Published online Jun 21, 2012. doi: 10.3748/wjg.v18.i23.2956
Revised: April 5, 2012
Accepted: April 10, 2012
Published online: June 21, 2012
AIM: To determine whether lentivirus-mediated shRNA targeting the X-linked inhibitor of apoptosis protein (XIAP) gene could be exploited in the treatment of pancreatic cancer.
METHODS: Human pancreatic cancer cells Panc-1, Mia-paca2, Bxpc-3 and SW1990, infected with lentivirus, were analyzed by real-time polymerase chain reaction (PCR). Western blotting was used to examine XIAP protein levels, survivin and p-Akt to confirm the result of real-time PCR and determine the possible mechanism. The 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was used to measure IC50 to determine chemosensitivity to the chemotherapeutic drugs 5-fluorouracil (5-FU) and gemcitabine. A colony assay, MTT assay and a tumorigenicity experiment were used to study cell proliferation in vitro and in vivo. Caspase-3/7 activity, 4',6-diamidino-2-phenylindole-staining and flow cytometric measurements were used to study apoptosis in SW1990 cells.
RESULTS: XIAP proteins were found to be differentially expressed among pancreatic cancer cell lines Panc-1, Mia-paca2, Bxpc-3 and SW1990. Data of real-time PCR and Western blotting showed that XIAP was reduced persistently and markedly by lentivirus-mediated shRNA. Downregulation of XIAP by transfection with XIAP shRNA resulted in decreased p-Akt expression. XIAP shRNA also inhibited the growth of pancreatic cancer cells in vitro and in vivo, enhanced drug-induced apoptosis and increased chemosensitivity to 5-FU and gemcitabine. Results also suggest that inhibition of XIAP and subsequent p-Akt depletion may have an anti-tumor effect through attenuating the ability of cancer cells to survive.
CONCLUSION: Lentivirus-mediated gene therapy is an attractive strategy in the treatment of pancreatic cancer and justifies the use of lentivirus in pancreatic cancer gene therapy studies.
- Citation: Jiang C, Yi XP, Shen H, Li YX. Targeting X-linked inhibitor of apoptosis protein inhibits pancreatic cancer cell growth through p-Akt depletion. World J Gastroenterol 2012; 18(23): 2956-2965
- URL: https://www.wjgnet.com/1007-9327/full/v18/i23/2956.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i23.2956
Pancreatic cancer is one of the most aggressive human malignancies, with an extremely poor prognosis and a 5-year survival rate of only approximately 5%[1], partially due to the very little possibility of surgical resection and resistance to chemo-radiotherapy. Disordered apoptosis and abnormal proliferation have been linked with development of malignancy and treatment resistance[2,3]. Apoptosis, also termed programmed cell death, occurs via extrinsic or intrinsic signal transduction pathways[4,5]. Therefore, further understanding of the molecular mechanisms, the relationship between pancreatic cancer chemoresistance and disordered apoptosis and abnormal proliferation, can be important in trying to circumvent resistance to cancer therapy[6].
To date, 8 human inhibitor of apoptosis protein (IAP) family members [X-linked IAP (XIAP), cIAP1, cIAP2, IAP-like protein 2, melanoma IAP, neuronal apoptosis inhibitory protein, survivin and baculovirus IAP repeats repeat-containing ubiquitin conjugating enzyme] have been identified. XIAP, a member of the IAP family, plays an important role in regulating both apoptosis and cell proliferation. XIAP is one of the most important members of the IAP family. It is highly expressed in malignant tumor cells and promotes tumor cell invasion, metastasis, growth, survival and chemoresistance. It is reported that XIAP antagonists such as second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI increase caspase activity, and not only directly induce apoptosis of many types of tumor cell lines in vitro, but also suppress growth of established tumors in xenograft models in mice in vivo, while displaying little toxicity to normal tissues. These findings validate XIAP as a target for cancer gene therapy. XIAP is a key factor in malignancy development and treatment resistance, which is associated with disordered cell apoptosis and abnormal proliferation. It is clear that XIAP is one of the most efficient caspase inhibitors of the 8 proteins, and inhibition of apoptosis by XIAP is mainly coordinated through binding to initiator caspase-9 and effector caspase-3 and caspase-7[7-9]. In addition to caspase inhibition, XIAP induces nuclear factor-κB and mitogen-activated protein kinase activation during transforming growth factor-β and bone morphogenetic protein receptor signaling and overexpression. Akt (protein kinase B) represents a subfamily of serine/threonine kinases that promotes cell survival[10]. XIAP and Akt are functionally linked in maintaining homeostasis between cell death and cell proliferation[11]. XIAP may be a core link between the cell apoptosis signaling pathway and the cell survival signaling pathway. Targeting XIAP might simultaneously influence cell apoptosis and proliferation.
RNA interference (RNAi) is a sequence specific posttranscriptional gene silencing factor, which has been extensively used in the study of gene function and gene therapy for cancer[12]. The use of chemically synthesized small interfering RNA (siRNA) or siRNA-encoding plasmids to produce RNAi in mammalian cells by transfection is still limited in clinical application due to some disadvantages including transient expression, and low transfection efficiency especially in non-dividing cells and when it is necessary to generate long-time gene silencing in vivo. A lentivirus-based vector is considered to be a promising gene delivery tool because of its ability for specific, highly stable and functional knockout of gene expression in both dividing and non-dividing cells compared with retroviral vectors. In addition, there is minimal immunogenicity associated with lentivirus vectors compared with adenoviral vectors[13-15].
In this study, we constructed lentivirus vectors encoding shRNA targeting the human XIAP gene to study the possible mechanisms of the XIAP gene in regulating apoptosis and proliferation in pancreatic cancer. There was an inhibitory effect of XIAP gene shRNA on the growth of SW1990 cells in vitro and in vivo, which would be useful for the development of gene therapy approaches for pancreatic cancer treatment in clinical application.
Three self-complementary hairpin DNA oligos targeting XIAP mRNA and a negative control were synthesized and cloned into a lentivirus vector. A self-inactivating lentivirus vector pGCSIL-PUR (Genechem, Shanghai, China) containing a cytomegalovirus-driven puromycin and a U6 promoter upstream of the cloning restriction sites (AgeI and EcoRI) was used. Three coding regions corresponding to targeting human XIAP (GenBank Accession No: NM_001167) were selected as shRNA target sequences under the guide of the shRNA design protocol. We constructed 3 shRNA-XIAP and negative control lentivirus vectors, namely Lv-X1, Lv-X2, Lv-X3 and Lv-Xnc, respectively (Table 1). Oligonucleotides were annealed and inserted between the AgeI and EcoRI restriction sites of the lentivirus vector. They were confirmed by restriction mapping and DNA sequencing. Lentivirus vector DNA and packaging vectors (pHelper1.0, pHelper2.0) were then transfected into 293T cells. Forty-eight hours later, the supernatant containing the lentivirus particles was collected, filtered through the 0.45 μm cellulose acetate filters, and the titer of lentiviruses was determined by hole-by-dilution titer assay. The virus titers produced was approximately 109 TU/mL.
| Name | Target sequences selected | Sequence cloned into the vector |
| Lv-X1 | GGTGAAGGTGATAAAGTAA | f: 5’-CCGGTAGGTGAAGGTGATAAAGTAATTCAAGAGATTACTTTATCACCTTCACCTA TTTTTG-3’ |
| r: 5’-AATTCAAAAATAGGTGAAGGTGATAAAGTAATCTCTTGAATTACTTTATCACCTTCACCTA-3’ | ||
| Lv-X2 | CTTGAGGAGTGTCTGGTAA | f: 5’-CCGGCACTTGAGGAGTGTCTGGTAATTCAAGAGATTACCAGACACTCCTCAAGTGTTTTTG-3’ |
| r: 5’-AATTCAAAAACACTTGAGGAGTGTCTGGTAATCTCTTGAATTACCAGACACTCCTCAAGTG-3’ | ||
| Lv-X3 | GTGGTAGTCCTGTTTCAGC | f: 5’-CCGGAAGTGGTAGTCCTGTTTCAGCTTCAAGAGAGCTGAAACAGGACTACCACTTTTTTTG-3’ |
| r: 5’-AATTCAAAAAAAGTGGTAGTCCTGTTTCAGCTCTCTTGAAGCTGAAACAGGACTACCACTT-3’ | ||
| Lv-Xnc | TTCTCCGAACGTGTCACGT | f: 5’-CCGGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTG-3’ |
| r: 5’-AATTCAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAA-3’ |
SW1990, Panc-1, Mia-paca2 and Bxpc-3 human pancreatic cancer cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and antibiotics (100 mg/mL streptomycin and 100 U/mL penicillin) at 37 °C in a humidified incubator containing 5% CO2. SW1990 cells were maintained in DMEM and was plated into 6-well plates at 1 × 105 cells per well. Overnight when the cells reached 30%-50% confluence, they were infected with viral particles in the presence of polybrene (5 μg/mL final concentration) and ENi.S (Genechem) for 8 h at a multiplicity of infection (MOI) of 50, and then added to fresh medium. The transfected SW1990 cells were subcultured at an appropriate density in fresh DMEM and 90% of the cells were transfected at 5 d post-transfection as indicated by the expression of green fluorescent protein (GFP) (pGCSIL-GFP empty vector was used to observe the transfection efficiency of lentivirus particles). Pooled stable transfectants were established using puromycin (Sigma-Aldrich, St Louis, MI, United States) selection. Puromycin was added into the medium to select stably transfected cells at a concentration of 1 μg/mL. Puromycin-resistant colonies were picked up 14 d after transfection and stable transfectant cells were maintained in medium containing 0.5 μg/mL puromycin.
Total RNA was isolated using Trizol (Invitrogen). M-MLV reverse transcriptase (Fermentas) was used to create cDNA according to the manufacturer’s instructions: 1 μg RNA, Oligo dt18 as primer, 42 °C for 60 min, 70 °C for 5 min. Quantitative real-time polymerase chain reaction (RT-PCR) assays were carried out using SYBR TAQ real-time kits (TaKaRa Biotechnology, Otsu, Japan) and RT-PCR amplification equipment ABI PRISM 7900HT (Applied Biosystems, Foster City, CA, United States). The PCR primers used to detect XIAP and β-actin were as follows: XIAP, upstream primer 5’-GACAGTATGCAAGATGAGTCAAGTCA-3’, downstream primer 5’-GCAAAGCTTCTCCTCTTGCAG-3’, with a product length of 93 bp; β-actin, upstream primer 5’-ACTCTTCCAGCCTTCCTTCC-3’, downstream primer 5’-GTACTTGCGCTCAGGAGGAG-3’, with a product length of 232 bp. The quantitative RT-PCR parameters and analysis of results were performed as normal.
The cell extracts were prepared with lysis buffer radio immunoprecipitation assay containing 50 mmol/L Tris-HCl, pH 7.3, 150 mmol/L NaCl, 2% NP-40, 0.5% deoxycholate, 2 mmol/L ethylenediaminetetraacetic acid, 2 mmol/L NaF, and 1% Protease Inhibitor Cocktail (Pierce, Rockford, IL, United States). Total protein concentration was measured using the BCA assay kit (Sigma, Inc.) with bovine serum albumin as a standard according to the manufacturer’s instructions. Western blotting was performed with primer and secondary antibodies: (1) Goat antihuman XIAP polyclonal antibody (R and D Systems Inc., Minneapolis, MN, United States) 1:2000, secondary antibody, 1:10 000; (2) Rabbit antihuman survivin antibody (Novus Biologicals, Inc.) 1:1000, secondary antibody, 1:10 000; (3) Rabbit antihuman p-Akt antibody (Abcam, Inc.) 1:300, secondary antibody, 1:10 000; and (4) Mouse antihuman β-actin monoclonal antibody (Sigma, Inc.) 1:10 000, secondary antibody, 1:10 000. Densitometry was performed by Quantity One image analysis software.
Cells were plated in 96-well plates at 5 × 103 (to test drug sensitivity) or 1 × 103 (for the growth curves) per well. Cell growth was examined by 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay after lentivirus transfection once a day for 8 d. The cells were cultured in DMEM overnight to ensure that they adhered to the wall of the plates. Various concentrations of 5-fluorouracil (5-FU) or gemcitabine were added to the medium. After incubation for 72 h, 20 μL of 5 mg/mL MTT (Sigma, Inc.) was added to the medium and cultured for another 4 h, then 150 μL of dimethyl sulfoxide (Sigma, Inc.) was added into each well and shaken for 10 min. Absorbance of each well was read using a Bio-Rad model 550-microplate reader (Bio-Rad Co., CA, United States) at a wavelength of 490 nm. Semilogarithmic curves were drawn for cell survival and the logarithm of the drug concentration by SPSS16.0. The 50% inhibitory concentration (IC50) was determined according to the curves.
Approximately 1 × 103 non-transfected control cells and SW1990 cells stably transfected with Lv-Xnc, Lv-X1, Lv-X2, Lv-X3 were plated in 10-cm culture dishes. After 28 d, cells were fixed with methanol and stained with 0.1% crystal violet. Colonies were counted by visual inspection.
Caspase-3/7 activity was evaluated using the Caspase-Glo 3/7 Assay (Promega, Madison, United States) according to the manufacturer’s instructions. Cells (1 × 105 cells per well) were seeded in a 6-well plate incubated at 37 °C overnight, then specific concentrations of 5-FU or gemcitabine were added into the medium. The ultimate concentrations for both 5-FU and gemcitabine were 1, 10 and 0.1, 1 μg/mL. Plates were further incubated at 37 °C for 72 h and luminescence was measured at 3 s delay-time, 10 s duration using SIRIUS Luminometer V3.2 (Berthold, Inc., Germany). Caspase-3/7 activity was normalized to the number of viable cells (as determined by trypan blue staining). Caspase-3/7-fold induction was determined as the ratio between caspase-3/7 activities in treated and control cells.
Cells were seeded into 24-well plates on sterile round glass coverslips at a density of 2 × 104 cells per well. The cells were incubated in the medium with various concentration of 5-FU or gemcitabine for 72 h. Cells were then washed once with phosphate-buffered saline (PBS) and fixed in PBS containing 4% paraformaldehyde and 10% sucrose at room temperature for 15 min in the dark. Cells were labeled with 4’,6-diamidino-2-phenylindole (DAPI) in PBS (1 μg DAPI/mL) at room temperature for 2 min in the dark. Thereafter, cells were washed twice with PBS and once with distilled water, and mounted in glycergel (60%, 4 μL). Staining was visualized via fluorescence microscope. The apoptosis index (AI) of cultured SW1990 cells with different lentivirus transfection was calculated using the following formula. AI (%) = apoptotic cells/total cells × 100%.
Apoptosis was measured with an annexin V-fluorescein isothiocyanate Apoptosis Detection Kit (Beyotime institute of biotechnology, China). Cells were seeded in 6-well culture plates and divided into the following groups: non-transfected control, SW1990 cells stably transfected with Lv-Xnc, Lv-X1; SW1990 + 5-FU, Lv-Xnc + 5-FU, Lv-X1 + 5-FU; SW1990 + gemcitabine, Lv-Xnc + gemcitabine, Lv-X1 + gemcitabine. Each group contained three culture flasks. When the cells were 70%-80% confluent, cells were added with 1 μg/mL 5-FU or 0.1 μg/mL gemcitabine. After 72 h, the cells were harvested and washed in cold PBS. Annexin V and PI staining were carried out using the Annexin V-FITC Apoptosis Detection Kit according to the manufacturer’s protocol. Apoptotic cells were immediately analyzed by fluorescence-activated cell sorting analysis.
To determine whether the Lv-X1 silence XIAP gene could inhibit tumor development in vivo, non-transfected control cells, Lv-Xnc control, Lv-X1 transfected SW1990 cells (1.5 × 107 cells in 200 μL DMEM) were injected subcutaneously into the left axilla of BALB/c nude mice (6 mice per group). Tumor growth was monitored every 4 d in 2 dimensions with a vernier caliper, and tumor size was calculated according to the formula V = a2b/2, where a and b are the shortest and longest diameters, respectively.
All data are expressed as mean ± SD. Analysis was performed using analysis of variance or the Student t test. The relationship between XIAP protein level and IC50 was analyzed by Pearson linear correlation analysis. The criterion for significance was P < 0.05. All the statistical analysis was performed by SPSS16.0.
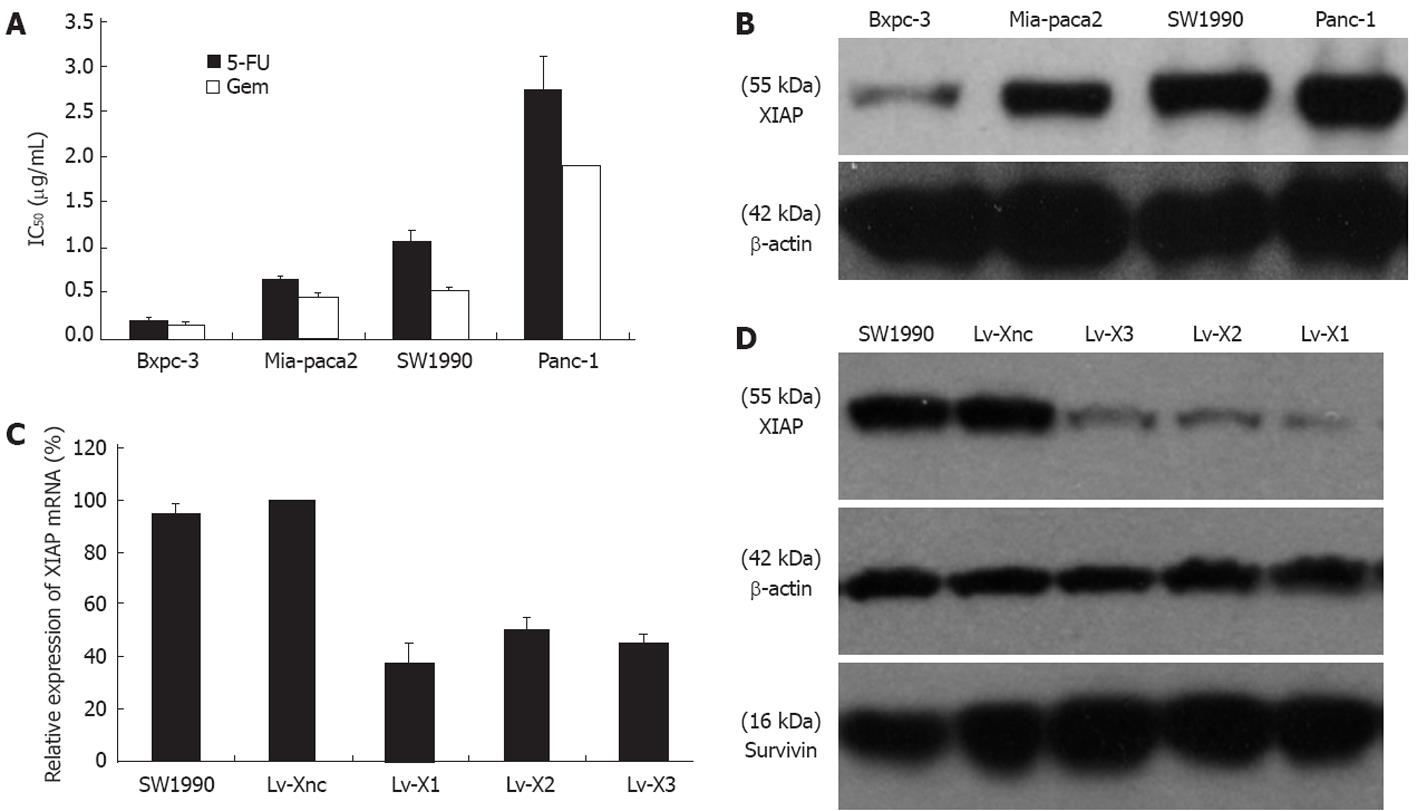
Levels of XIAP expression were highest in Panc-1 and SW1990 cell lines with a higher degree of 5-FU and gemcitabine chemoresistance than Mia-paca2 and Bxpc-3, which expressed XIAP at relatively lower levels (Figure 1A and B).
In order to exclude an off-target silencing effect mediated by specific shRNA, we designed 3 different sequences targeting XIAP and selected the most effective Lv-shRNA in this study. Real-time RT-PCR was performed after transfection and selection with puromycin. The XIAP mRNA expression in Lv-X1, Lv-X2 and Lv-X3 transfected SW1990 cells were reduced by 62.48% ± 7.67%, 49.62% ± 4.7% and 54.47% ± 2.7%, respectively, compared with the Lv-Xnc transfected control (P < 0.05). In addition, no difference was observed between the Lv-Xnc control and the SW1990 control (P > 0.05) (Figure 1C). Western blotting revealed that the inhibition efficiencies on XIAP protein expression by Lv-X1, Lv-X2, and Lv-X3 lentivirus were consistent with that on the targeted genes’ mRNA expression. XIAP protein was knocked down in Lv-X1, Lv-X2 and Lv-X3 transfected SW1990 cells, its expression demonstrated a significant reduction in Lv-X1 (5.98% ± 0.7%), Lv-X2 (12.32% ± 0.9%) and Lv-X3 (13.52% ± 2.2%) transfected SW1990 cells compared with the Lv-Xnc transfected control (P < 0.05). In addition, no difference was observed between the Lv-Xnc control and the SW1990 control (P > 0.05) (Figure 1D). According to the results of RT-PCR and Western blotting, Lv-X1 was the most effective lentivirus vector and thus we used it in the following research. To validate the specificity of RNAi targeting XIAP, we also determined the level of anther IAP family protein, survivin. The results showed that survivin was not affected by any constructed lentivirus (Figure 1D).
To determine the IC50, cells were exposed to 1000, 100, 10, 1, 0.1, 0.01 and 0.001 μg/mL 5-FU or gemcitabine for 72 h. The IC50 was calculated from MTT cytotoxicity assay data. Lv-X1 (0.2 ± 0.01 μg/mL for 5-FU, 0.14 ± 0.03 μg/mL for gemcitabine), Lv-X2 (0.72 ± 0.08 μg/mL for 5-FU, 0.42 ± 0.02 μg/mL for gemcitabine), Lv-X3 (0.5 ± 0.05 μg/mL for 5-FU, 0.28 ± 0.01 μg/mL for gemcitabine) inhibited the IC50 significantly (P < 0.05 vs Lv-Xnc), and Lv-X1 was the most effective. Lv-Xnc control had no effect on the IC50 (P > 0.05 vs SW1990 control) (Figure 2A). When compared with SW1990 and Lv-Xnc control cells, Lv-X1, Lv-X2 and Lv-X3 infected SW1990 transfected cells showed much slower growth, especially Lv-X1 infected SW1990 transfected cells. XIAP shRNA lentivirus transfection knockdown of XIAP significantly inhibited the proliferation of cultured SW1990 cells in vitro at the time points from day 4 to day 8 (P < 0.05) (Figure 2B). The result of the colony formation assay indicated that the number of colonies of Lv-X1 cells (55.26% ± 3.74%) were much less than that of Lv-Xnc (100.6% ± 5.07%) and non-transfected cells (100% ± 6.04%) (P < 0.05) (Figure 2C). To determine whether Lv-X1 knockout of the XIAP gene could inhibit tumor development in vivo, SW1990 control cells, Lv-Xnc control, and Lv-X1 infected SW1990 cells were injected into BALB/c nude mice and tumor growth was monitored every 4 d. At the time points of day 16 to day 28 after SW1990 implantation, the tumorigenicity experiments revealed that suppression of XIAP in the Lv-X1 group significantly inhibited the growth of the transplanted tumor in nude mice, which was consistent with the results achieved from the experiments in vitro (P < 0.05). For example, the tumor volume in nude mice at day 28 after the inoculation of SW1990 cells in groups of non-transfected, Lv-Xnc and Lv-X1 were 1223.28 ± 176.14, 1173.45 ± 149.61 and 532.83 ± 84.59 mm3, respectively. When compared with SW1990 and Lv-Xnc control cells, Lv-X1 infected SW1990 transfected cells developed much smaller tumors in the nude mice (P < 0.05) (Figure 2D).
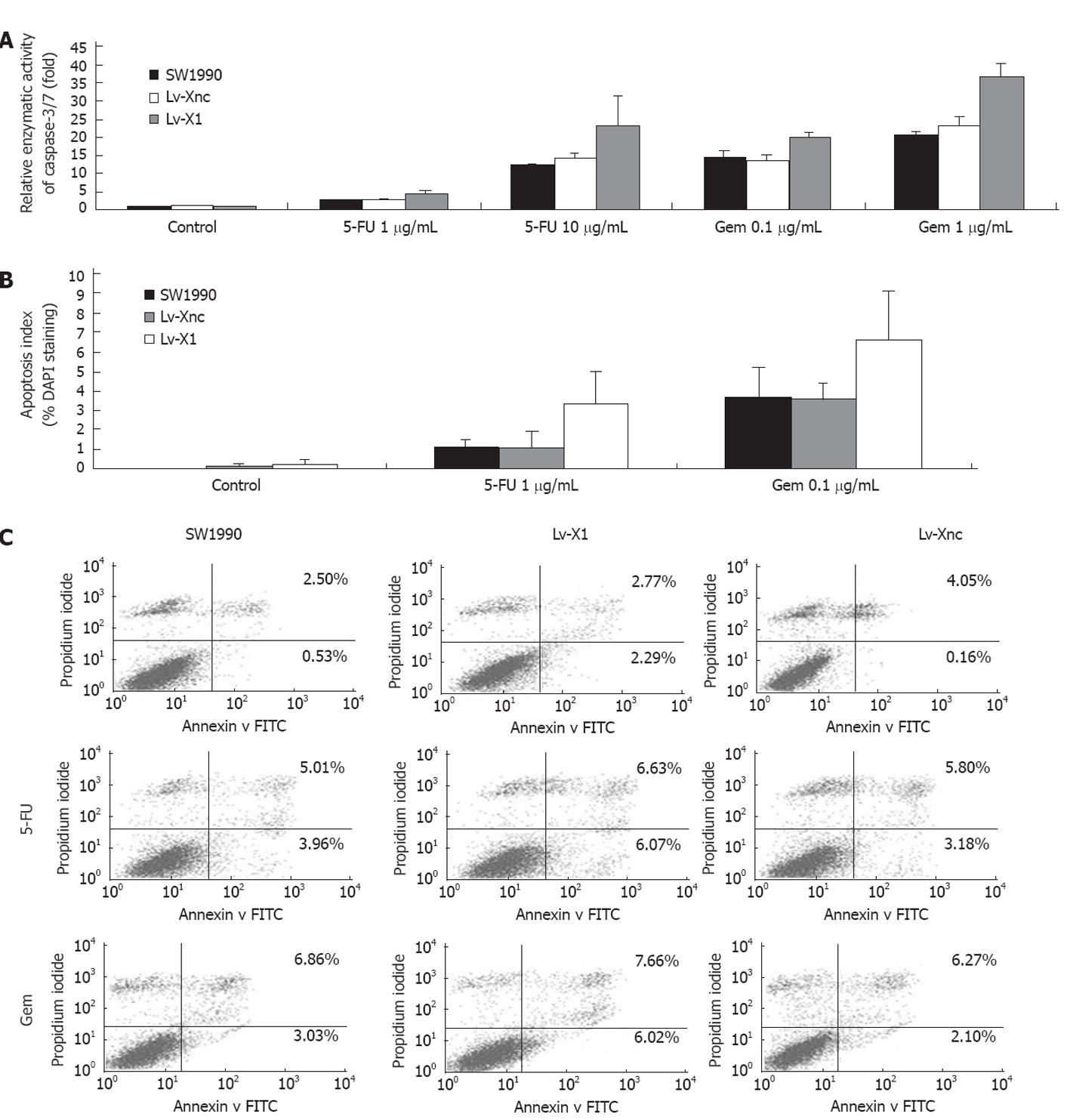
5-FU or gemcitabine induced apoptosis in cancer cells, via caspase activation and inhibition of apoptosis by XIAP, was mainly coordinated through binding to effector caspase-3 and caspase-7. Thus, we sought to determine the effect of XIAP silencing on caspase activities after exposure to different concentrations of 5-FU or gemcitabine for 72 h. 5-FU or gemcitabine-induced caspase-3/7 activity was markedly increased after transfection of Lv-X1 (4.41 ± 0.51-fold, 23.31 ± 8.14-fold at 1, 10 μg/mL 5-FU, respectively; 20.26 ± 0.96-fold, 36.62 ± 3.77-fold at 0.1, 1 μg/mL gemcitabine, respectively), but was unaffected after transfection of Lv-Xnc control. In addition, blank Lv-X1 (1.07 ± 0.03-fold) and Lv-Xnc (1.03 ± 0.01-fold) caspase-3/7 activity, although slightly increased, were not significantly different compared with SW1990 control (Figure 3A), which was also confirmed by DAPI staining and flow cytometry (FCM) (Figure 3B and C). We used DAPI staining to observe the morphological changes of apoptosis and the apoptosis index of DAPI staining analysis was consistent with caspase-3/7 activity (Figure 3B). To validate the results of DAPI staining, SW1990 and stably transfected SW1990 cells (Lv-Xnc, Lv-X1) were stained with annexin V and PI and analyzed by FCM. Cell apoptosis analysis indicated that downregulation of XIAP was not associated with a significantly increased spontaneous apoptosis rate (there were no obvious differences in apoptosis rates among SW1990 (3.03% ± 0.49%), Lv-X1 (5.06% ± 0.54%) and Lv-Xnc (4.21% ± 0.36%) (P > 0.05)), while the apoptosis of Lv-X1 + 5-FU (12.7% ± 0.50%) or Lv-X1 + Gem (13.68% ± 0.56%) was significantly increased compared with SW1990 and Lv-Xnc control cells (P < 0.05) (Figure 3C). All these results showed that the lentivirus-mediated inhibition of XIAP expression did not lead to acceleration of the apoptosis of SW1990 cells.
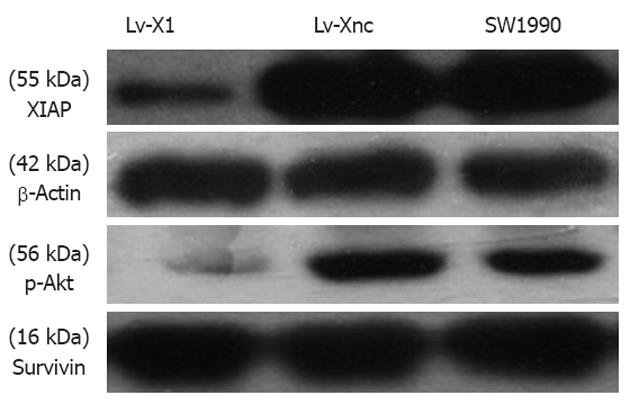
We observed that decreased expression of XIAP resulted in inhibition of cell proliferation according to the results of the growth curves and colony formation assay of different stably transfected cell lines (Figure 2B-D). Caspase-3/7 activity, DAPI staining and FCM analysis of Lv-X1 groups although slightly increased, showed no significant differences compared with controls (Figure 3A-C). It is reported that apoptotic pathways in cancer functionally crossover with survival pathways PI3K/Akt, and compared with the Lv-Xnc group, XIAP protein expression levels in cells transfected with Lv-X1 were reduced by 92.55% ± 0.78% (P < 0.05), and p-Akt protein expression in Lv-X1 transfection groups was reduced by 94.63% ± 0.32% (P < 0.05) (Figure 4). We found that downregulation of XIAP with Lv-X1 decreased p-Akt levels, which might explain the phenomenon.
It has been reported that XIAP is overexpressed in many human malignancies such as ovarian carcinoma, laryngeal cancer, esophageal cancer, breast cancer, hepatoma cells, colon cancer, and pancreatic cancer. Upregulated levels of XIAP expression have been correlated with tumor resistance to chemotherapy or radiotherapy, and some researchers have reported that inhibition of XIAP by siRNA, plasmid or adenovirus mediated-shRNA can reduce tumor cell growth, induce apoptosis and enhance the sensitivity of tumor cells to chemotherapeutic or radiotherapeutic agents[16-21]. Though chemically synthesized siRNA can be introduced into cells via traditional delivery strategies including liposomes, polyethyleneimine, or electroporation, these methods have short gene silencing effects and the effects cannot be passed to cell progeny. Other gene delivery systems such as adenovirus showed transient expression of the transgene and immunogenicity. Lentivirus vectors were developed to overcome these disadvantages. Recently, the lentivirus vector-mediated gene therapy including RNAi or overexpression has shown great promise in pancreatic cancer and other tumors because of its long-term gene expression and high efficiency in transducing dividing and nondividing cells[22-25].
As we know, Panc-1 and Mia-paca2 are poorly differentiated; SW1990 and Bxpc-3 are moderately differentiated. In our study, human pancreatic cancer cell lines with higher levels of XIAP protein displayed greater 5-FU or gemcitabine chemoresistance (Figure 1A and B).
In this study, we found that lentivirus-based vectors were extremely efficient in transducing SW1990 cells. In vitro, 79% of the cells expressed GFP at a MOI of 50. In addition, GFP expression was stable up to 6 mo in culture (data was not shown). To determine whether lentivirus-mediated shRNA could stably inhibit the expression of XIAP, pooled clones were expanded, and stably transfected cells were lysed for Western blotting analysis 6 mo later. The results showed that the expression of XIAP in Lv-X1 cell was still markedly reduced (Figure 4). It indicated that lentivirus-based shRNA resulted in a persistent gene knockdown instead of a transient inhibitory effect.
Furthermore, the strong inhibition of XIAP by lentivirus could inhibit proliferation of SW1990 cells (Figure 2B-D), enhance drug-induced apoptosis and promote chemosensitivity to chemotherapeutic drugs, which also were reported in other studies (Figure 3A-C)[26,27], and some drugs induce apoptosis through downregulation of cell survival proteins and upregulation of death receptors via the reactive oxygen species-mediated upregulation of the CHOP (CCAAT/enhancer-binding protein-homologous protein) pathway[28]. Some studies showed that silencing the XIAP gene resulted in a significant induction of apoptosis in pancreatic cancer cells Mia-paca2 and AsPC-1[29]. In this study, we did not find that downregulation of XIAP was associated with a significantly increased spontaneous apoptosis rate (Figure 3A-C), which was consistent with some other previous studies[30]. To validate the specificity of RNAi targeting XIAP and to avoid off-target phenomenon, we transfected the cells with lentivirus carrying 3 different shRNA sequences against XIAP and determined the level of another IAP family protein survivin (Figures 1-4). The results showed that survivin was not affected by any constructed lentivirus, which was not consistent with previous studies[31].
One of the greatest challenges for researchers in new treatment strategies for pancreatic cancer is the obvious need to test them in preclinical in vivo animal models that have a good probability of being predictive of similar activity in humans. The most used models are xenografts of human tumors grown subcutaneously in immunodeficient mice such as BALB/c nude mice[32]. In this study, we determined whether SW1990 cells stably transfected with lentivirus have reduced tumorigenicity. In the xenograft model, BALB/c nude mice that received injection of Lv-X1 cells developed tumors of a smaller size compared with control, and also showed higher levels of XIAP protein and greater tumorigenicity (Figure 2D). These findings were consistent with some previous studies in xenograft models, in which tumors from the parental pancreatic carcinoma cells with stable XIAP knockdown clones showed growth retardation[33].
Akt, which has been shown to have both prosurvival and antiapoptotic functions, is a serine/threonine kinase that is known to have at least 3 isoforms (Akt1, Akt2 and Akt3), all of which are activated by phosphoinositide 3-kinase (PI3K), and p-Akt is an activity form of Akt. It is reported that total Akt (phosphorylated + non-phosphorylated) was not altered by XIAP, nor was the expression of the p85 subunit of PI3K, suggesting a direct influence of XIAP upon Akt activation rather than an upregulation of Akt expression[34]. One of the Akt substrates identified to have antiapoptotic effects is Bad, which is the proapoptotic Bcl-2 family member that initiates apoptosis via binding antiapoptotic Bcl-2 family members and results in the release of cytochrome c from mitochondria. Akt has also been shown to directly phosphorylate and inactivate caspase-9[10,35], which is coordinated through binding to XIAP. Recently, XIAP was added to the list of Akt substrates. Akt has been shown to prevent the ubiquitination and degradation of XIAP via phosphorylation both in vitro and in vivo, and XIAP is a downstream target of Akt and a potentially important mediator of the effect of Akt on cell survival[36]. XIAP is also thought to promote Akt activity, XIAP acting as an E3 ubiquitin ligase for PTEN and promotes Akt activity by regulating PTEN content and compartmentalization, while XIAP silencing reduces constitutive mono- and poly-ubiquitination of PTEN, increases PTEN protein levels, and prevents nuclear accumulation of PTEN[37]. Furthermore, it has been reported that suppression of XIAP by either siRNA or antisense adenovirus of XIAP induced apoptosis and inhibited Akt-stimulated cell survival in ovarian cancer cells and uterine cancer cells[11,34]. These results are significant because they suggest a feedback regulation of XIAP and Akt. In this study, we found stable downregulation of XIAP with Lv-X1 in SW1990 pancreatic cancer cells markedly decreased the p-Akt protein level (Figure 4). Perhaps XIAP is a potentially important mediator of the effect of Akt on cell survival in pancreatic cancer cells as well as in ovarian and uterine cancer cells. While XIAP upregulates Akt phosphorylation and requires Akt for its function, so stable downregulation of XIAP markedly decreases the p-Akt protein level in SW1990 pancreatic cancer cells. Thus, it appears that there is an intricate, coordinated regulatory system at play between XIAP and the PI3K/Akt signaling pathway. Additional studies are necessary to determine the precise molecular mechanism by which XIAP regulates the Akt survival pathway.
In summary, our findings demonstrate for the first time that suppression of XIAP expression via lentivirus-mediated shRNA represents a novel strategy for chemosensitizing pancreatic cancer cells to chemotherapeutic drugs. Our study indicates that lentivirus-mediated inhibition of XIAP is an attractive therapeutic strategy in the treatment of pancreatic cancer and justifies the use of lentivirus in cancer gene therapy studies. However, emergence of replication competent lentivirus in vivo, transcriptional targeting affected by the chromosomal integration site and risk of oncogene activation by the lentivirus are current problems, and an effective and safe protocol should be developed. Thus, there remains a long road before lentivirus-mediated shRNA targeting XIAP can be introduced into clinical use.
Pancreatic cancer is one of the most aggressive human malignancies with an extremely poor prognosis and a 5-year survival rate of only approximately 5%, partially because of the low possibility of surgical resection and resistance to chemo-radiotherapy.
It has been reported that X-linked inhibitor of apoptosis protein (XIAP) is overexpressed in many human malignancies. The upregulated levels of XIAP expression have been correlated with tumor resistance to chemotherapy or radiotherapy. Lentivirus vector-mediated gene therapy has great promise in pancreatic cancer because of its long-term gene expression and high efficiency. Thus it is necessary to determine whether lentivirus-mediated shRNA targeting XIAP gene could be exploited in the treatment of pancreatic cancer.
XIAP proteins were found to be differentially expressed among pancreatic cancer cell lines. Downregulation of XIAP by transfection with XIAP shRNA resulted in decreased p-Akt expression. Moreover, it could inhibit the growth of pancreatic cancer cells in vitro and in vivo and enhance drug-induced apoptosis and promote chemosensitivity to chemotherapeutic drugs 5-fluorouracil and gemcitabine. Results also suggest that inhibition of XIAP and subsequent p-Akt depletion may have an anti-tumor effect through attenuating the ability of cancer cells to survive. Perhaps XIAP is a potentially important mediator of the effect of Akt on cell survival in pancreatic cancer cells. It appears that there is an intricate, coordinated regulatory system at play between XIAP and the phosphatidylinositol 3-kinases/Akt signaling pathway.
The results suggest that suppression of XIAP expression via lentivirus-mediated shRNA represents a novel strategy for chemosensitizing pancreatic cancer to chemotherapeutic drugs. Lentivirus-mediated inhibition of XIAP is an attractive therapeutic strategy in the treatment of pancreatic cancer and justifies the use of lentivirus in cancer gene therapy studies.
XIAP: A member of the IAP family, plays an important role in regulating both apoptosis and cell proliferation; Apoptosis or programmed cell death: Disordered apoptosis and abnormal proliferation have been linked to malignancy development and treatment resistance; Akt: Also termed protein kinase B, represents a subfamily of serine/threonine kinases that promotes cellular survival. RNAi: RNA interference, a sequence specific posttranscriptional gene silencing process, which has been extensively used in the study of gene function and gene therapy for cancer.
This is an interesting manuscript. The data is solid, but it is still a long way from lentivirus-mediated shRNA targeting XIAP to clinical use.
Peer reviewer: Run Yu, MD, PhD, Division of Endocrinology, Diabetes and Metabolism, Cedars-Sinai Medical Center, 8700 Beverly Blvd, B-131, Los Angeles, CA 90048, United States
S- Editor Cheng JX L- Editor Cant MR E- Editor Li JY
| 1. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [PubMed] [Cited in This Article: ] |
| 2. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [PubMed] [Cited in This Article: ] |
| 3. | Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ. Apoptosis and cancer: mutations within caspase genes. J Med Genet. 2009;46:497-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 461] [Cited by in F6Publishing: 480] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 4. | Qiao L, Wong BC. Targeting apoptosis as an approach for gastrointestinal cancer therapy. Drug Resist Updat. 2009;12:55-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Schimmer AD. Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004;64:7183-7190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 353] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Kim R, Tanabe K, Uchida Y, Emi M, Inoue H, Toge T. Current status of the molecular mechanisms of anticancer drug-induced apoptosis. The contribution of molecular-level analysis to cancer chemotherapy. Cancer Chemother Pharmacol. 2002;50:343-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 202] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Danson S, Dean E, Dive C, Ranson M. IAPs as a target for anticancer therapy. Curr Cancer Drug Targets. 2007;7:785-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543-1568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 404] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 9. | Lopes RB, Gangeswaran R, McNeish IA, Wang Y, Lemoine NR. Expression of the IAP protein family is dysregulated in pancreatic cancer cells and is important for resistance to chemotherapy. Int J Cancer. 2007;120:2344-2352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905-2927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3190] [Cited by in F6Publishing: 3220] [Article Influence: 128.8] [Reference Citation Analysis (0)] |
| 11. | Gagnon V, Van Themsche C, Turner S, Leblanc V, Asselin E. Akt and XIAP regulate the sensitivity of human uterine cancer cells to cisplatin, doxorubicin and taxol. Apoptosis. 2008;13:259-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Hannon GJ. RNA interference. Nature. 2002;418:244-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3058] [Cited by in F6Publishing: 2840] [Article Influence: 129.1] [Reference Citation Analysis (0)] |
| 13. | Manjunath N, Wu H, Subramanya S, Shankar P. Lentiviral delivery of short hairpin RNAs. Adv Drug Deliv Rev. 2009;61:732-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2007;39:803. [PubMed] [Cited in This Article: ] |
| 15. | Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8:573-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 495] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 16. | Wang R, Li B, Wang X, Lin F, Gao P, Cheng SY, Zhang HZ. Inhibiting XIAP expression by RNAi to inhibit proliferation and enhance radiosensitivity in laryngeal cancer cell line. Auris Nasus Larynx. 2009;36:332-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Zhang S, Ding F, Luo A, Chen A, Yu Z, Ren S, Liu Z, Zhang L. XIAP is highly expressed in esophageal cancer and its downregulation by RNAi sensitizes esophageal carcinoma cell lines to chemotherapeutics. Cancer Biol Ther. 2007;6:973-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Zhang Y, Wang Y, Gao W, Zhang R, Han X, Jia M, Guan W. Transfer of siRNA against XIAP induces apoptosis and reduces tumor cells growth potential in human breast cancer in vitro and in vivo. Breast Cancer Res Treat. 2007;103:129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Yamaguchi Y, Shiraki K, Fuke H, Inoue T, Miyashita K, Yamanaka Y, Saitou Y, Sugimoto K, Nakano T. Targeting of X-linked inhibitor of apoptosis protein or survivin by short interfering RNAs sensitize hepatoma cells to TNF-related apoptosis-inducing ligand- and chemotherapeutic agent-induced cell death. Oncol Rep. 2005;14:1311-1316. [PubMed] [Cited in This Article: ] |
| 20. | Dai Y, Qiao L, Chan KW, Yang M, Ye J, Zhang R, Ma J, Zou B, Lam CS, Wang J. Adenovirus-mediated down-regulation of X-linked inhibitor of apoptosis protein inhibits colon cancer. Mol Cancer Ther. 2009;8:2762-2770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Li Y, Jian Z, Xia K, Li X, Lv X, Pei H, Chen Z, Li J. XIAP is related to the chemoresistance and inhibited its expression by RNA interference sensitize pancreatic carcinoma cells to chemotherapeutics. Pancreas. 2006;32:288-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Jiang G, Li J, Zeng Z, Xian L. Lentivirus-mediated gene therapy by suppressing survivin in BALB/c nude mice bearing oral squamous cell carcinoma. Cancer Biol Ther. 2006;5:435-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Wang F, Chen L, Mao ZB, Shao JG, Tan C, Huang WD. Lentivirus-mediated short hairpin RNA targeting the APRIL gene suppresses the growth of pancreatic cancer cells in vitro and in vivo. Oncol Rep. 2008;20:135-139. [PubMed] [Cited in This Article: ] |
| 24. | Liau SS, Ashley SW, Whang EE. Lentivirus-mediated RNA interference of HMGA1 promotes chemosensitivity to gemcitabine in pancreatic adenocarcinoma. J Gastrointest Surg. 2006;10:1254-1262; discussion 1263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Ravet E, Lulka H, Gross F, Casteilla L, Buscail L, Cordelier P. Using lentiviral vectors for efficient pancreatic cancer gene therapy. Cancer Gene Ther. 2010;17:315-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Shrikhande SV, Kleeff J, Kayed H, Keleg S, Reiser C, Giese T, Büchler MW, Esposito I, Friess H. Silencing of X-linked inhibitor of apoptosis (XIAP) decreases gemcitabine resistance of pancreatic cancer cells. Anticancer Res. 2006;26:3265-3273. [PubMed] [Cited in This Article: ] |
| 27. | Vogler M, Walczak H, Stadel D, Haas TL, Genze F, Jovanovic M, Gschwend JE, Simmet T, Debatin KM, Fulda S. Targeting XIAP bypasses Bcl-2-mediated resistance to TRAIL and cooperates with TRAIL to suppress pancreatic cancer growth in vitro and in vivo. Cancer Res. 2008;68:7956-7965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Sung B, Park B, Yadav VR, Aggarwal BB. Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors. J Biol Chem. 2010;285:11498-11507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Rückert F, Samm N, Lehner AK, Saeger HD, Grützmann R, Pilarsky C. Simultaneous gene silencing of Bcl-2, XIAP and Survivin re-sensitizes pancreatic cancer cells towards apoptosis. BMC Cancer. 2010;10:379. [PubMed] [Cited in This Article: ] |
| 30. | McManus DC, Lefebvre CA, Cherton-Horvat G, St-Jean M, Kandimalla ER, Agrawal S, Morris SJ, Durkin JP, Lacasse EC. Loss of XIAP protein expression by RNAi and antisense approaches sensitizes cancer cells to functionally diverse chemotherapeutics. Oncogene. 2004;23:8105-8117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Zhu Y, Roshal M, Li F, Blackett J, Planelles V. Upregulation of survivin by HIV-1 Vpr. Apoptosis. 2003;8:71-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 32. | Céspedes MV, Casanova I, Parreño M, Mangues R. Mouse models in oncogenesis and cancer therapy. Clin Transl Oncol. 2006;8:318-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Mohr A, Albarenque SM, Deedigan L, Yu R, Reidy M, Fulda S, Zwacka RM. Targeting of XIAP combined with systemic mesenchymal stem cell-mediated delivery of sTRAIL ligand inhibits metastatic growth of pancreatic carcinoma cells. Stem Cells. 2010;28:2109-2120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Asselin E, Mills GB, Tsang BK. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 2001;61:1862-1868. [PubMed] [Cited in This Article: ] |
| 35. | Straszewski-Chavez SL, Abrahams VM, Aldo PB, Romero R, Mor G. AKT controls human first trimester trophoblast cell sensitivity to FAS-mediated apoptosis by regulating XIAP expression. Biol Reprod. 2010;82:146-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, Tsang BK, Cheng JQ. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J Biol Chem. 2004;279:5405-5412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 37. | Van Themsche C, Leblanc V, Parent S, Asselin E. X-linked inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J Biol Chem. 2009;284:20462-20466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |