Published online Jul 7, 2011. doi: 10.3748/wjg.v17.i25.3060
Revised: March 23, 2011
Accepted: March 30, 2011
Published online: July 7, 2011
AIM: To determine the effects of duodenogastric juice pH on the development of esophageal adenocarcinoma (EAC).
METHODS: An animal model of duodenogastroesophageal reflux was established using Sprague-Dawley (SD) rats undergoing esophagoduodenostomy (ED). The development of EAC was investigated in rats exposed to duodenogastric juice of different pH. The rats were divided into three groups: low-pH group (group A), high-pH group (group B) and a sham-operated group as a control (group C) (n = 30 rats in each group). The incidence of esophagitis, Barrett’s esophagus (BE), intestinal metaplasia with dysplasia and EAC was observed 40 wk after the treatment.
RESULTS: The incidence rate of esophagitis, BE, intestinal metaplasia with dysplasia and EAC was higher in groups A and B compared with the control group after 40 wk (P < 0.01), being 96% and 100% (P > 0.05), 88% and 82.4% (P > 0.05), 20% and 52.1% (P < 0.05), and 8% and 39% (P < 0.05), respectively.
CONCLUSION: Non-acidic refluxate increases the occurrence of intestinal metaplasia with dysplasia and EAC while the low-pH gastric juice exerts a protective effect in the presence of duodenal juice. The non-acid reflux is particularly important in the progression from BE to cancer. Therefore, control of duodenal reflux may be an important prophylaxis for EAC.
- Citation: Cheng P, Li JS, Gong J, Zhang LF, Chen RZ. Effects of refluxate pH values on duodenogastroesophageal reflux-induced esophageal adenocarcinoma. World J Gastroenterol 2011; 17(25): 3060-3065
- URL: https://www.wjgnet.com/1007-9327/full/v17/i25/3060.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i25.3060
The incidence rate of esophageal adenocarcinoma (EAC) has been increasing more rapidly than that of other malignancies[1]. This rapid increase may be related to the increasing occurrence of gastroesophageal reflux disease (GERD) and Barrett’s esophagus (BE)[2,3]. BE is the main risk factor and an acquired condition for these tumors[4]. Gastric acid has been regarded as the major risk factor for GERD, and acid suppression is the first-line treatment[5]. However, the role of the gastric acid in the development of GERD remains controversial.
Gastric juice refluxing into the esophagus contains gastric, biliary and pancreatic secretions that have refluxed into the stomach from the duodenum. Early studies showed that reflux of combined duodenal and gastric juices into the esophagus caused severe esophagitis[6], and reflux of duodenal juice alone resulted in a similar degree of esophageal injury[7]. It has been shown[8-12] that esophageal exposure to duodenal juice is a key factor in the genesis of BE and EAC. Some researchers have suggested that the obvious increase in the incidence of EAC might be related to the acid suppression[13]. However, to dynamically monitor the duodenal juices and clarify the role of duodenal juice reflux in the pathologic process has attracted much attention. Some studies have confirmed that duodenal juice reflux could induce BE and EAC in rats[10].
Improved animal models are therefore needed to examine the role of non-acidic reflux in EAC induced by duodenal juice reflux in the absence of exogenous carcinogens. The aim of this study was to investigate the roles of gastric and duodenal juices in the genesis of EAC in a rat model.
Ninety 8-wk-old Sprague-Dawley (SD) rats weighing 200-250 g were purchased from the Experimental Animal Center of Xi’an Jiao Tong University. The male pairing female rats were randomly divided into three groups, each with 30 rats.
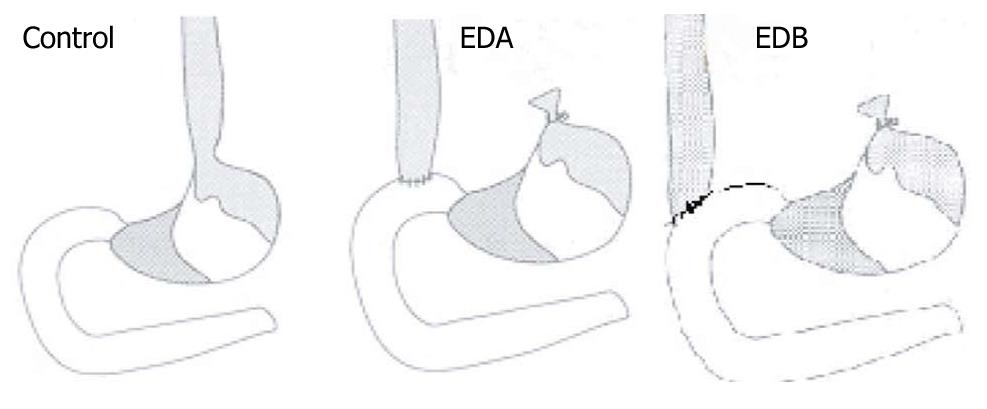
A rat model of duodenogastroesophageal reflux, with different pH values of reflux contents, was established in accordance with the method of Zhang et al[14]. Surgical diversion of duodenal secretions into the esophagus in the experimental group was induced by end-to-side esophagoduodenostomy (ED). The rats were divided into a low-pH group (group A) and a high-pH group (group B), with 30 rats in each group. A sham-operated group (group C, n = 30) was used as a control group (Figure 1).The esophagus was separated from the posterior vagal trunk and left gastric vessels, tied with silk at the gastroesophageal junction, and dissected 2 mm proximal to the tie. The anterior vagus nerve was protected from damage when the esophagus was cut with 16 interrupted stitches of 7-0 polypropylene. The purpose of the anastomosis was to induce the reflux of both gastric and duodenal juices into the esophagus. In group A, the anterolateral wall of the duodenum at the distal end 1 cm from the pylorus was opened longitudinally, and anastomosed with the cut end of the esophagus. In group B, the anterolateral wall of the duodenum at the distal end 2 cm from the pylorus was opened longitudinally and anastomosed with the cut end of the esophagus. In group C, the lower esophagus and the first portion of the duodenum were dissociated. Surgery was performed after an acclimatization period of 4 d. Rats were kept in hanging cages under a 12 h light-dark cycle at a temperature of 21°C and a humidity of 60%. Water and standard chow were provided ad libitum. Food was discontinued the evening before surgery, and water was discontinued on the morning of surgery. Rats were anesthetized with an intramuscular injection of xylazine hydrochloride (18 mg/kg) and ketamine (72 mg/kg), with further doses administered intraperitoneally during surgery, as required. Before closure, 0.5-1.5 mL of 0.9% sodium chloride was instilled into the peritoneal cavity. Water was permitted when the rats awoke, and chow was provided the next day. The rats were housed in cages at 22-25°C with free access to standard rat pellet food and water for 40 wk. Rats were treated following the guidelines for the care and use of laboratory animals of the National Animal Welfare Committee.
Intraluminal pH was measured using a pH glass electrode of Digitrapper MK Portable pH Monitor(Sweden Medtronic Synectics Company, Stockholm, Sweden). It was positioned in the distal end of the esophagus, the forestomach, the opisthogaster and the duodenum 1 and 2 cm from the pylorus in the process of esophagoduodenostomy. It was also measured after rats were killed 40 wk after operation.
Rats were killed 40 wk after operation. The esophagus was opened longitudinally and gross pathologic changes were observed. Esophagitis, BE and EAC were differentiated, and samples of the three abnormal tissues (0.2 × 0.2 cm2) were removed, and fixed in formalin. Paraffin sections were stained with hematoxylin-eosin and observed under a light microscope.
The incidence rates of esophagitis, BE and EAC between the groups were analyzed and compared using χ2 tests with SPSS software, and differences in numerical data were compared between groups using t test. The level of significance was set at P < 0.05.
The number of the surviving rats in the three groups was 25, 23 and 29, respectively. The overall mortality was 14.4%.
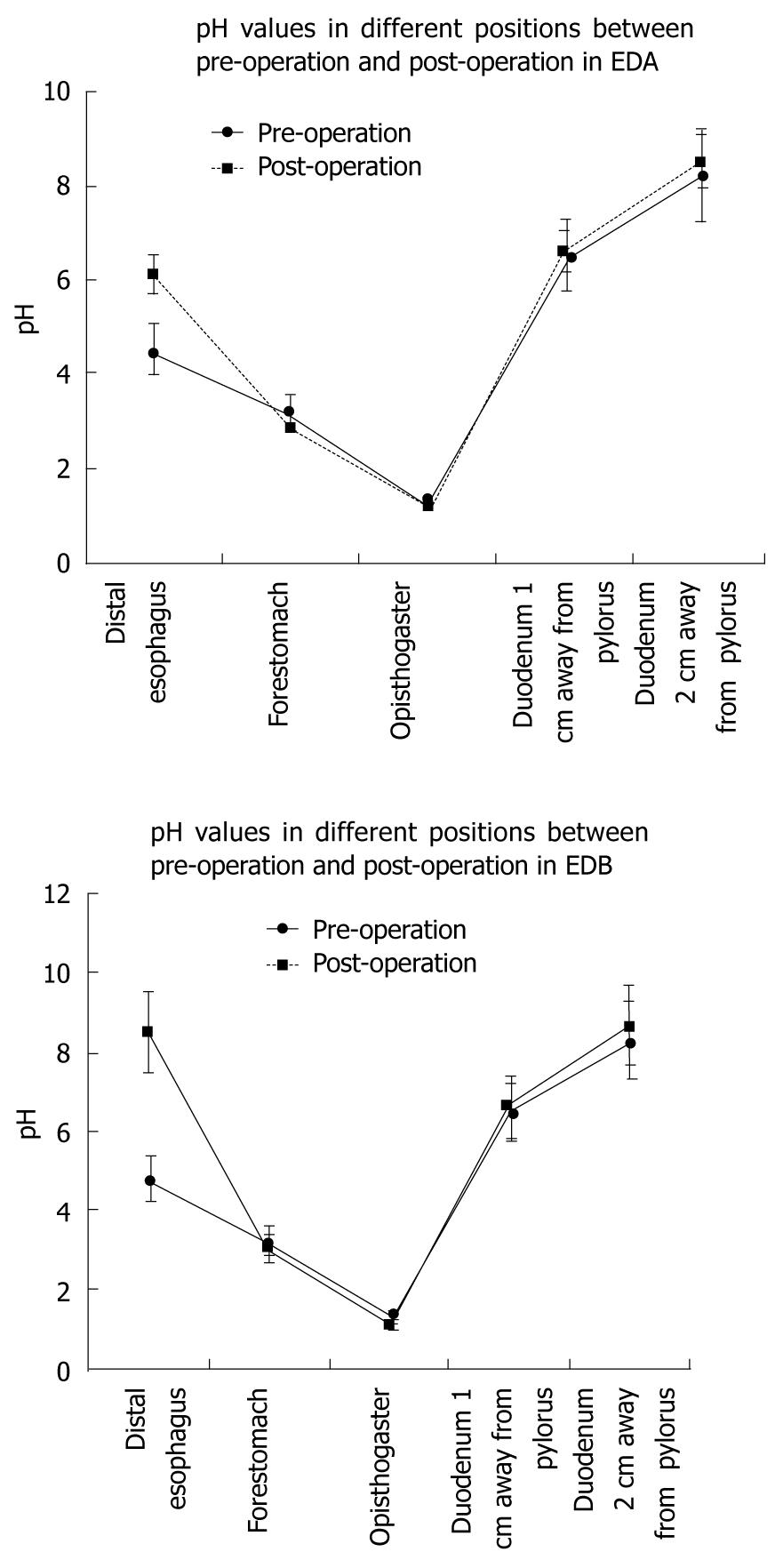
The pH values increased from the proventriculus down to the duodenum 2 cm away from the pylorus (P < 0.05), (Figure 2). The preoperative pHs at the distal end of the esophagus in groups A and B were significantly lower than the postoperative values (P < 0.05).
The pH value in the distal esophagus in group A, in which the duodenum was cut 1 cm from the pylorus, was 6.14 ± 0.36, which was significantly lower than that in group B (8.27 ± 0.46, P < 0.01). There was no significant difference in preoperative pH values in the distal esophagus between the two groups (P = 0.12).
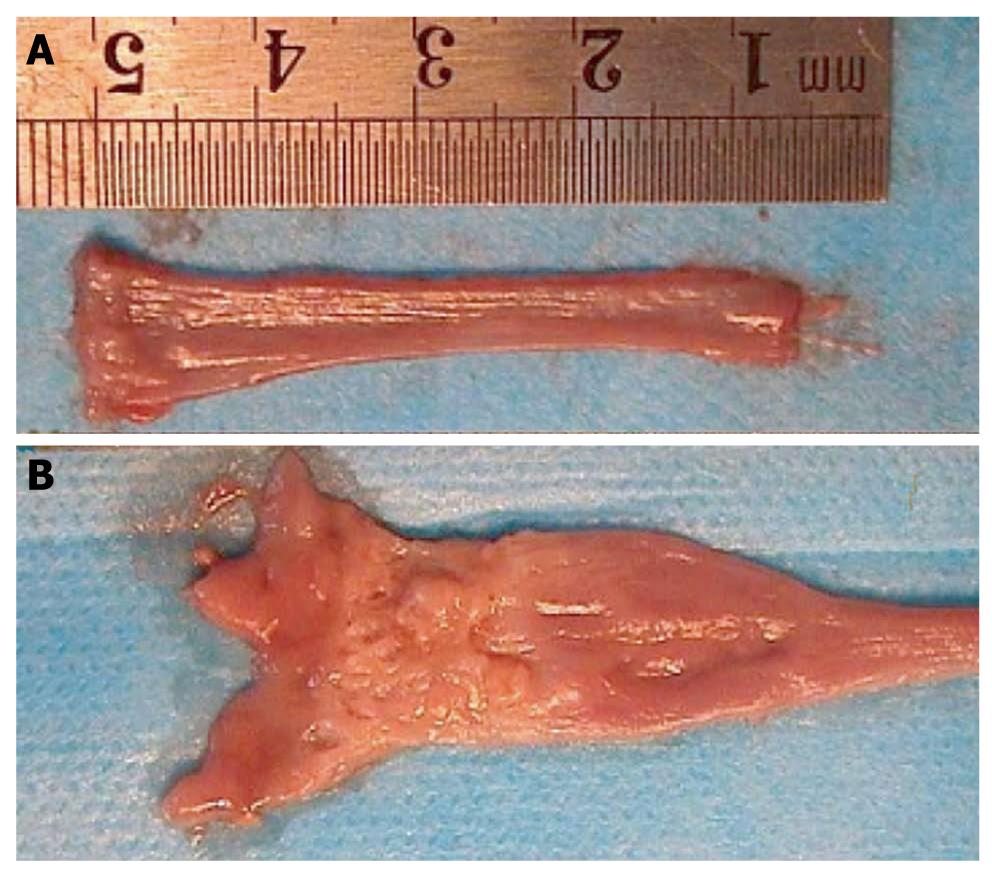
In the sham-operated group, the esophageal wall was thin, with a smooth mucosa, and the esophageal lumen was uniform in size along its length. Blood vessels were visible below the mucous membrane, and congestive inflammation was occasionally visible in the distal esophagus. In most animals in both groups, the lumen of the middle and the lower parts of the esophagus was dilated. Esophagitis appeared as mucosal hyperplasia, with a thickened, rough surface with small and large kernels in longitudinal rows, becoming less pronounced from the distal to the proximal end. Hyperemia, edema, mild erosion and indistinct blood vessels below the mucous membrane were visible at the proximal end. BE occurred mostly in the distal esophagus at the stoma between the esophagus and duodenum, and appeared as an unclear boundary between the esophagus and the duodenal mucosa. The esophagus was inflamed at the proximal end, smooth and velvet-like, with a clear boundary from the duodenum. The area of BE generally extended for about 0.5-2 cm, with chronic proliferation and inflammation at the proximal end of the esophageal mucosa. Small sheets of BE pathology were seen in some cases of esophagitis. The upper esophagus was normal in BE, and all esophageal adenocarcinomas (EACs) developed near the proximal end of the stoma in BE, with nodular hyperplasia, ulcer and fish-like appearance. The esophagus at the upper end of the tumor was obstructed, with obvious dilation and changes in the features of BE. The hyperplasia was reduced at the proximal end of the obstruction, and appeared as congestion of 1-2 mm and presented edema changes (Figure 3).
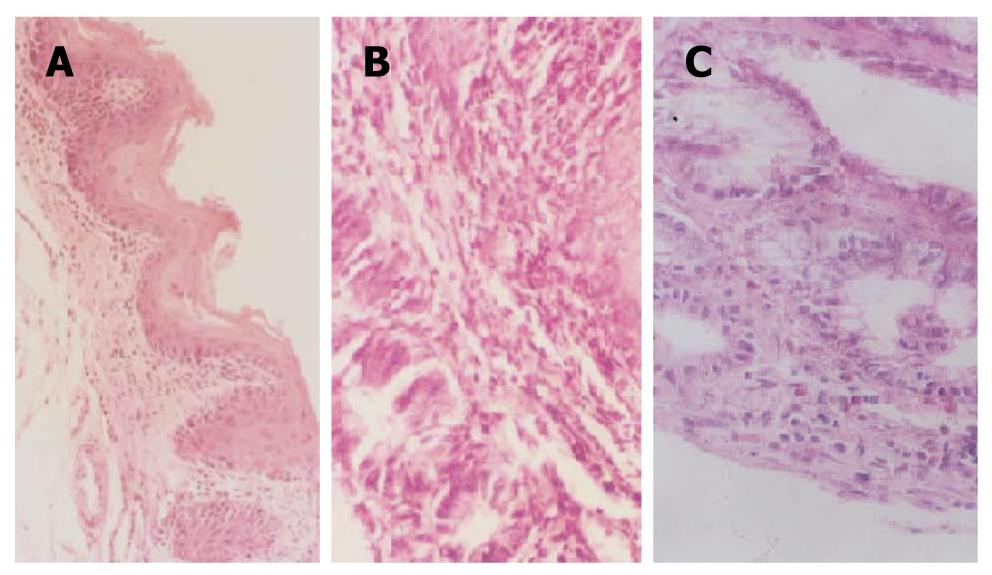
Normal esophageal epithelium appeared as stratified squamous epithelium, with neat rows, some showing keratinization. Esophagitis appeared as hyperplasia of the scaly epithelial basal cells, excessive keratinization and papillomatosis, visible neutrophilic granulocytes, infiltrated lympho-epithelioid cells, mucosal erosion and edema of the submucosa in the mucosa and the lower layers of the mucosa. BE showed replacement of the squamous mucosa with simple columnar epithelium. Intestinal metaplasia with dysplasia showed replacement of the mucosa with simple columnar epithelium, changes in the size and shape of hyperplastic cells, with large, hyperchromatic nuclei and increased nucleoplasm, irregular arrangement of the cells, disappearance of cell polarity, and irregular shape and arrangement of the glandular cells. However, these changes were not characteristic of cancer and no obviously abnormal cells could be seen and no pathologic invasion to the basilar membrane. EAC showed severe intestinal metaplasia with dysplasia, pathologic invasion to the basilar membrane, and some to the blood or lymphatic vessels (Figure 4).
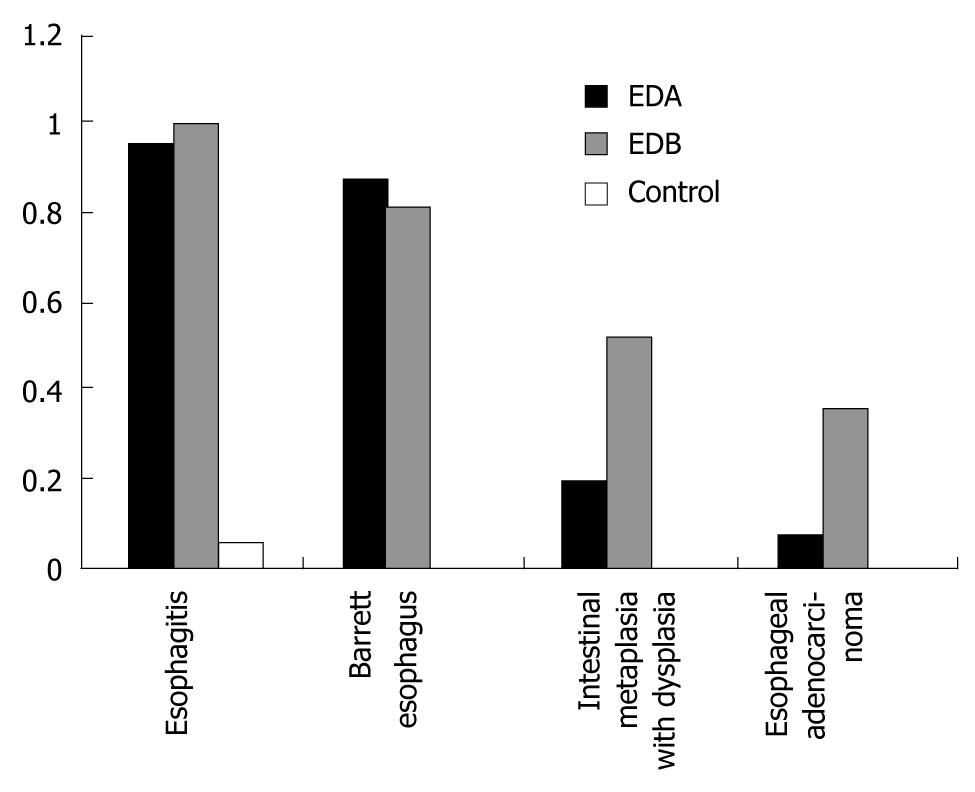
The incidence rate of esophagitis in groups A and B 40 wk after the treatment was 96% and 100%, respectively (χ2 = 0.930, P = 0.330). The equivalent incidence rate of BE was 88% and 82.4%, respectively χ2 =0.280, P = 0.60), and of intestinal metaplasia with dysplasia was 20% and 52.1%, respectively (χ2 = 5.420, P = 0.02). The incidence rate of EAC was 8% and 39% in the two groups, respectively (χ2 = 6.570, P = 0.01). All these rates were significantly higher than in the sham-operated control group (P < 0.001) (Figure 5).
GERD occurs when the contents of the stomach and duodenum are regurgitated into the esophagus, causing pathologic lesions of the mucosa, and pathologic changes in the esophagus[15]. Gastroesophageal reflux can result in the development of EAC. The incidence rate of EAC has increased significantly in recent years, more rapidly than that of other tumors[16], and the annual increase in the incidence rate of GERD reflects the increasing incidence rate of EAC. Clinical epidemiological studies have shown that gastroesophageal reflux correlates closely with EAC[17].
The mechanism that gastroesophageal reflux induces EAC has been the subject of numerous studies[18], and a recent research has shown that reflux of both gastric and duodenal juices can damage the esophageal mucosa[19]. However, the contributions of the specific components of gastroesophageal reflux to the development of EAC remains unclear[20]. The current study used an animal model, in which the pH values of the duodenogastric reflux could be varied, to investigate the effects of gastric acid and duodenal juice on the EAC induced by gastroesophageal reflux, with the aim of identifying the specific responsible factors.
Gastric acid is believed to be an important contributory factor in reflux esophagitis[10,21]. However, recent studies suggest that the role of other refluxes in the morbidity of gastroesophageal reflux cannot be ignored[22]. With the development of biliary monitoring technology, the effects of duodenogastric reflux can be better understood. The duodenal contents include bile, pancreatic juice, and intestinal juice, of which cholic acid, trypsin and hemolytic lecithin could damage the esophageal mucosa[23,24]. Exposure to acid and duodenal contents for a prolonged period would lead to the damage of the esophageal mucosa, the development of BE, and even EAC. Animal experiments have shown that bile can damage the esophageal mucosa[9]. Cholic acid synergizes with gastric acid, and could damage the esophageal mucosa by increasing the acidic environment, thus reinforcing the damaging effects of acid and pepsase, instead of being destroyed by acid after combining cholic acid and trypsin[25].
An animal model was established in this study to investigate the duodenogastric reflux of contents with different pH values, their damage to the esophageal mucosa and their effects on the development of BE and EAC. The pH value of the duodenogastric reflux 2 cm away from the pylorus was significantly higher than that 1 cm away (P < 0.05). This difference was related to the relative proportions of sodium bicarbonate secreted by the pancreas and gastric acid secreted by the stomach. The pH values differed depending on the position in the esophagus relative to the anastomosis of the esophagus and duodenum; the pH value 2 cm away from the pylorus was higher than that 1 cm away from the pylorus (P < 0.01).
These results confirm that the esophagus was stimulated by the contents of the stomach and duodenum with different pH values; a higher pH indicated a higher proportion of duodenal juice, while a lower pH indicated a higher proportion of gastric juice.
Esophagitis, BE, intestinal metaplasia with dysplasia and EAC developed in both the treated groups after 40 wk. The incidence rates of intestinal metaplasia with dysplasia and EAC were higher in the high-pH group, compared with the low-pH group (P < 0.01). There were no significant differences in the incidence rate of esophagitis or BE.
The results of this study showed that the reflux of gastric juice and duodenal contents could induce EAC in rats. More acidic duodenogastric reflux was associated with lower incidence rate of intestinal metaplasia with dysplasia and EAC, compared with more basic duodenogastric reflux. These results suggest that duodenal juice reflux increases the incidence rate of intestinal metaplasia with dysplasia and EAC, thus playing an important role in the pathogenesis of EAC, while gastric juice regurgitation had an opposite effect. The results imply that non-acid reflux is particularly important in the progression from BE to cancer. Therefore, control of duodenal reflux may be an important prophylaxis of the EAC.
The incidence rate of esophageal adenocarcinoma (EAC) is rising faster than that of any other cancers. Clinical epidemiological studies have shown that gastroesophageal reflux correlates closely with EAC. However, the relationship between the specific reflux components and the induction of EAC remains unclear.
Gastroesophageal reflux can cause EAC, and the mechanisms have been the subject of extensive research. The specific gastroesophageal reflux components responsible for EAC remain largely unknown. In this study, the authors demonstrated that non-acidic reflux increases the incidence rates of intestinal metaplasia with dysplasia and EAC, while acidic reflux had an opposite effect.
Recent reports have highlighted the importance of duodenal juice in the pathogenesis of EAC. The duodenum contains bile, pancreatic juice, and intestinal juice, of which cholic acid, trypsin and hemolytic lecithin could damage the esophageal mucosa. This is the first study to report a relationship between the pH of the duodenogastric refluxate and the incidence of EAC. The results of this study therefore suggest that duodenal juice plays an important role in the pathogenesis of EAC and gastric juice had an opposite effect.
Better understanding of the roles of the specific esophageal reflux components in the pathogenesis of EAC may represent a future strategy for the prevention of EAC.
The article is original and well-thought. The topic of the research is important, as it would add to the body of evidence regarding the role of alkaline reflux in esophageal carcinoma. The manuscript is clearly laid out and well written. The methodology/design is suitable to answer the questions posed.
Peer reviewer: Juan L Iovanna, Professor , Centre de Recherche INSERM, Unité 624, Stress Cellulaire, Parc Scientifique et Technologique de Luminy case 915, 13288 Cedex 9 Marseille, France
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH
| 1. | Lagergren J. Adenocarcinoma of oesophagus: what exactly is the size of the problem and who is at risk? Gut. 2005;54 Suppl 1:i1-i5. [Cited in This Article: ] |
| 2. | Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24:729-735. [Cited in This Article: ] |
| 3. | Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142-146. [Cited in This Article: ] |
| 4. | Lee IS, Choi SC, Shim KN, Jee SR, Huh KC, Lee JH, Lee KJ, Park HS, Lee YC, Jung HY. Prevalence of Barrett’s esophagus remains low in the Korean population: nationwide cross-sectional prospective multicenter study. Dig Dis Sci. 2010;55:1932-1939. [Cited in This Article: ] |
| 5. | Theisen J, Peters JH, Stein HJ. Experimental evidence for mutagenic potential of duodenogastric juice on Barrett’s esophagus. World J Surg. 2003;27:1018-1020. [Cited in This Article: ] |
| 6. | Fujikawa H, Saijyo T, Ito S, Ii K. [Studies of experimental model of reflux esophagitis in rats by ligature on both lower portion of duodenum and most of forestomach]. Nippon Shokakibyo Gakkai Zasshi. 1994;91:829-838. [Cited in This Article: ] |
| 7. | Orel R, Vidmar G. Do acid and bile reflux into the esophagus simultaneously? Temporal relationship between duodenogastro-esophageal reflux and esophageal pH. Pediatr Int. 2007;49:226-231. [Cited in This Article: ] |
| 8. | Kauer WK, Stein HJ. Emerging concepts of bile reflux in the constellation of gastroesophageal reflux disease. J Gastrointest Surg. 2010;14 Suppl 1:S9-S16. [Cited in This Article: ] |
| 9. | Chen KH, Mukaisho K, Sugihara H, Araki Y, Yamamoto G, Hattori T. High animal-fat intake changes the bile-acid composition of bile juice and enhances the development of Barrett’s esophagus and esophageal adenocarcinoma in a rat duodenal-contents reflux model. Cancer Sci. 2007;98:1683-1688. [Cited in This Article: ] |
| 10. | Miyashita T, Ohta T, Fujimura T, Ninomiya I, Fushida S, Hattori T, Miwa K. Duodenal juice stimulates oesophageal stem cells to induce Barrett’s oesophagus and oesophageal adenocarcinoma in rats. Oncol Rep. 2006;15:1469-1475. [Cited in This Article: ] |
| 11. | Miwa K, Miyashita T, Hattori T. [Reflux of duodenal or gastroduodenal contents induces esophageal carcinoma in rats]. Nippon Rinsho. 2004;62:1433-1438. [Cited in This Article: ] |
| 12. | Freedman J, Ye W, Näslund E, Lagergren J. Association between cholecystectomy and adenocarcinoma of the esophagus. Gastroenterology. 2001;121:548-553. [Cited in This Article: ] |
| 13. | Theisen J, Peters JH, Fein M, Hughes M, Hagen JA, Demeester SR, Demeester TR, Laird PW. The mutagenic potential of duodenoesophageal reflux. Ann Surg. 2005;241:63-68. [Cited in This Article: ] |
| 14. | Zhang T, Zhang F, Han Y, Gu Z, Zhou Y, Cheng Q, Zhu Y, Zhang C, Wang Y. A rat surgical model of esophageal metaplasia and adenocarcinoma-induced by mixed reflux of gastric acid and duodenal contents. Dig Dis Sci. 2007;52:3202-3208. [Cited in This Article: ] |
| 15. | Armstrong D, Sifrim D. New pharmacologic approaches in gastroesophageal reflux disease. Gastroenterol Clin North Am. 2010;39:393-418. [Cited in This Article: ] |
| 16. | Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2-9. [Cited in This Article: ] |
| 17. | Buxbaum JL, Eloubeidi MA. Endoscopic evaluation and treatment of esophageal cance. Minerva Gastroenterol Dietol. 2009;55:455-469. [Cited in This Article: ] |
| 18. | Herbella FA, Patti MG. Gastroesophageal reflux disease: From pathophysiology to treatment. World J Gastroenterol. 2010;16:3745-3749. [Cited in This Article: ] |
| 19. | Lahiri S, Singh P, Singh S, Rasheed N, Palit G, Pant KK. Melatonin protects against experimental reflux esophagitis. J Pineal Res. 2009;46:207-213. [Cited in This Article: ] |
| 20. | Grotenhuis BA, van Lanschot JJ, Dinjens WN, Wijnhoven BP. The pathogenesis of Barrett’s metaplasia and the progression to esophageal adenocarcinoma. Recent Results Cancer Res. 2010;182:39-63. [Cited in This Article: ] |
| 21. | Miner PB. Review article: physiologic and clinical effects of proton pump inhibitors on non-acidic and acidic gastro-oesophageal reflux. Aliment Pharmacol Ther. 2006;23 Suppl 1:25-32. [Cited in This Article: ] |
| 22. | Rubenstein JH, Scheiman JM, Sadeghi S, Whiteman D, Inadomi JM. Esophageal adenocarcinoma incidence in individuals with gastroesophageal reflux: synthesis and estimates from population studies. Am J Gastroenterol. 2011;106:254-260. [Cited in This Article: ] |
| 23. | Burnat G, Majka J, Konturek PC. Bile acids are multifunctional modulators of the Barrett’s carcinogenesis. J Physiol Pharmacol. 2010;61:185-192. [Cited in This Article: ] |
| 24. | Pera M, Trastek VF, Carpenter HA, Fernandez PL, Cardesa A, Mohr U, Pairolero PC. Influence of pancreatic and biliary reflux on the development of esophageal carcinoma. Ann Thorac Surg. 1993;55:1386-1392; discussion 1386-1392. [Cited in This Article: ] |
| 25. | Sital RR, Kusters JG, De Rooij FW, Kuipers EJ, Siersema PD. Bile acids and Barrett’s oesophagus: a sine qua non or coincidence? Scand J Gastroenterol Suppl. 2006;243:11-17. [Cited in This Article: ] |