-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 178] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | van Helden DF, Imtiaz MS, Nurgaliyeva K, von der Weid P, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol. 2000;524:245-265. [PubMed] [Cited in This Article: ] |
| 5. | Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393-1403. [PubMed] [Cited in This Article: ] |
| 6. | Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 221] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Vanderwinden JM, Rumessen JJ, Liu H, Descamps D, De Laet MH, Vanderhaeghen JJ. Interstitial cells of Cajal in human colon and in Hirschsprung's disease. Gastroenterology. 1996;111:901-910. [PubMed] [Cited in This Article: ] |
| 8. | Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal in human gut and gastrointestinal disease. Microsc Res Tech. 1999;47:344-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 9. | Sanders KM, Koh SD, Ordög T, Ward SM. Ionic conductances involved in generation and propagation of electrical slow waves in phasic gastrointestinal muscles. Neurogastroenterol Motil. 2004;16 Suppl 1:100-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Rehfeld JF. Clinical endocrinology and metabolism. Cholecystokinin. Best Pract Res Clin Endocrinol Metab. 2004;18:569-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Lal S, McLaughlin J, Barlow J, D'Amato M, Giacovelli G, Varro A, Dockray GJ, Thompson DG. Cholecystokinin pathways modulate sensations induced by gastric distension in humans. Am J Physiol Gastrointest Liver Physiol. 2004;287:G72-G79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 12. | Brennan IM, Little TJ, Feltrin KL, Smout AJ, Wishart JM, Horowitz M, Feinle-Bisset C. Dose-dependent effects of cholecystokinin-8 on antropyloroduodenal motility, gastrointestinal hormones, appetite, and energy intake in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E1487-E1494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Miyasaka K, Funakoshi A. Cholecystokinin and cholecystokinin receptors. J Gastroenterol. 2003;38:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Patterson LM, Zheng H, Ward SM, Berthoud HR. Immunohistochemical identification of cholecystokinin A receptors on interstitial cells of Cajal, smooth muscle, and enteric neurons in rat pylorus. Cell Tissue Res. 2001;305:11-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Herranz R. Cholecystokinin antagonists: pharmacological and therapeutic potential. Med Res Rev. 2003;23:559-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Straub SV, Giovannucci DR, Bruce JI, Yule DI. A role for phosphorylation of inositol 1,4,5-trisphosphate receptors in defining calcium signals induced by Peptide agonists in pancreatic acinar cells. J Biol Chem. 2002;277:31949-31956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | MacIntosh CG, Andrews JM, Jones KL, Wishart JM, Morris HA, Jansen JB, Morley JE, Horowitz M, Chapman IM. Effects of age on concentrations of plasma cholecystokinin, glucagon-like peptide 1, and peptide YY and their relation to appetite and pyloric motility. Am J Clin Nutr. 1999;69:999-1006. [PubMed] [Cited in This Article: ] |
| 18. | Si XM, Huang L, Paul SC, An P, Luo HS. Signal transduction pathways mediating CCK-8S-induced gastric antral smooth muscle contraction. Digestion. 2006;73:249-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Zhang W, Segura BJ, Mulholland MW. Cholecystokinin-8 induces intracellular calcium signaling in cultured myenteric neurons from neonatal guinea pigs. Peptides. 2002;23:1793-1801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Morton MF, Welsh NJ, Tavares IA, Shankley NP. Pharmacological characterization of cholecystokinin receptors mediating contraction of human gallbladder and ascending colon. Regul Pept. 2002;105:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Varga G, Bálint A, Burghardt B, D'Amato M. Involvement of endogenous CCK and CCK1 receptors in colonic motor function. Br J Pharmacol. 2004;141:1275-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 22. | Lee KJ, Tack J. Duodenal implications in the pathophysiology of functional dyspepsia. J Neurogastroenterol Motil. 2010;16:251-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | El-Salhy M, Vaali K, Dizdar V, Hausken T. Abnormal small-intestinal endocrine cells in patients with irritable bowel syndrome. Dig Dis Sci. 2010;55:3508-3513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Fornai M, Colucci R, Antonioli L, Crema F, Buccianti P, Chiarugi M, Baschiera F, Ghisu N, Tuccori M, Blandizzi C. Cholecystokinin CCK2 receptors mediate the peptide's inhibitory actions on the contractile activity of human distal colon via the nitric oxide pathway. Br J Pharmacol. 2007;151:1246-1253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Washington MC, Murry CR, Raboin SJ, Roberson AE, Mansour MM, Williams CS, Sayegh AI. Cholecystokinin-8 activates myenteric neurons in 21- and 35-day old but not 4- and 14-day old rats. Peptides. 2011;32:272-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Takaki M, Suzuki H, Nakayama S. Recent advances in studies of spontaneous activity in smooth muscle: ubiquitous pacemaker cells. Prog Biophys Mol Biol. 2010;102:129-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Mostafa RM, Moustafa YM, Hamdy H. Interstitial cells of Cajal, the Maestro in health and disease. World J Gastroenterol. 2010;16:3239-3248. [PubMed] [Cited in This Article: ] |
| 29. | Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008;586:5047-5061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 264] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 30. | Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 31. | Janiak R, Wilson SM, Montague S, Hume JR. Heterogeneity of calcium stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2001;280:C22-C33. [PubMed] [Cited in This Article: ] |
| 32. | Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901-930. [PubMed] [Cited in This Article: ] |
| 33. | Soulsby MD, Wojcikiewicz RJ. The type III inositol 1,4,5-trisphosphate receptor is phosphorylated by cAMP-dependent protein kinase at three sites. Biochem J. 2005;392:493-497. [PubMed] [Cited in This Article: ] |
| 34. | Murthy KS. cAMP inhibits IP(3)-dependent Ca(2+) release by preferential activation of cGMP-primed PKG. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1238-G1245. [PubMed] [Cited in This Article: ] |
| 35. | Montero M, Lobatón CD, Gutierrez-Fernández S, Moreno A, Alvarez J. Modulation of histamine-induced Ca2+ release by protein kinase C. Effects on cytosolic and mitochondrial [Ca2+] peaks. J Biol Chem. 2003;278:49972-49979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Hirose K, Kadowaki S, Tanabe M, Takeshima H, Iino M. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science. 1999;284:1527-1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 401] [Article Influence: 16.0] [Reference Citation Analysis (0)] |