Published online Sep 28, 2010. doi: 10.3748/wjg.v16.i36.4549
Revised: June 2, 2010
Accepted: June 9, 2010
Published online: September 28, 2010
AIM: To investigate whether the clinicopathologic features of infantile hemangioendothelioma (IHE) of the liver in a Chinese population are similar to the features observed in other races.
METHODS: The clinical data, radiological findings, histopathological changes and outcome of 12 cases of IHE diagnosed by the Department of Pathology, West China Hospital over the last 10 years were analyzed retrospectively. Immunohistochemical studies were carried out using antibodies against CD31, CD34, Factor VIII, cytokeratin 8 and cytokeratin 18.
RESULTS: The 12 patients were aged from fetal to 5 years (three males and nine females). The tumor was presented with different clinical manifestations, mainly as an asymptomatic, palpable, upper abdominal mass, except for the two fetuses who were detected antenatally by ultrasound. In one patient, this presentation was accompanied by an initial severe pneumothorax. No symptoms of congestive heart failure were present and neither congenital abnormalities nor vascular tumors in the skin or other organs were found. Laboratory abnormalities included leukocytosis (40%), anemia (60%), thrombocytosis (60%), hyperbilirubinemia (16.7%), abnormal liver function (50%) and increased α-fetoprotein (80%). Based on radiological findings and gross specimens, the tumor presented as a solitary lesion or a multifocal space-occupying lesion. The tumor size ranged from 5.0 cm × 3.5 cm × 2.0 cm to 13.8 cm × 9.0 cm × 7.7 cm, and the 0.2-1.1 cm nodules were diffusely distributed within the multifocal tumor. Seven cases were surgically resected, three cases underwent biopsy and the two fetuses were aborted. Histologically, nine cases were classified as type I and three as type II, presenting aggressive morphologic features, immature vessels, active mitosis and necrosis. An inflammatory component, predominantly eosinophilic granulocytes, sometimes obscured the nature of the tumor. Ten patients are alive after a follow-up of 1-9 years. Based on immunohistochemistry, the endothelial cells in all cases were positive for CD31, CD34 and polyclonal factor VIII antigen, whereas the scattered hyperplasia bile ducts were positive for cytokeratin 8 and cytokeratin 18.
CONCLUSION: The clinical manifestations of IHE are non-specific. There is no significant correlation between histological type and prognosis. The clinicopathologic features of IHE in Chinese patients may provide a clue to further evidence-based studies.
- Citation: Zhang Z, Chen HJ, Yang WJ, Bu H, Wei B, Long XY, Fu J, Zhang R, Ni YB, Zhang HY. Infantile hepatic hemangioendothelioma: A clinicopathologic study in a Chinese population. World J Gastroenterol 2010; 16(36): 4549-4557
- URL: https://www.wjgnet.com/1007-9327/full/v16/i36/4549.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i36.4549
Infantile hemangioendothelioma (IHE) of the liver is a rare mesenchymal tumor, but it is the most common benign vascular tumor of the liver in infancy. IHE has been described as a “tumor malformation” when it occurs in the uterus, and patients usually manifest symptoms or syndromes prior to the first year of life. Kunstadter et al[1] was the first to review and describe IHE in 1933. Since then, a series of cases have been reported in the literature. Previously, IHE was known as “multinodular hemangiomatosis of the liver”[2]. To date, most cases reported in the literature are IHEs mainly in the Caucasians. However, diseases affecting individuals of the Mongoloid race often present with unique characteristics. The purpose of this study was to examine the clinical and pathologic features of IHE in Chinese patients and to correlate these features with the treatment and prognosis. Some reports have suggested that a few IHEs may be metastatic and undergo malignant degeneration into angiosarcoma[3], while most of the cases undergo involution and regression. These findings make the treatment controversial. In addition, we have reviewed adequately documented reports of similar tumors from the literature and compared them with our findings. This is the first and largest population of patients examined from a single institution in China in a period of 10 years.
The records of 12 infants and children (including two aborted fetuses) with IHE of the liver seen at West China Hospital over the last 10 years between 2000 and 2010 were retrieved from the files of the Department of Pathology. West China Hospital is a Medical Center with the largest number of beds in China. The clinical data, laboratory examinations, radiographic findings, surgical findings, treatment, histopathological changes and outcome of these cases were retroactively reviewed. The surgical specimens from partial hepatectomies and biopsies were taken for histopathological evaluation and the morphological characteristics were reviewed according to the standard of diagnosis described by Dehner et al[4]. Follow-up information was available for all patients. Immunohistochemistry (IHC) using antibodies against CD31, CD34, Factor VIII, cytokeratin 8 and cytokeratin 18 was performed on paraffin-embedded tissue specimens. IHC was performed using an Envision kit on a Dako automatic stainer (Dako, Carpinteria, CA). Positive (internal) controls were included. Sequential tissue sections treated with sera from the same species as the primary antibody were used as negative controls (Table 1).
| Antibody | Clone/PAD | Company and Cat. No. | Staining pattern | Dilution used |
| CD31 | JC70A | Dako, M0823 | Membrane | 1:50 |
| CD34 | QBEnd/10 | Zymed, ZM-0046 | Membrane | 1:25 |
| Factor VIII | Polyclonal serum | Zymed, ZA0111 | Cytoplasmic | 1:50 |
| CK8 | C51 | Zymed, ZM-0310 | Cytoplasmic | 1:100 |
| CK18 | DC-10 | Zymed, ZM-0073 | Cytoplasmic | 1:100 |
The clinical features of the IHE are listed in Table 2. The age of the 12 patients ranged from fetal to 5 years. The patients were referred to our hospital for different clinical manifestations. Ten (83.33%) were seen at diagnosis, prior to 6 mo of age, with a mean age of 10 mo at presentation. There were three males and nine females, with a ratio of 1:3. Eight had asymptomatic palpable abdominal masses detected during a routine physical examination. Additional symptoms included abdominal distention and pain caused by a large tumor, jaundice, dyspnea, neonatal pneumonia and fever. A 5-year-old girl presented with repeated abdominal pain combined with a pneumothorax for 2 mo. No congenital defect was observed and no vascular tumor was found in the skin or other organs. No symptoms of congestive heart failure (CHF) were present in any of the patients. The primary physical finding was an upper abdominal mass or hepatomegaly, with some extending across the midline, with a smooth surface and sharp edges. No family history was reported in any case. No proof of maternal exposure to dangerous environmental factors during pregnancy was ascertained. The fetuses with IHE had no chorioangioma or intrauterine growth retardation detected antenatally using ultrasound.
| Case | Sex/age | Presenting features | Solitary or multicentric lesion; site/size (cm) | Leuko cytosis | Anemia | Thrombocytosis | Hyperbiliru binemia | Abnormalliverfunction | Increase AFP | Histological type | Treatment | Follow-up (yr) |
| 1 | M/2 mo | AM | S; LL; 9.0 × 9.0 × 7.5 | No | Yes | Yes | No | No | NA | I | CR | Alive, 9 |
| 2 | F/17 d | Abdominal distention | S; LL and RL; 11.0 × 8.0 × 6.5 | No | No | No | Yes | Yes | NA | I | BO | Alive, 6.75 |
| 3 | F/2 mo | AM, neonatal pneumonia | S; LL; 5.0 × 3.5 × 2.0 | Yes | No | Yes | NA | Yes | Yes | I | CR | Alive, 6.25 |
| 4 | F/6 mo | AM | S; LL; 11.0 × 10.0 × 8.0 | No | Yes | Yes | NA | No | NA | I | CR | Alive, 5 |
| 5 | F/5 yr | Abdominal pain, dyspnea | M; LL and RL; Oblique diameter of right hepatic: 9.7 | Yes | No | Yes | No | Yes | NA | II | BO | Alive, 4 |
| 6 | M/5 mo | AM | S; LL; 10.5 × 7.5 × 7.0 | Yes | Yes | Yes | NA | No | NA | II | CR | Alive, 3.75 |
| 7 | F/6 mo | AM | S; RL; 10.0 × 10.0 × 8. 0 | No | Yes | No | No | No | Yes | I | CR | Alive, 3.5 |
| 8 | F/3 yr | Jaundice, fever | S; LL, RL and CL; 13.8 × 9.0 × 7.7 | Yes | Yes | No | NA | Yes | No | II | CR | Alive, 2.25 |
| 9 | F/5 mo | AM | S; RL; 6.2 × 6.0 × 3.5 | No | No | No | No | No | Yes | I | CR | Alive, 1.5 |
| 10 | F/2 mo | AM | S; CL; 6.0 × 5.0 × 4.0 | No | Yes | Yes | No | Yes | Yes | I | BO | Alive, 0.25 |
| 11 | F/AF | AM | S; RL; 8.2 × 6.5 × 7.8 | NA | NA | NA | NA | NA | NA | I | Aborted | |
| 12 | M/AF | AM | S; LL; 7.6 × 7.4 × 7.2 | NA | NA | NA | NA | NA | NA | I | Aborted |
Laboratory data, including hemograms and liver function tests were available for all patients, except for the aborted fetuses. Leukocytosis, anemia and thrombocytosis were found. One of six cases had hyperbilirubinemia and displayed jaundice. An elevated aspartate aminotransferase level was seen in five cases. The serum α-fetoprotein (AFP) concentration was abnormal in four cases. The serum hepatitis virus markers and blood coagulation system were normal in all patients. The laboratory examinations are summarized in Table 2.
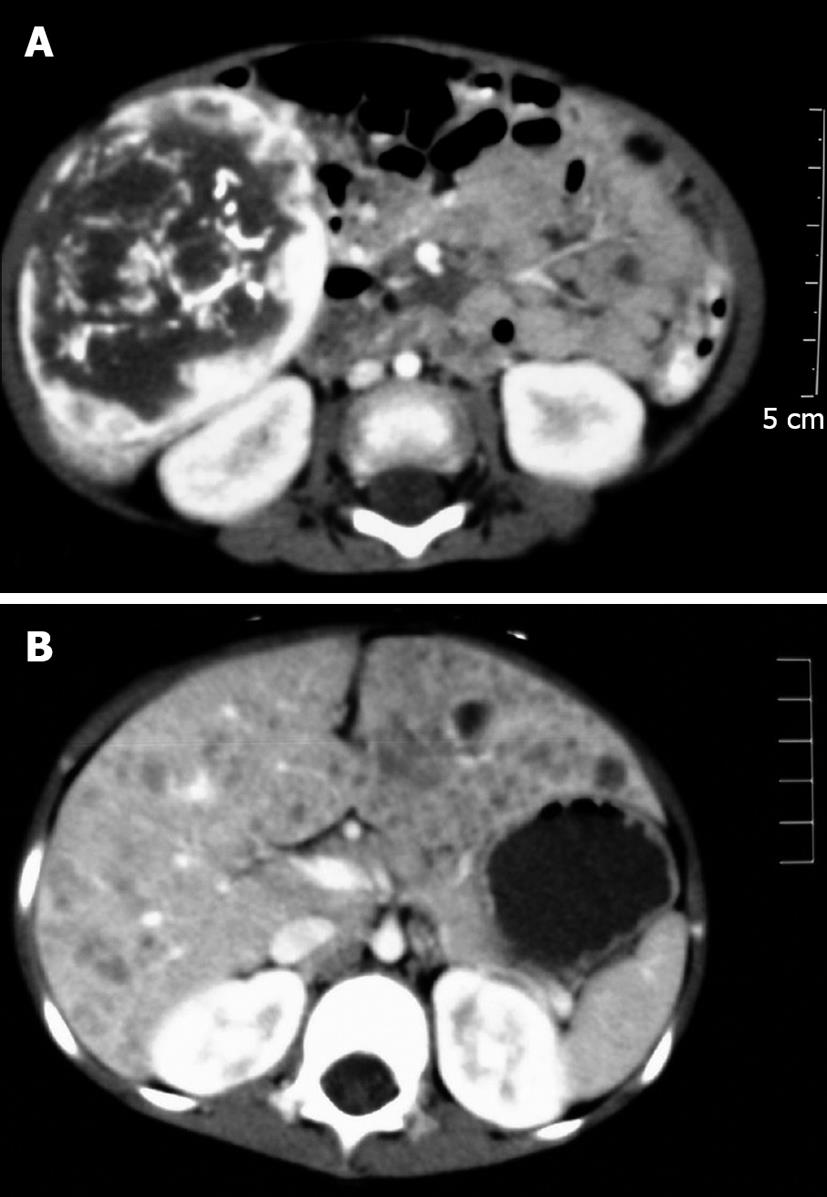
Abdominal ultrasonography, computed tomography (CT), contrast-enhanced arterial phase CT and magnetic resonance imaging scans demonstrated different features in the solitary lesions and multiple lesions (Figure 1). Local calcification was prevalent in this tumor and presented as a fine-speckled pattern. In the thoracic CT scans, the pneumothorax (case 5) appeared as lung consolidation, the left lung was compressed by air, and the mediastinum moved to the right.
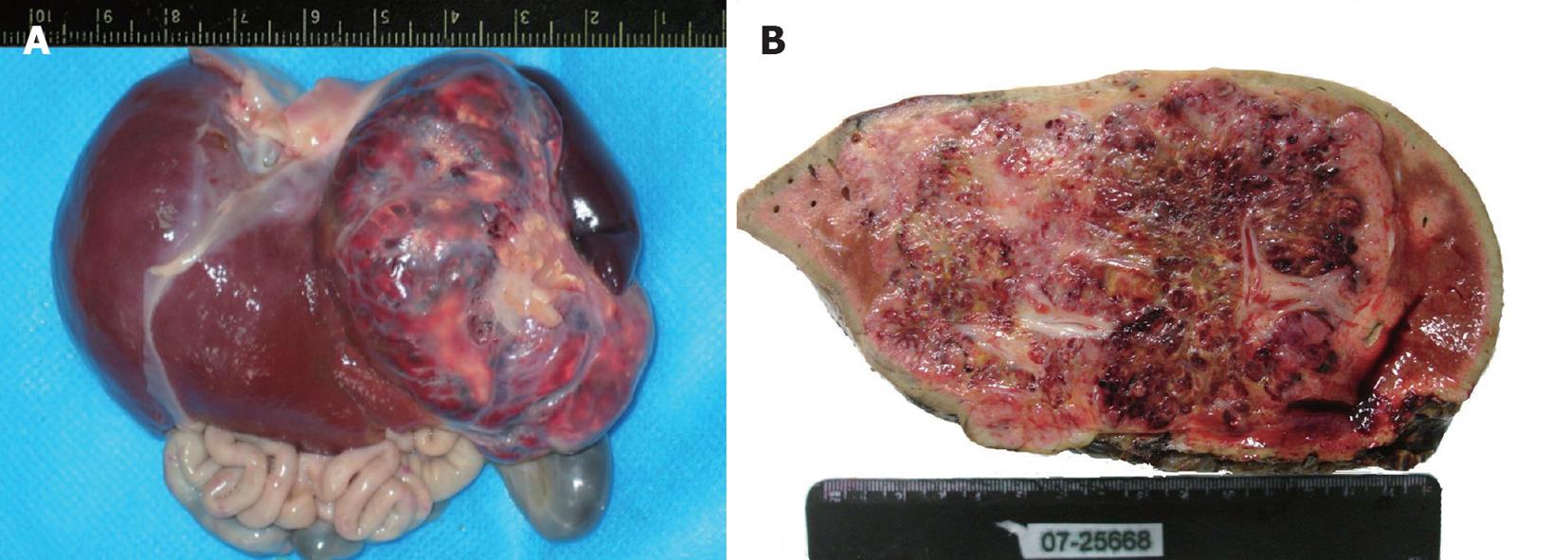
Nine cases presented with a solitary lesion in one lobe. The lesion was restricted to the right or left lobe of liver in five and three patients, respectively; one case had the tumor in the caudate lobe. Case 2 and case 8 had a huge mass arising from the right lobe that extended to the left and caudate lobe. The size of solitary lesions ranged from 5.0 cm × 3.5 cm × 2.0 cm to 13.8 cm × 9.0 cm × 7.7 cm and the lesion presented as a large, well-circumscribed, red-brown tumor, with a smooth and glittery appearance and a capsule containing abundant blood vessels of different sizes. On the cut surface, the tumor was more or less homogeneous, red-brown and partially ill-circumscribed (Figure 2). Sometimes the tumor had partially stranded vessels and a central fibrosis or infarction. The lesion in case 5 was diffuse and multifocal, and both lobes were involved. Extensive multiple masses in the swelling liver made the surface irregular, and the nodules were too numerous to count. The maximum diameter of the nodules was 1.1 cm. On the cut surface, the liver was replaced by diffuse multiple, solid or cystic, reddish-gray masses partially surrounded by a thin rim but without evidence of hemorrhage.
All patients underwent surgical interventions. The tumor was completely removed surgically in seven patients, whereas segmental resection or left/right lobectomy was performed according to the size and location of the tumor. Three exploratory laparotomies with liver biopsies were carried out for the patients with multifocal and solitary lesions, but subsequent therapies were not accepted by their parents. In our series, the two aborted fetuses were delivered following induced labor.
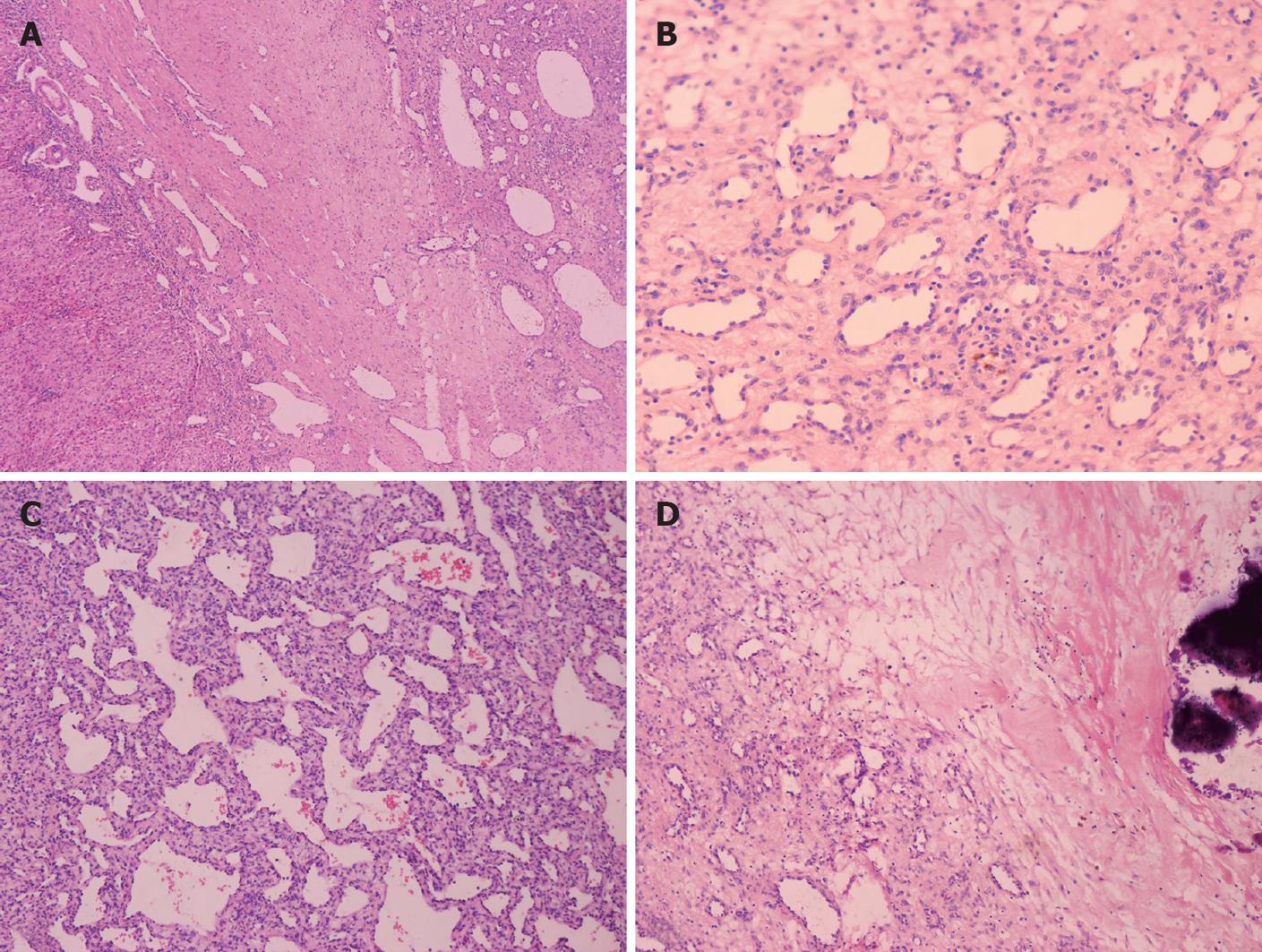
Because of the broad variety of histological features, the growth pattern of the tumors was subdivided into two subtypes: type I and type II. The nine cases of type I consisted of vascular channels with some variations in structure and a single layer of endothelial cells without polymorphisms. Dilated capillaries were interspersed among loose, edematous, myxoid connective tissues. Disorganized elongated branching bile ducts and scanty cavernous structures were also observed in the lesion. A lesion with infiltrating inflammatory cells mimicked granulation tissues. Infarction, extramedullary hematopoiesis and foci of calcification were more prominent in the type I cases (Figure 3). No bile plug or thrombus hemorrhage was observed.
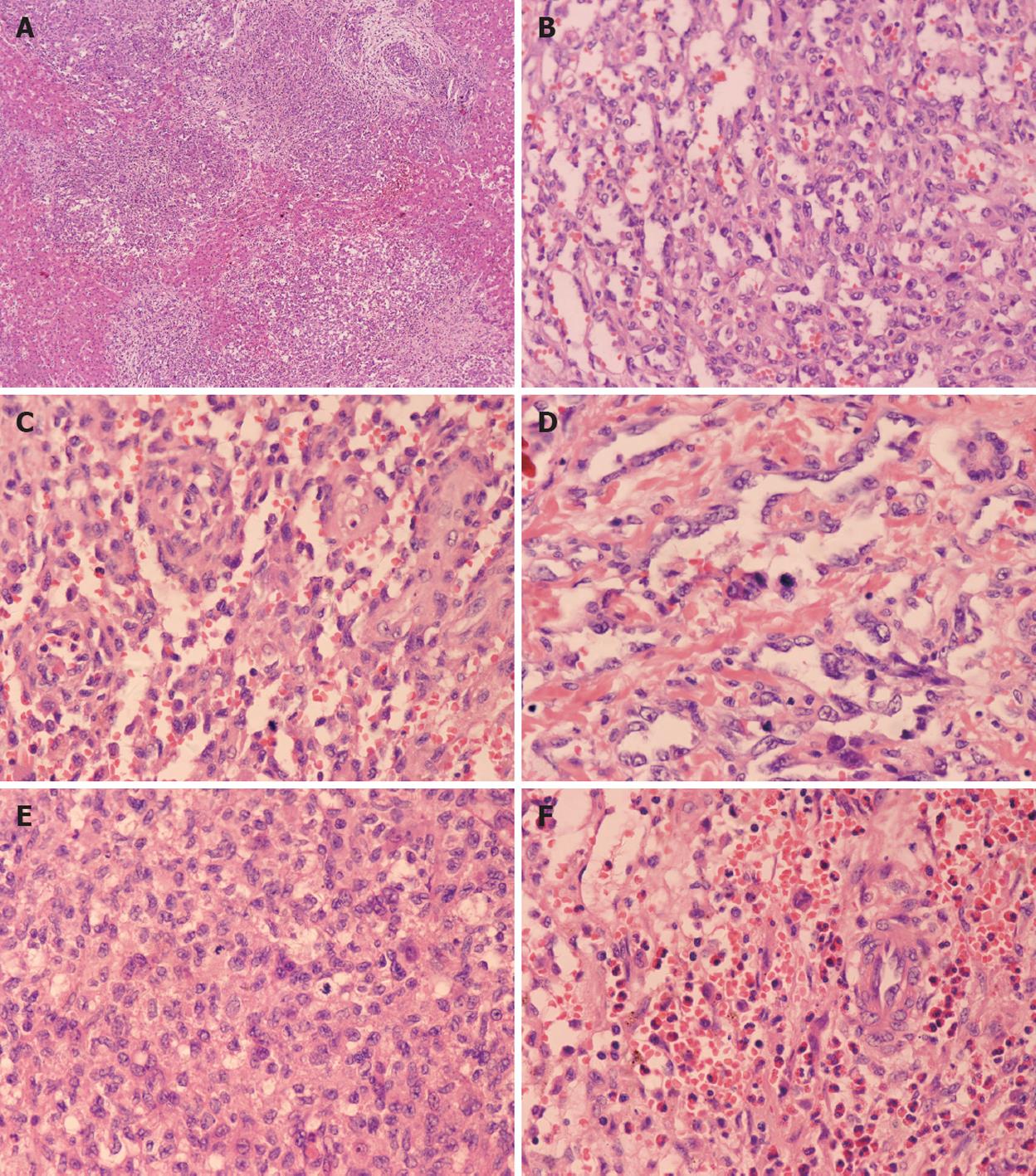
Three cases were classified as type II. Although two cases appeared as a solitary lesion, based on their histological morphology, they were classified as type II. The remaining case was a multifocal lesion that diffusely spread throughout the hepatic parenchyma. The multifocal and sinusoidal parts in these lesions were more prominent than in type I. At the periphery, the nodules were not encapsulated. The tumor nodules intermixed with the hepatic plate, and the normal hepatic parenchymal cells surrounding the nodules were compressed and infiltrated by projections of the tumor. The vascular spaces were twisting with irregular budding, branching and anastomosing structures. The endothelial cells were plump and more hyperchromatic and pleomorphic than those in type I cases. Mitosis in the endothelial cells demonstrated a high rate of proliferation. Most of the bile ducts were located in the central region of the nodules. The presence of eosinophilic granulocytes as the predominant inflammatory component in case 5 resulted in an initial misdiagnosis as a parasite infection (Figure 4).
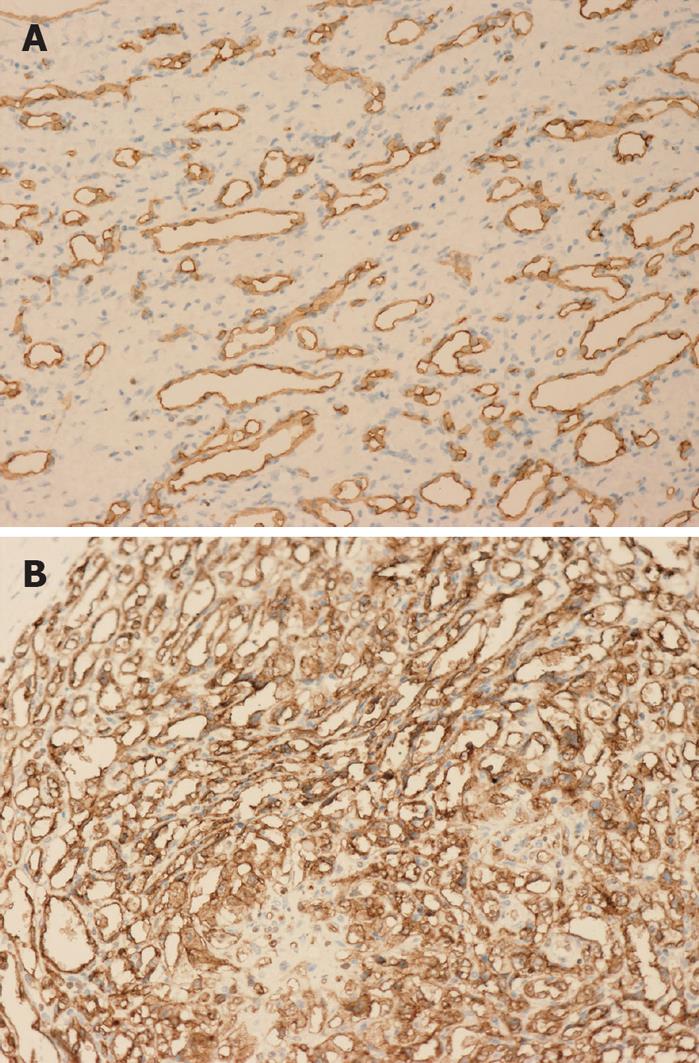
IHC revealed that the dilated vessels were vascular spaces. The endothelial nature of the cells surrounding the vascular spaces was supported by the expression of the vascular markers CD34 (Figure 5), CD31 and Factor VIII. Cytokeratin 8 and cytokeratin 18 staining verified the presence of scattered bile ducts.
All of our patients, except for the aborted fetuses, are alive and have presented with no symptoms suggestive of recurrence during the follow-up of 1-9 years. Follow-up ultrasonographic evaluation of the tumor volume and Doppler examination of tumor perfusion were performed in several cases; however, no evidence of tumor recurrence has been detected. The three patients (cases 2, 5 and 10) who underwent surgical biopsy only and without subsequent treatment were closely followed, and a regression in tumor size was noted by ultrasonography without complications.
In recent years, there have been important advances in the conceptual understanding of vascular tumors and malformations of the skin in infants. However, the study of hepatic IHE has lagged far behind skin lesions, in part because of its low incidence. Although IHE is the third most common type of hepatic tumor and the most common benign vascular tumor of the liver in infants, hepatic tumors are relatively rare in children, accounting for 2%-3% of all pediatric tumors[5]. Many diagnostic tests have been established for hepatic tumors in adults, but there are significant differences in pediatric tumors[6-8]. Over the last 10 years, we have diagnosed 12 cases of IHE (including two cases diagnosed at autopsy), which accounted for 38.8% of liver tumors and 80% of benign liver tumors in children in our institution. Pediatric hepatic vascular tumors are usually divided into IHEs and cavernous hemangiomas. IHE of the liver has been recognized for over 70 years, and to date, most cases reported were in Caucasians. The current study for the first time details the characteristics of hepatic IHE in a Chinese population.
The patients in our series ranged in age from fetal to 5 years. Most (83.33%) of the lesions were diagnosed within 6 mo of birth[9,10]. The sex ratio was 1:3 (male:female), consistent with other studies that demonstrated a clear female preponderance [9]. An asymptomatic abdominal mass or hepatomegaly found in a routine physical examination has been by far the most frequent symptom observed. Cutaneous and mucosal hemangiomas were not detected. The observed symptoms in Chinese individuals correct the misconceptions regarding the previously described associations with IHE, namely the classic triad of hepatomegaly, high-output CHF and cutaneous hemangioma[11]. There were no instances of CHF resulting from an arteriovenous shunt as other cases described in Mongoloid individuals[12]. Some previously described cases also had a variety of associated congenital abnormalities or hypothyroidism[13,14]. In our group, a 5-year-old girl had prominent signs of dyspnea caused by a pneumothorax, which is an unusual combined lesion. As similar reports have not been reported to date, we are not sure about the correlation between the pneumothorax and IHE. Our assumption is that IHE (hepatomegaly) caused a portion of the right lung to become compressed, which led to the compensatory expansion of the left lung. This in turn resulted in a local pneumothorax.
The serum AFP level, which often increases in primary hepatic malignancies, has been used as an important tumor marker for hepatoblastoma, hepatocellular carcinoma and some germ cell tumors. In this study, the serum AFP level was increased in four of five infants (80%), although it is seldom elevated in IHE[15,16]. Based on the data from laboratory examinations, we conclude that laboratory tests are neither nonspecific nor indicative of the diagnosis. As these lesions were excised or regressed, all the abnormal data subsided. These phenomena could be helpful in monitoring the recurrence of the tumor.
Imaging studies showed the presence of a space-occupying lesion in the liver and may provide a diagnosis or a differential diagnosis[17]. However, some cases undergoing involution may contain calcifications, fibrosis and vascular channel deficiencies which make it difficult to differentiate from other hepatic neoplasms. Therefore, biopsy of the mass is necessary for a definitive diagnosis. IHE presented as a solitary mass or multicentric nodules based on the radiographic findings and gross specimens. The former was more common in our study. It is noteworthy that the two solitary tumors in this group presented features of type II lesions, whereas in the literature, the diffuse multifocal lesions were all classified as type II. Therefore, solitary tumors are not exclusively type I, and it is necessary to determine the histological type using microscopy. There seems no close correlation between the size of the tumor, solitary or multinodular appearance and poor prognosis in this study.
IHE must be histologically distinguished from cavernous hemangioma, epithelioid hemangioendothelioma, angiosarcoma and mesenchymal hamartoma[4]. Cavernous hemangioma is less common than IHE in children. In cavernous hemangioma, the capillary and sinusoidal portions are invisible and are replaced by widely dilated nonanastomotic thin-walled vascular spaces lined with flat endothelial cells and supported by fibrous issues. Epithelioid hemangioendothelioma is regarded in the World Health Organization (WHO) classification as an intermediate grade tumor, composed of epithelioid or spindle cells growing in myxoid stroma. This tumor often infiltrates extensively and forms intracellular vascular lumina that may contain erythrocytes. Angiosarcoma is an extremely rare malignant tumor during childhood. The dilated vascular channels are lined by pseudopapillary processes, and the disrupted liver cells act as scaffolding for the pleomorphic, neoplastic endothelial cells. Giant cell formation, solid sarcomatous foci, intrasinusoidal spread and invasion of portal or hepatic veins within the liver are often observed in angiosarcomas. A history of exposure to toxins, age, immature vascular differentiation, malignant cell morphology, pathologic karyokinesis and vascular infiltration all suggest a diagnosis of angiosarcoma. The differential diagnosis between IHE and mesenchymal hamartomas is difficult. The latter is a mixture of bile ducts, mesenchymal tissue and blood vessels. Mesenchymal tissue presents as myxomatous stroma with loosely arranged stellate cells, and the bile ducts display a ductal plate malformation. In case 5, eosinophilic granulocytes were the predominant inflammatory component. This concealed the vascular nature of the lesion and led to the misdiagnosis of a parasite infection. The formation of vascular channels, the absence of exposure to pathogens and the absence of antibodies to parasites could be considered to avoid such a misdiagnosis.
Based on the results of our study, it is critical to remind pathologists that it is not possible to predict the clinical behavior of these tumors based on morphologic criteria alone as there was no significant correlation between the histological type (type I or type II) and prognosis. Dehner et al[4] subdivided the tumor histologically into type I and II[4,18]. Type I tumors are composed of capillary, sinusoidal and cavernous parts lined by plump endothelial cells with a bland cytological appearance[19]. Type II tumors have areas composed of papillate tufting vascular channels that are lined by larger pleomorphic and hyperchromatic cells. Type II tumors were thought to have a poorly formed and more aggressive microscopic appearance, but the presence of extensive endothelial cell proliferation, active mitosis and an infiltration margin do not indicate malignant characteristics compared with other pediatric tumors. Selby explained the cellular pleomorphism as a degenerative phenomenon in the setting of IHE, which is supported by other encouraging follow-up data[9]. The outcome in case 5, case 6 and case 8 in our study, which were classified as type II tumors, demonstrated that histological type II was not a significant indicator of poor prognosis, and the histological divisions have not been mentioned by the WHO classification[20]. In contrast, fibrosis, calcification and the formation of cavernous hemangiomatous foci are representative of regression or maturation. We propose to classify IHE into solitary and multifocal lesions rather than into type I and type II. However, to test the validity of this classification, a large number of cases and long-term follow-up will be needed.
IHE has been regarded as a benign vascular tumor by most authors, although a few have reported cases in which the IHE was malignant and developed into an angiosarcoma with eventual metastasis (especially type II)[3,21,22]. All of the IHE cases in our study, except for the aborted fetuses, were associated with a good prognosis. In the natural course of our patients, the tumors grew rapidly for a period after they first appeared and then spontaneously involuted in the ensuing years. The natural evolution of this tumor, which is similar to that of cutaneous hemangioma during infancy, indicates a possible homogeneous histogenesis and functional differentiation. The prognosis is considered excellent except for some patients with severe complications, such as thrombocytopenia, hemolytic anemia, intravascular consumption coagulopathy and CHF that substantially contribute to the mortality associated with the tumor. However, these adverse risk factors are corrected once the tumor is resected. The more complications that occur, the poorer the prognosis will be. Therefore, pediatricians should pay a close attention to the complications to determine the best treatment strategy.
Because IHE frequently coexists and shares some biological features with cutaneous hemangioma, they may be related diseases. We presume that the simultaneous occurrence of hemangiomas in other organs can be attributed to a multicentric origin rather than metastasis[2,9,23]. “Kaposiform hemangioendothelioma”, “retiform hemangioendothelioma”, “composite hemangioendothelioma” and “epithelioid hemangioendothelioma” all demonstrate an intermediate or malignant clinical course. We propose that IHE should be designated “infantile capillary hepatic hemangioma”. This name reflects its benign nature, which is better than “hemangioendothelioma” that often refers to the intermediate vascular tumor.
The treatment of IHE is controversial and the effects of the various forms of therapy are diverse and inconclusive[24]. The high survival rate in the IHE reported in this study, in contrast to the high mortality rates reported in the literature, illustrates the significant improvement made in the management of these tumors[4]. Although the tumor can spontaneously regress, it is necessary to guard against potentially life-threatening complications that may become a therapeutic challenge. Based on our experience, if IHE is discovered as a solitary asymptomatic mass and is a large lesion in a suitable location, partial hepatectomy should be the first line therapy[25]. There is no need for over-treatment of asymptomatic multicentric lesions. Supportive care, controlling severe complications and close follow-up are all necessary for the treatment of these lesions. The resolution of the hemangioma is best monitored by ultrasound. Additionally, there are reports of treating diffuse lesions with steroids, interferon or vincristine, but these treatments may have adverse effects. If there is severe heart failure, digitalis and diuretics, transfusion and steroids should be used before surgical intervention. The use of therapeutic radiation and cyclophosphamide still remains controversial[26]. Transplantation is not recommended, unless the IHE is complicated with severe liver failure[27]. In a word, integrating the clinical manifestations with pathological features is appropriate in the treatment of tumors.
In summary, this retrospective study covered 12 cases of IHE of the liver seen over the past 10 years. All of the patients with a solitary lesion remained alive following surgery, and the multifocal cases underwent spontaneous regression without additional treatment. All of the IHE cases in our study were benign vascular tumors and were associated with a good prognosis despite some aggressive histological appearances. There was no correlation between the clinical behavior and the morphologic features in these cases. This is the first and largest population of patients with IHE examined from a single institution in China over a period of 10 years. A rigorous, evidence-based study in the future will guide in the selection of therapies for this disease.
Infantile hemangioendothelioma (IHE) of the liver is a rare mesenchymal tumor, but it is the most common benign vascular tumor of the liver in infancy. Since the first article to review and describe IHE in 1933, a series of cases have been reported in the literature.
To date, most cases reported in the literature have been IHEs mainly in the Caucasians. However, diseases affecting individuals of the Mongoloid race often present with unique characteristics. The purpose of this study was to examine the clinical and pathologic features of IHE in Chinese individuals and to correlate these features with the treatment and prognosis.
This is the first and largest population of patients with IHE examined from a single institution in China over a period of 10 years. All of the IHE cases in this study were benign vascular tumors and were associated with a good prognosis despite some aggressive histological appearances.
The clinicopathologic features of IHE in Chinese patients may provide a clue to further evidence-based studies and guide the selection of therapies.
IHE of the liver is a rare mesenchymal tumor in infancy. Type I and type II are subtypes of IHE classified according to the histological characteristics. CD34, CD31 and factor VIII are antibodies that are expressed mainly in vascular tumors. CK8 and CK18 are antibodies that are expressed in epithelial cells, including bile ducts.
In this study, the authors investigated the clinicopathological features of 12 IHE cases. They describe that this is the largest series of IHE from a single institution of China. The prognoses of their cases were fairly good; this is a different result from that of previous reports.
Peer reviewers: Qin Su, Professor, Department of Pathology, Cancer Hospital and Cancer Institute, Chinese Academy of Medical Sciences and Peking Medical College, PO Box 2258, Beijing 100021, China; Yoshihisa Takahashi, MD, Department of Pathology, Teikyo University School of Medicine, 2-11-1 Kaga, Itabashi-ku, Tokyo 173-8605, Japan
S- Editor Tian L L- Editor Ma JY E- Editor Zheng XM
| 1. | Kunstadter RH. Hemangio-endothelioma of liver in infancy: case report and review of literature. Am J Dis Child. 1933;46:803-810. [Cited in This Article: ] |
| 2. | McLean RH, Moller JH, Warwick WJ, Satran L, Lucas RV Jr. Multinodular hemangiomatosis of the liver in infancy. Pediatrics. 1972;49:563-573. [Cited in This Article: ] |
| 3. | Nazir Z, Pervez S. Malignant vascular tumors of liver in neonates. J Pediatr Surg. 2006;41:e49-e51. [Cited in This Article: ] |
| 4. | Dehner LP, Ishak KG. Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol. 1971;92:101-111. [Cited in This Article: ] |
| 5. | Finegold MJ, Egler RA, Goss JA, Guillerman RP, Karpen SJ, Krishnamurthy R, O'Mahony CA. Liver tumors: pediatric population. Liver Transpl. 2008;14:1545-1556. [Cited in This Article: ] |
| 6. | Assy N, Nasser G, Djibre A, Beniashvili Z, Elias S, Zidan J. Characteristics of common solid liver lesions and recommendations for diagnostic workup. World J Gastroenterol. 2009;15:3217-3227. [Cited in This Article: ] |
| 7. | Koike N, Cho A, Nasu K, Seto K, Nagaya S, Ohshima Y, Ohkohchi N. Role of diffusion-weighted magnetic resonance imaging in the differential diagnosis of focal hepatic lesions. World J Gastroenterol. 2009;15:5805-5812. [Cited in This Article: ] |
| 8. | Numata K, Isozaki T, Morimoto M, Sugimori K, Kunisaki R, Morizane T, Tanaka K. Prospective study of differential diagnosis of hepatic tumors by pattern-based classification of contrast-enhanced sonography. World J Gastroenterol. 2006;12:6290-6298. [Cited in This Article: ] |
| 9. | Selby DM, Stocker JT, Waclawiw MA, Hitchcock CL, Ishak KG. Infantile hemangioendothelioma of the liver. Hepatology. 1994;20:39-45. [Cited in This Article: ] |
| 10. | Prokurat A, Kluge P, Chrupek M, Kościesza A, Rajszys P. Hemangioma of the liver in children: proliferating vascular tumor or congenital vascular malformation? Med Pediatr Oncol. 2002;39:524-529. [Cited in This Article: ] |
| 11. | Sevinir B, Ozkan TB. Infantile hepatic hemangioendothelioma: clinical presentation and treatment. Turk J Gastroenterol. 2007;18:182-187. [Cited in This Article: ] |
| 12. | Moon SB, Kwon HJ, Park KW, Yun WJ, Jung SE. Clinical experience with infantile hepatic hemangioendothelioma. World J Surg. 2009;33:597-602. [Cited in This Article: ] |
| 13. | Skopec LL, Lakatua DJ. Non-immune fetal hydrops with hepatic hemangioendothelioma and Kasabach-Merritt syndrome: a case report. Pediatr Pathol. 1989;9:87-93. [Cited in This Article: ] |
| 14. | Kalpatthi R, Germak J, Mizelle K, Yeager N. Thyroid abnormalities in infantile hepatic hemangioendothelioma. Pediatr Blood Cancer. 2007;49:1021-1024. [Cited in This Article: ] |
| 15. | Sari N, Yalçin B, Akyüz C, Haliloglu M, Büyükpamukçu M. Infantile hepatic hemangioendothelioma with elevated serum alpha-fetoprotein. Pediatr Hematol Oncol. 2006;23:639-647. [Cited in This Article: ] |
| 16. | Kim TJ, Lee YS, Song YS, Park CK, Shim SI, Kang CS, Lee KY. Infantile hemangioendothelioma with elevated serum alpha fetoprotein: report of 2 cases with immunohistochemical analysis. Hum Pathol. 2010;41:763-767. [Cited in This Article: ] |
| 17. | Horton KM, Bluemke DA, Hruban RH, Soyer P, Fishman EK. CT and MR imaging of benign hepatic and biliary tumors. Radiographics. 1999;19:431-451. [Cited in This Article: ] |
| 18. | Ishak KG, Goodman ZD, Stocker JT. Tumors of the liver and intrahepatic bile ducts. Atlas of tumor pathology, 3rd series, Fascicle 31. Washington: Armed Forces Institute of Pathology 2001; . [Cited in This Article: ] |
| 19. | Yasunaga C, Sueishi K, Ohgami H, Suita S, Kawanami T. Heterogenous expression of endothelial cell markers in infantile hemangioendothelioma. Immunohistochemical study of two solitary cases and one multiple one. Am J Clin Pathol. 1989;91:673-681. [Cited in This Article: ] |
| 20. | Hamilton SR, Aaltonen LA, editors . World Health Organization classification of tumours: pathology and genetics of tumours of the digestive system. Lyon: IARC Press 2000; . [Cited in This Article: ] |
| 21. | Stocker JT. Hepatic tumors in children. Clin Liver Dis. 2001;5:259-281, viii-ix. [Cited in This Article: ] |
| 22. | Noronha R, Gonzalez-Crussi F. Hepatic angiosarcoma in childhood. A case report and review of the literature. Am J Surg Pathol. 1984;8:863-871. [Cited in This Article: ] |
| 23. | Nord KM, Kandel J, Lefkowitch JH, Lobritto SJ, Morel KD, North PE, Garzon MC. Multiple cutaneous infantile hemangiomas associated with hepatic angiosarcoma: case report and review of the literature. Pediatrics. 2006;118:e907-e913. [Cited in This Article: ] |
| 24. | Emre S, McKenna GJ. Liver tumors in children. Pediatr Transplant. 2004;8:632-638. [Cited in This Article: ] |
| 25. | Stanley P, Geer GD, Miller JH, Gilsanz V, Landing BH, Boechat IM. Infantile hepatic hemangiomas. Clinical features, radiologic investigations, and treatment of 20 patients. Cancer. 1989;64:936-949. [Cited in This Article: ] |
| 26. | Seo HI, Jo HJ, Sim MS, Kim S. Right trisegmentectomy with thoracoabdominal approach after transarterial embolization for giant hepatic hemangioma. World J Gastroenterol. 2009;15:3437-3439. [Cited in This Article: ] |
| 27. | Grabhorn E, Richter A, Fischer L, Krebs-Schmitt D, Ganschow R. Neonates with severe infantile hepatic hemangioendothelioma: limitations of liver transplantation. Pediatr Transplant. 2009;13:560-564. [Cited in This Article: ] |