Published online May 28, 2010. doi: 10.3748/wjg.v16.i20.2484
Revised: February 1, 2010
Accepted: February 8, 2010
Published online: May 28, 2010
AIM: To investigate whether semi-vegetarian diet (SVD) has a preventive effect against relapse of Crohn’s disease (CD) in patients who have achieved remission, who are a high-risk group for relapse.
METHODS: A prospective, single center, 2-year clinical trial was conducted. Twenty-two adult CD patients who achieved clinical remission either medically (n = 17) or surgically (n = 5) and consumed an SVD during hospitalization were advised to continue with an SVD and avoid known high-risk foods for inflammatory bowel disease. The primary endpoint was clinical relapse defined as the appearance of active symptoms of CD. Kaplan-Meier survival analysis was used to calculate the cumulative proportion of patients who had a relapse. A 2-year analysis of relapse rates of patients who followed an SVD and those who did not (an omnivorous diet group) was undertaken.
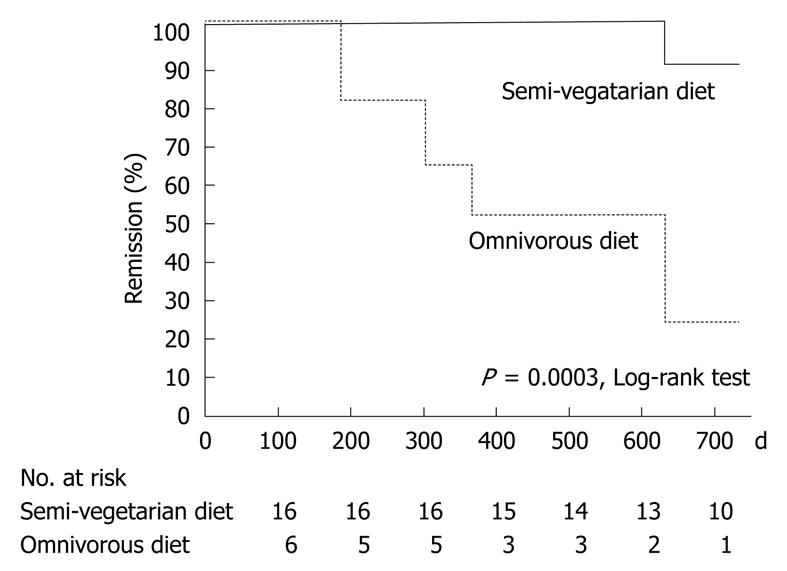
RESULTS: SVD was continued by 16 patients (compliance 73%). Remission was maintained in 15 of 16 patients (94%) in the SVD group vs two of six (33%) in the omnivorous group. Remission rate with SVD was 100% at 1 year and 92% at 2 years. SVD showed significant prevention in the time to relapse compared to that in the omnivorous group (P = 0.0003, log rank test). The concentration of C-reactive protein was normal at the final visit in more than half of the patients in remission who were taking an SVD, who maintained remission during the study (9/15; 60%), who terminated follow-up (8/12; 67%), and who completed 2 years follow-up (7/10; 70%). There was no untoward effect of SVD.
CONCLUSION: SVD was highly effective in preventing relapse in CD.
- Citation: Chiba M, Abe T, Tsuda H, Sugawara T, Tsuda S, Tozawa H, Fujiwara K, Imai H. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World J Gastroenterol 2010; 16(20): 2484-2495
- URL: https://www.wjgnet.com/1007-9327/full/v16/i20/2484.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i20.2484
The etiology of inflammatory bowel disease (IBD), which is a collective term for Crohn’s disease (CD) and ulcerative colitis (UC), is unknown but is believed to be multifactorial, whereby development of the disease in genetically susceptible subjects is triggered by environmental factors[1]. IBD is a complex polygenic disease in which the contribution of each gene to the onset of disease is small[2]. IBD incidence increases along with wealth[3,4]. The above two main features of IBD are exactly the same as those for other chronic diseases such as diabetes mellitus, coronary heart disease, and obesity in developed countries[5]. A large part of chronic diseases is related to lifestyle, including food and being sedentary. Some of the environmental factors that have been observed in association with IBD include cigarette smoking, the use of oral contraceptives, and appendectomy[3]. However, these environmental factors are thought to play only a mediating role in IBD. A real key environmental factor has not been identified. Consequently, there is no particular recommendation on lifestyle in guidelines except for smoking in CD[6-9].
Recent studies have clarified that the presence of gut indigenous microflora is a prerequisite for gut inflammation[10,11], and susceptible IBD genes identified are thought to play a role in recognition of microbial agents, immunoregulation, and inflammation[2]. Therefore, IBD is thought to result from an inappropriate response of the mucosal immune system to the intestinal microflora in a genetically susceptible individual[2]. An imbalance of gut microflora, a decrease in beneficial (preventive) bacteria, and an increase in potentially pathogenic bacteria, called dysbiosis, is observed in patients with IBD[12-14]. Dysbiosis can be a trigger for onset and relapse of IBD[15].
There is enough evidence to indicate that IBD is a diet-related disease. Epidemiology shows that IBD is prevalent in wealthy nations[3,4,16] where dietary westernization inevitably occurs[17,18]. Dietary westernization is characterized by increased consumption of animal protein, animal fat, and sugar, with decreased consumption of grains. A dietary study during dietary transition in Japan incriminated an increased intake of animal fat and animal protein in the increase in CD[19]. Pre-illness case-control studies, including those in Japan, have reported increased intake of sugar[20-23], fast foods, chocolate, and cola drinks in IBD[24,25], and a decrease in total fruit and vegetable fiber in CD[20,26]. Case-control studies in Japan are consistent; western foods including bread are a risk factor, whereas traditional Japanese foods are a preventive factor[27-29]. The same tendency has recently been reported in pediatric CD cases in Canada: a positive association with a western diet (meats, fatty foods, and desserts) and an inverse association with a prudent diet (vegetables, fruits, olive oil, grains, and nuts)[30]. Diets rich in animal protein and animal fat cause a decrease in beneficial bacteria in the intestine[31,32]. Probiotics that consist of beneficial bacteria can prevent and are effective against pouchitis, which is ileal inflammation after proctocolectomy in UC[33,34]. These pieces of evidence are consistent with the notion that a westernized diet is linked to an increase in IBD. Therefore, we regard IBD as a lifestyle-related disease that is mediated by mainly a westernized diet[35,36]. Consequently, if a suitable diet is identified and patients stick to the diet, we believe that the majority of IBD patients could be free from relapse without medication.
The conventional recommended diet for IBD is a low-residue diet[37]. A fear of irritating the bowel with dietary fiber has led to a low-residue diet. However, there is no evidence that such a diet is ideal for IBD. A low-residue diet that lacks non-digestible carbohydrates might accelerate the dysbiosis in IBD. Meals per se are thought to cause gut inflammation. Therefore, about half of the daily energy intake is provided by an elemental diet, which is a standard regimen in quiescent CD in Japan[38,39]. At present, it is unknown what kind of diet is suitable for IBD.
Therefore, we designed a diet that hopefully increases the number of beneficial bacteria. Limited foods are known to increase beneficial bacteria: green tea and unrefined brown rice[40,41]. However, most prebiotics are extracts of plants[42]. Therefore, we thought that a vegetarian diet would be suitable for IBD. Considering that excessive restriction in foods can be less acceptable, a semi-vegetarian diet (SVD) could be appropriate. Therefore, an SVD has been provided to IBD patients in our hospital since 2003[35,36]. SVD, which is rich in dietary fiber, is quite opposite to conventional low-residue diets in IBD[37].
The biggest problem in practice in IBD is a lack of a safe, long-lasting modality for relapse prevention. Relapse in CD is far more untenable than in UC[43]. The risk of relapse in CD is influenced by the interval of remission: a higher risk among patients with recently achieved remission than among patients in remission[44].
In this study, an SVD was provided throughout the induction phase in CD patients, or after intestinal surgery. After their discharge, they were immediately followed up in the IBD outpatient department. The aim of this study was to investigate whether an SVD has a preventive effect against relapse in high-risk patients who have recently achieved remission.
This was a prospective clinical trial with 2 years follow-up to establish whether an SVD has a preventive effect against relapse in high-risk patients who have recently achieved remission. This study was approved by the Ethical Committee of Nakadori General Hospital and all patients gave written informed consent.
SVD was first introduced in our institution in 2003, and it has been provided to all patients with CD. From April 2003 to December 2008, 25 active adult CD patients over 19 years old were admitted to the Division of Gastroenterology, Nakadori General Hospital, Akita, Japan. Diagnosis of CD was made by established criteria[45]. Two patients did not achieve remission: one patient was referred to another hospital for surgery, and the other patient was discharged part way through treatment at his request. There were three smokers. Two of them accepted the doctor’s advice and stopped smoking after admission. One patient resumed smoking again after discharge, and this patient was excluded from this study. Therefore, 22 patients who achieved clinical remission medically (n = 17, No. 1-17, Table 1) or surgically (n = 5, No. 18-22, Table 1) were included in this study. Clinical remission was defined as the disappearance of active symptoms of CD. Male patients predominated over female patients (14:8). Ages ranged from 19 to 77 years old (median: 26.5 years). The number of patients with enterocolitis, enteritis, and colitis was 11, 1, and 10, respectively. Fourteen patients had perianal fistulas and/or anal tags. Eleven patients with anal fistulas had draining pus. Disease duration of medically treated patients ranged from 1 to 74 mo (median: 8.0 mo). That of surgically treated patients was > 8 years except for case 19, who underwent surgery on his initial visit after 2 years with the disease. Two of the 22 patients underwent previous intestinal resection for CD. Thirteen cases were initial onset and nine were relapses. Crohn’s disease activity index (CDAI)[46] on admission in medically treated patients ranged from 52 to 679 (median: 211). Five cases showed CDAI < 150, which is arbitrarily defined to be remission in many studies, but they were definitely suffering from active symptoms. Two cases showed very low CDAI, i.e. 88 in case 6 and 52 in case 13, but they had active symptoms: diarrhea for 2 mo and loose stools and lower abdominal pain for 3 mo, respectively. In surgically treated patients, CDAI score on transfer to our division after surgery was determined. The details such as sex, age, clinical types by disease location, anal lesions, disease duration, initial onset or relapse, and C-reactive protein (CRP) concentration are shown in Table 1.
| Case No. | Sex | Age (yr) | Type of disease | Anal lesion | Disease duration | Previous segmental resection | Initial onset or relapse | Hospitalization | |||||||
| Fistula | Tag | On admission | Main medication | Duration of SVD (d) | On discharge | ||||||||||
| CDAI | CRP (mg/dL) | CDAI | CRP (mg/dL) | Morphology | |||||||||||
| 1 | F | 52 | C | - | - | < 1 mo | - | I | 161 | 0.6 | Sulfasalazine | 58 | 25 | 0.9 | Active (CS) |
| 2 | M | 22 | C | + | + | 4 yr and 3 mo | + | R | 264 | 3.1 | Infliximab | 55 | 62 | 0 | NT |
| 3 | F | 21 | EC | + | - | 5 yr and 8 mo | - | R | 133 | 6.3 | Infliximab | 48 | 38 | 0 | NT |
| 4 | M | 22 | EC | + | - | 5 yr and 3 mo | - | R | 679 | 5.9 | Infliximab | 82 | 72 | 0.1 | Remission (CS) |
| 5 | M | 23 | EC | + | - | < 2 mo | - | I | 235 | 1.5 | Infliximab | 55 | 98 | 0.1 | Near remission (CS) |
| 6 | M | 28 | C | - | - | 2 mo | - | I | 88 | 2.5 | Infliximab | 68 | 59 | 0.5 | Near remission (CS) |
| 7 | F | 77 | C | - | - | 1 yr and 10 mo | - | R | 233 | 13.8 | Infliximab | 49 | 39 | 0 | Remission (CS and BE) |
| 8 | M | 30 | C | + | - | 6 mo | - | I | 281 | 4.6 | Infliximab | 50 | 39 | 0.1 | Remission (CS) |
| 9 | M | 55 | C | + | + | 8 mo | - | I | 172 | 1.0 | Infliximab | 46 | 67 | 0.5 | Remission (CS and BE) |
| 10 | M | 19 | EC | + | - | 4 mo | - | I | 147 | 0.8 | Infliximab | 43 | 40 | 0 | Remission (CS and BE) |
| 11 | F | 21 | EC | - | + | 3 mo | - | I | 335 | 6.0 | Infliximab | 49 | 47 | 0.3 | Improved still active (CS and BE) |
| 12 | F | 50 | E | - | - | 1 mo | - | I | 488 | 5.1 | Infliximab | 43 | 53 | 0 | Remission (CS) |
| 13 | M | 28 | C | - | - | 3 mo | - | I | 52 | 0.6 | Infliximab | 50 | 2 | 0 | Remission (CS) |
| 14 | M | 19 | EC | + | + | 2 yr and 1 mo | - | I | 193 | 0.8 | Infliximab | 43 | 35 | 0 | Near remission (CS and BE) |
| 15 | M | 29 | C | + | - | 1 yr and 4 mo | - | I | 126 | 0.2 | Infliximab | 43 | 8 | 0 | Improved still active (CS) |
| 16 | M | 30 | C | + | - | 1 yr and 1 mo | - | I | 211 | 11.8 | Infliximab | 47 | 28 | 0.8 | Near remission (CS and BE) |
| 17 | F | 21 | EC | - | + | 6 yr and 2 mo | - | R | 544 | 3.7 | Infliximab | 46 | 64 | 0.3 | Improved still active (BE) |
| 18 | F | 45 | EC | - | - | 13 yr | + | R, PO 21st d | 143 | 0.2 | Metronidazole | 19 | 105 | 0.1 | NT |
| 19 | M | 21 | EC | + | + | 2 yr | - | I, PO 13th d | 217 | 1.1 | Metronidazole | 18 | 166 | 0.1 | NT |
| 20 | M | 34 | EC | - | - | 17 yr | - | R, PO 12th d | 146 | 0 | Metronidazole | 24 | 108 | 0.1 | NT |
| 21 | M | 25 | C | - | + | 13 yr | - | R, PO 17th d | 372 | 7.1 | Metronidazole | 21 | 149 | 0 | NT |
| 22 | F | 23 | EC | - | - | 8 yr | - | R, PO 25th d | 151 | 8.0 | Metronidazole | 83 | 141 | 0.8 | NT |
Medical induction of remission: The main medication was infliximab in all medically treated patients except for case 1. Case 1 was the earliest case in this study with mild symptoms and was treated with sulfasalazine 3 g/d. Metronidazole 750 mg/d was given after admission. Patients received liquid infusion of 1500 mL/d via a peripheral vein for about 1 wk, without meals. Meanwhile, morphological studies including colonoscopy or contrast barium enema study, enteroclysis, and esophagogastroduodenoscopy were performed to assess clinical types and stenosis. Laboratory data were also obtained. Then, infliximab (5 mg/kg body weight; Remicade, Centocor, Malvern, PA, USA) was infused over 3 h[47]. Infliximab was further infused at 2 and 6 wk according to the standard recommendation[47]. After about 1 mo of metronidazole administration, it was switched to mesalamine 1.5 g/d or sulfasalazine 2 g/d. After the third infusion of infliximab, patients were discharged. Iron (60 mg/d saccharated ferric oxide) was given to patients with moderately severe anemia, but none of patients received a blood transfusion. No steroid hormone, immunosuppressant, or antibiotic other than metronidazole was administered.
The CDAI score of medically treated patients was significantly decreased from 255 ± 169 (mean ± SD) on admission to 46 ± 24 at week 6 after the first infusion of infliximab, i.e. before the third infusion of infliximab (paired t test, P < 0.0001, Table 1). The concentration of CRP also significantly decreased from 4.0 ± 3.9 to 0.2 ± 0.3 mg/dL (P = 0.001). Morphological studies by colonoscopy and/or contrast barium enema study were performed before discharge in 15 patients (Table 1). Morphological remission was achieved in seven patients. Near remission was achieved in four patients in whom redness and/or a few small aphthoid lesions without ulcer were present. In three patients, active lesions were improved, but active lesions (ulcer) were still present. In case 1, there was no improvement in active lesions. Nutritional data in patients who showed hypoalbuminemia (< 3.8 g/dL, n = 7) or hypocholesterolemia (< 120 mg/dL, n = 3) on admission were improved to a normal level at week 6: from 3.2 ± 0.6 to 3.9 ± 0.6 g/dL (P = 0.0086) and from 99 ± 4 to 148 ± 31 mg/dL, respectively (Table 2). Low serum cholinesterase (< 3200 IU/L, n = 5) improved: from 1983 ± 855 to 3149 ± 1039 (P = 0.0233). There was little change in body mass index (BMI) at week 6 in patients who showed abnormally low levels (< 18.5 kg/m2, n = 5) on admission: from 17.4 ± 0.2 to 17.5 ± 1.0. Anemia (male < 13.8 g/dL, female < 12.0 g/dL, n = 11) improved from 10.7 ± 2.0 to 12.3 ± 1.6 (P = 0.0058). Details of individual data are presented in Table 2.
| Case No. | Dietary pattern beforehospitalization | Hospitalization | |||||||||
| On admission | On discharge | ||||||||||
| BMI (kg/m2) | Albmin(g/dL) | Chol(mg/dL) | ChE(IU/mL) | Hemoglobin(g/dL) | BMI (kg/m2) | Albmin(g/dL) | Chol(mg/dL) | ChE(IU/mL) | Hemoglobin(g/dL) | ||
| 1 | Pro-Japanese | 21.1 | 4.7 | 155 | 4929 | 14.8 | 20.5 | 4.1 | 145 | 4959 | 13.0 |
| 2 | Standard | 17.2 | 3.8 | 140 | 4059 | 14.0 | 17.1 | 4.5 | 145 | 4170 | 15.5 |
| 3 | Japanese | 17.2 | 3.7 | 102 | 2003 | 8.3 | 19.2 | 4.0 | 152 | 4117 | 11.1 |
| 4 | Pro-Japanese | 18.5 | 2.8 | 100 | 789 | 8.6 | 17.9 | 3.0 | 116 | 1876 | 12.7 |
| 5 | Pro-western | 17.7 | 4.8 | 132 | 5592 | 15.8 | 17.0 | 4.2 | 135 | 5057 | 14.4 |
| 6 | Pro-western | 21.3 | 3.0 | 122 | 2876 | 10.8 | 20.7 | 4.4 | 192 | 4321 | 10.8 |
| 7 | Pro-Japanese | 17.2 | 2.1 | 95 | 1549 | 7.5 | 16.8 | 3.2 | 177 | 2633 | 11.6 |
| 8 | Pro-western | 19.2 | 3.2 | 128 | 3441 | 12.6 | 18.3 | 4.2 | 114 | 4911 | 14.3 |
| 9 | Japanese | 19.7 | 3.9 | 190 | 5094 | 13.0 | 18.2 | 4.1 | 221 | 4602 | 14.1 |
| 10 | Pro-western | 17.6 | 4.0 | 147 | 4505 | 13.5 | 17.6 | 4.5 | 154 | 4725 | 13.7 |
| 11 | Standard | 19.8 | 3.7 | 147 | 4081 | 9.5 | 18.4 | 3.9 | 125 | 4837 | 10.3 |
| 12 | Standard | 24.1 | 4.4 | 149 | 5156 | 11.0 | 21.4 | 4.3 | 158 | 6626 | 10.7 |
| 13 | Standard | 24.4 | 4.6 | 151 | 4599 | 16.2 | 22.5 | 4.4 | 143 | 4376 | 15.1 |
| 14 | Pro-western | 19.0 | 4.7 | 167 | 5722 | 15.0 | 18.8 | 4.4 | 138 | 4861 | 16.0 |
| 15 | Pro-western | 24.1 | 4.6 | 188 | 5755 | 15.4 | 22.4 | 4.5 | 137 | 5643 | 15.8 |
| 16 | Pro-western | 22.5 | 3.7 | 138 | 3757 | 12.3 | 21.4 | 4.4 | 116 | 4304 | 14.3 |
| 17 | Standard | 18.8 | 3.8 | 187 | 2700 | 10.6 | 18.1 | 4.1 | 209 | 2798 | 11.8 |
| 18 | Pro-Japanese | 21.6 | 4.1 | 126 | 7943 | 10.7 | 21.9 | 3.9 | 129 | 9098 | 10.8 |
| 19 | Pro-western | 20.1 | 4.1 | 150 | 3320 | 11.0 | 20.1 | 4.2 | 152 | 3426 | 11.8 |
| 20 | Polymeric diet | 18.1 | 3.9 | 100 | 3512 | 11.0 | 17.9 | 4.1 | 130 | 3757 | 13.4 |
| 21 | Standard | 15.6 | 3.5 | 116 | 3387 | 10.2 | 15.6 | 4.0 | 138 | 3870 | 11.9 |
| 22 | Pro-Japanese | 16.5 | 3.2 | 58 | 2320 | 9.9 | 16.0 | 2.9 | 78 | 3355 | 9.8 |
Surgical induction of remission: Five patients, cases 18-22, who had recently undergone intestinal resection for intestinal obstruction, were transferred to our division when they could take solid meals. This occurred on postoperative day 12-15 (Table 1). They took metronidazole 750 mg/d for about 1 mo along with meals[48].
Patients’ dietary habits: Patients’ dietary habits and lifestyles before onset or relapse of the disease were obtained immediately after admission, prior to providing information about the SVD, by means of a food-frequency questionnaire[49]. The questionnaire included 45 questions that covered almost all foods or food groups in Japan. There was a question about dietary type that listed six types: western, pro-western, standard/mixed, pro-Japanese, Japanese, and SVD. A definition of dietary types was not set. There was a variety of dietary patterns before the onset or relapse of CD: pro-western (n = 8), standard/mixed of western and Japanese diet (n = 6), pro-Japanese (n = 5), and Japanese (n = 2) (Table 2). Case 20 had a 2000 kcal/d polymeric diet without meals. None of our patients’ dietary pattern was SVD or a western diet. Based on the questionnaire, a summarized table was made that showed both a patient’s hitherto and future recommended lifestyle and dietary habits. Such a table created for case 8 is shown in Table 3. Recommended dietary habits were consistent with SVD in the following. This table was used by the dietitian when giving dietary guidance, and it was given to the patient by the chief investigator (Chiba M) during hospitalization.
| Every day | 3-5 times/wk | 1-2 times/wk | Rare | None | |
| Smoking (No. of cigarettes/d) | 20 ≤ | 6-19 | 1-5 | Rare | P |
| Regular exercise | R | R | R | P | |
| Alcohol | P | ||||
| Eating between meals | P | R | R | ||
| Type of diet (D) | Semi-vegetarian (R) | Japanese | Pro-Japanese | Standard/mixed | Pro-western (P) |
| Food | |||||
| Rice | P | ||||
| Miso soup | P | ||||
| Pulses | R | P | |||
| Vegetables | R | P | |||
| Udon/soba (Japanese noodles) | P | ||||
| Ramen (Chinese noodles) | P | R | |||
| Bread (D) | P | R | |||
| Tea, coffee | Canned coffee (P) | R | |||
| (Sugar in tea or coffee) (D) | Large amount (P) | Average amount | Small amount | Rare | None (R) |
| Juice | P | R | R | ||
| Cola/soda | P | ||||
| Beef | P | R | |||
| Pork/chicken (D) | P | R | |||
| Minced or processed meat | P | R | |||
| Fish | P | ||||
| Cheese/butter/margarine | P | R | |||
| Sweets (D) | P | R | R | ||
| Ice cream/milk shake | P | ||||
| Yoghurt (plain) | R | P | |||
| Green tea (D) | R | P | |||
| Potatoes/starches (D) | R | P | |||
| Fruits (D) | R | P |
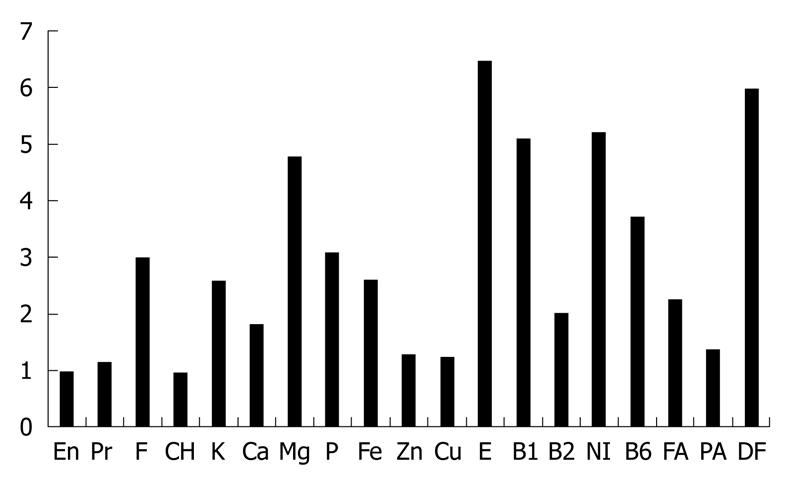
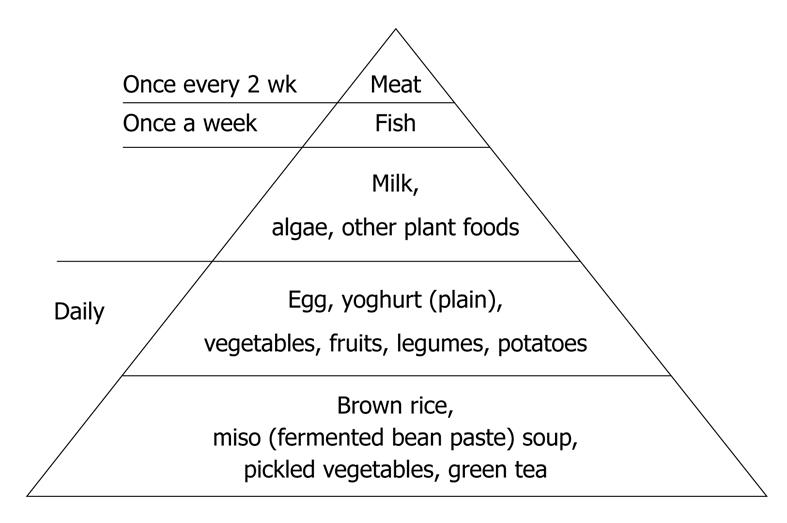
SVD: SVD was initiated on the same day as infusion of infliximab in medically treated patients. About 800 or 1100 kcal/d was given in the beginning, and calories were gradually increased to a maximum of about 30 kcal/kg standard body weight. White rice was served first for 2-5 d followed by mixed rice (70% white rice and 30% unrefined whole brown rice) for 2-5 d, and finally brown rice was served. Unrefined brown rice contains more vitamins and minerals than white rice[50] (Figure 1) and is reported to increase significantly beneficial bacteria compared to well-milled white rice[41]. Eggs and milk were used. In other words, our diet was a lacto-ovo-vegetarian diet[51]. Miso (fermented bean paste) soup, vegetables, fruits, legumes, potatoes, pickled vegetables, and plain yoghurt were served daily. Fish was served once a week and meat once every 2 wk, both at about a half the average amount. Patients were provided with several different 4-wk menus on a rotational basis. Figure 2 shows what an SVD looks like. Details of the contents of nutritional elements including minerals, vitamins, and fatty acids in an SVD are shown in Table 4. These figures were obtained by HOPE/COMETY-NT (Fujitsu, Tokyo, Japan). The rate of fat in total calories (18.6%) was < 20%, which is the lower limit of Dietary Reference Intakes for Japanese (DRI)[52]. The amounts of dietary fiber and iron in the SVD were above the DRI. Those of most of the other elements in the SVD were comparable to DRI (Table 4). Coarse tea was served along with the meal service. During hospitalization, foods other than the meal service were discouraged. Drinking of green tea was encouraged. Participant days of SVD ranged from 43 to 82 d (median: 49 d) in medically treated patients (Table 1). In surgically treated patients, they were shorter, about 3 wk. Case 22 was exceptionally long, 83 d, due to a postoperative complication of subcutaneous abscess formation. In all 22 cases, there were no adverse effects such as gaseous distress, abdominal discomfort, or diarrhea as a result of the SVD.
| SVD | DRI | |
| Energy | ||
| Protein (%) | 16.1 ± 0.5 | < 20 |
| Fat (%) | 18.6 ± 1.4 | 20-30 |
| Carbohydrate (%) | 66.1 ± 1.6 | 50-70 |
| Content | ||
| Protein (g) | 80.3 ± 3.0 | M 60, F 50 |
| Fat (g) | 41.4 ± 3.6 | |
| Carbohydrate (g) | 330.2 ± 11.4 | |
| Dietary fiber (g) | 32.4 ± 2.1 | M 20, F 17 |
| Soluble dietary fiber (g) | 6.8 ± 0.7 | |
| Insoluble dietary fiber (g) | 23.3 ± 1.6 | |
| Calcium (mg) | 873.7 ± 65.2 | M 600-650, F 600 |
| Phosphorus (mg) | 1882.7 ± 101.8 | M 1050, F 900 |
| Iron (mg) | 19.3 ± 1.3 | M 7.5, F 10.5 |
| Sodium (mg) | 4492.9 ± 342.1 | 600 |
| Kalium (mg) | 4281.7 ± 250.6 | M 2000, F 1600 |
| Vitamin A (μgRE) | 1416.6 ± 360.9 | M 750, F 600 |
| Vitamin B1 (mg) | 2.0 ± 0.2 | M 1.4, F 1.1 |
| Vitamin B2 (mg) | 1.3 ± 0.2 | M 1.6, F 1.2 |
| Niacin (mg) | 26.6 ± 3.1 | M 15, F 12 |
| Vitamin C (mg) | 133.1 ± 23.4 | 100 |
| Vitamin D (μg) | 3.2 ± 1.0 | 5 |
| Vitamin E (mg) | 12.3 ± 1.9 | M 9, F 8 |
| Cholesterol (mg) | 285.9 ± 72.1 | M < 700, F < 600 |
| NaCl (g) | 10.9 ± 0.8 | M < 10, F < 8 |
| Polyunsaturated fatty acid (g) | 13.9 ± 1.9 | |
| Monounsaturated fatty acid (g) | 9.3 ± 0.9 | |
| Saturated fatty acid (g) | 7.1 ± 0.9 | |
| P:S ratio | 2.0 ± 0.2 |
At the end of hospitalization, a qualified dietitian gave dietary guidance to the patient and the meal preparer. It included how to boil brown rice. The responsible doctor (Chiba M) also gave patients an SVD guide (Figure 3) and advised them to continue the diet after discharge. Foods that have been shown to be a risk factor for IBD in or outside Japan, including sweets[20-23,28-30], bread[27], cheese[27-29], margarine[27], fast foods, carbonated beverages, and juices[24,25,29], were discouraged. Healthy habits were encouraged: no smoking, regular physical activity, moderate or no use of alcohol, regularity of meals, and not eating between meals[53].
Twenty-two patients who achieved remission either medically or surgically were followed up for 2 years. Medication was mesalamine or sulfasalazine (Table 5). None of the patients took immunosuppressants such as azathioprine or 6-mercaptopurine. They visited the IBD outpatient department (responsible doctor: Chiba M) every 8 wk. Blood samples for measurement of CRP, complete blood counts, albumin, and transaminases were taken at each visit. The food-frequency questionnaire was obtained at month 3, at years 1 and 2, or when a patient relapsed. When remission was maintained for 1 year and a patient seemed to continue the SVD, medication was stopped if the patient so desired. Primary end point was clinical relapse that was defined as the appearance of active symptoms of CD that required treatment. Duration of remission was calculated as the number of days between discharge and the appearance of symptoms of relapse. Although the responsible doctor (Chiba M) similarly advised patients to continue the SVD, some patients did not follow the advice or abandoned it at some point. Relapse in 2 years was compared between two groups of patients: SVD group and the omnivorous diet group.
| Case No. | Main medication | Dietary pattern | Days of remission | Reason for termination | On final attendance | |||||||||
| Month 3 | Year 1 | Year 2 | On relapse | CDAI | CRP (mg/dL) | BMI (kg/m2) | Alb (g/dL) | Chol (mg/dL) | ChE (IU/mL) | Hemoglobin (g/dL) | ||||
| 1 | SSZ for 1 yr | SVD | SVD | SVD | 730 | Completion | 0 | 0.1 | 23.1 | 4.5 | 235 | 5684 | 14.4 | |
| 2 | Mesalamine | SVD | Omni | Omni | 301 | Relapse | 193 | 8.2 | 17.5 | 3.8 | 151 | 3262 | 13.8 | |
| 3 | Mesalamine | SVD | Omni | Omni | 367 | Relapse | 167 | 11.3 | 21.1 | 3.5 | 133 | 3551 | 10.0 | |
| 4 | Mesalamine | SVD | SVD | SVD | 730 | Completion | 128 | 5.0 | 18.7 | 2.8 | 114 | 1113 | 9.3 | |
| 5 | SSZ for 1 yr | SVD | SVD | 523 | Moving | 39 | 0 | 19.4 | 4.5 | 194 | 5509 | 15.0 | ||
| 6 | Mesalamine | Omni | Omni | 191 | Relapse | 192 | 5.7 | 21.7 | 3.9 | 134 | 3052 | 7.9 | ||
| 7 | None | SVD | SVD | 442 | INFX for RA | 61 | 2.0 | 19.6 | 4.0 | 158 | 5068 | 10.9 | ||
| 8 | Mesalamine | SVD | SVD | SVD | 635 | Relapse | 174 | 7.6 | 19.1 | 3.1 | 88 | 2678 | 11.3 | |
| 9 | Mesalamine | Omni | Omni | Omni | 730 | Completion | 48 | 0.2 | 19.8 | 4.3 | 171 | 5461 | 15.2 | |
| 10 | Mesalamine | SVD | SVD | SVD | 730 | Completion | 18 | 0.1 | 18.2 | 5.1 | 181 | 4659 | 16.3 | |
| 11 | Mesalamine | SVD | SVD | SVD | 730 | Completion | 11 | 0 | 19.7 | 4.7 | 147 | 5215 | 13.9 | |
| 12 | Mesalamine | SVD | SVD | SVD | 730 | Completion | 45 | 0 | 20.2 | 4.7 | 163 | 6268 | 11.3 | |
| 13 | Mesalamine | SVD | SVD | SVD | 730 | Completion | 0 | 0 | 22.8 | 4.6 | 171 | 4770 | 15.6 | |
| 14 | Mesalamine | SVD | SVD | 666 | Ongoing | 27 | 0.1 | 19.3 | 4.7 | 188 | 6084 | 15.1 | ||
| 15 | Mesalamine | SVD | Omni | 560 | Ongoing | 0 | 0 | 22.6 | 4.8 | 185 | 5671 | 16.8 | ||
| 16 | Mesalamine | SVD | SVD | 440 | Ongoing | 19 | 1.9 | 21.8 | 4.4 | 141 | 5146 | 14.2 | ||
| 17 | Mesalamine | SVD | SVD | 397 | Ongoing | 32 | 2.0 | 22.5 | 3.6 | 216 | 2683 | 11.1 | ||
| 18 | Mesalamine | SVD | SVD | SVD | 730 | Completion | 25 | 0.4 | 22.6 | 3.7 | 151 | 10222 | 11.1 | |
| 19 | Mesalamine | SVD | SVD | SVD | 730 | Completion | 62 | 0 | 20.1 | 4.7 | 139 | 4391 | 13.3 | |
| 20 | None | SVD | SVD | SVD | 730 | Completion | 37 | 0.7 | 18.2 | 4.5 | 156 | 5655 | 13.2 | |
| 21 | SSZ | SVD | SVD | SVD | 730 | Completion | 16 | 0.1 | 19.0 | 4.9 | 205 | 6424 | 14.4 | |
| 22 | Mesalamine | SVD | Omni | Omni | 630 | Relapse | 118 | 5.1 | 22.1 | 3.5 | 84 | 3596 | 10.1 | |
Assessment of dietary pattern in outpatients: Dietary pattern was classified into two groups: SVD and omnivorous diet. When the following two conditions were fulfilled, it was regarded as SVD in this study. One is that a patient follows the principle of SVD: daily intake of rice, vegetables, and fruits, and occasional intake of fish, meat, and other animal-based foods. The other one is that a patient refrains from foods reported as risk factors for IBD in or outside Japan as stated above[20-30]. A diet that did not fulfill these two conditions was regarded as an omnivorous diet.
Kaplan-Meier survival analysis was used to calculate the cumulative proportion of patients who had a relapse. Patients who stopped the treatment for any reason other than relapse were considered censored at the time of their last observation. Comparison of cumulative relapse rates between the SVD and omnivorous groups was tested by the log rank test. A P value of 0.05 or less was considered to indicate a statistically significant difference. Statistical analysis was performed using JMP 8 (SAS Institute Inc., Cary, NC, USA) software.
Eighteen patients terminated the follow-up: completion of 2-year observation in 11, relapse in five, and early termination for other reason in two (moving and infliximab therapy for associated rheumatoid arthritis in one each). Four patients are ongoing (Table 5). Dietary types that patients marked in the food-frequency questionnaire during the follow-up study were judged appropriate by other foods or food groups marked. SVD was maintained by 16 patients but not by six (compliance 73%) (Table 5). There was no untoward effect of SVD. The reason for the discontinuance of SVD in six patients was not a detrimental effect of SVD but their preference for sweets, meat, and/or fish. Case 2 continued SVD for the first 3 mo but stopped thereafter. Cases 3 and 22 consumed vegetables and fruits only once a week and ate sweets frequently. Case 6 cooked and added meat to the SVD that was prepared by his mother. Case 9 had alcohol at supper every night and frequently ate fish and sweets. Case 15 consumed animal foods more often than the standard for an SVD.
Five patients relapsed. Four were medically treated and one was surgically treated. On the final study visit, they showed elevated CRP concentration and a CDAI of > 100 (Table 5). Seventeen patients maintained remission. Among them, 11 with normal CRP concentration showed almost normal values in other nutritional data (BMI, albumin, cholesterol, and cholinesterase) and hemoglobin. Their CDAI was extremely low. Meanwhile, most of the six patients with elevated CRP, although in remission, showed some abnormality in these indices. The details are shown in Table 5.
Among the 16 patients who continued with the SVD, 15 maintained remission and one relapsed (Table 5): the remission rate was 100% (16/16) at 1 year and 92% at 2 years (Figure 4 and Table 6). Among six patients who were on an omnivorous diet, two maintained remission and four relapsed (Table 5): the remission rate was 67% (4/6) at 1 year and 25% at 2 years (Figure 4 and Table 6). Life table analysis showed that the cumulative relapse rate at 2 years was significantly lower in the SVD group than in the omnivorous group (P = 0.0003) (Figure 4). The concentration of CRP was normal at the final visit in more than half of the patients in remission on an SVD; in those who maintained remission during the study (9/15; 60%); terminated follow-up (8/12; 67%), or completed 2 years follow-up (7/10; 70%).
| Induction therapy | Maintenance therapy | ||||
| Subjects | Regimen | Regimen | Duration | ||
| 1 yr | 2 yr | ||||
| Candy et al[55], 1995 | CDAI > 200 | PS (diminishing dose) + AZA 12 wk | AZA 2.5 mg/kg | Remission rate 42% (14/33) | |
| Placebo | 7% (2/30) | ||||
| Hanauer et al[56], 2002 | CDAI ≥ 220 | Gr (1) and Gr (2) | Gr (1) INFX 5 mg/kg every 8 wk | Remission rate 25% (28/113) | |
| Responder to a single infusion of infliximab | INFX 5 mg/kg at weeks 0, 2, 6 | Gr (2) INFX 10 mg/kg every 8 wk | 33% (37/112) | ||
| Gr (3) INFX 5 mg/kg then placebo infusion at weeks 2, 6 | Gr (3) placebo infusion every 8 wk | 11% (12/110) | |||
| Sandborn et al[58], 2005 | Mild to moderate CD | Budesonide 6 mg or 3 mg | Budesonide 6 mg or 3 mg | Relapse rate 60%-70% | |
| Placebo | (In all 3 groups) | ||||
| Takagi et al[39], 2006 | Active CD | TPN (25 cases), TEN (22 cases), | ED (half) + a free diet (half) | Relapse rate 26.9% (7/26) | |
| PS (1 case), INFX (3 cases) | A free diet | 64.0% (16/25) | |||
| Regueiro et al[59], 2009 | CD patients who had surgery | Ileocolonic resection | INFX 5 mg/kg at weeks 0, 2, 6 | Remission rate 80% (8/10) | |
| then every 8 wk | |||||
| Placebo | 58% (7/13) | ||||
| Present study | Active CD | INFX 5 mg/kg at weeks 0, 2, 6 (16 cases), intestinal resection (5 cases), SSZ (1 case) | Semi-vegetarian diet | Remission rate 100% (16/16) | 92% |
| Omnivorous diet | 67% (4/6) | 25% | |||
We used mesalamine or sulfasalazine in the follow-up phase. Considering that the relapse-preventive effect of mesalamine or sulfasalazine is absent or modest at most[54], it is reasonable to conclude that the SVD protected patients from relapse but an omnivorous diet did not.
Azathioprine and 6-mercaptopurine are known to be effective and used for maintenance of remission in patients with steroid-dependent CD[6-9]. They are also used in the induction phase together with prednisolone and then used for maintenance. In such situations, Candy et al[55] have reported that the remission rate at 1 year was 42% (placebo control, 7%) (Table 6). Recently, scheduled infliximab therapy every 8 wk has been shown to be effective as maintenance treatment in both adults and pediatric patients[56,57]. Remission rate at 1 year in adults was 25%-33% (placebo control, 11%) (Table 6)[56]. An elemental diet that consisted of about a half the daily energy intake has been shown recently to have a relapse-preventive effect in the Japanese study group: 26.9% relapse rate at 1 year (control free diet, 64.0%) (Table 6)[39]. Remission rates with the SVD in the present study were far better than those reported previously: 100% (16/16) remission rate at 1 year and 92% at 2 years. To the best of our knowledge, this is the best result in relapse prevention. Although our study population included mild cases, our excellent results cannot be explained solely by a difference in severity in study population, because even mild to moderate disease relapse rates are 60%-70% at 1 year (Table 6)[58]. More recently, scheduled infliximab therapy every 8 wk has been shown to be effective in postoperative patients: 80% remission rate at 1 year (placebo control, 58%) (Table 6)[59]. Successful scheduled maintenance therapy with infliximab, however, could encounter difficulty over longer periods due to a waning of efficacy, and side effects. In 2 years, more than half the patients need a shortening in the infliximab dosing interval and about 10% of patients need cessation of therapy[60]. Although the number of patients in our study was small, all four postoperative patients on the SVD maintained remission for 2 years (Table 5).
When we began thinking of IBD as a lifestyle-related disease mediated mainly by dietary westernization, we changed our modality of treatment to emphasize diet (Table 7). The menu of a conventional low-residue diet for IBD is shared by UC and CD. However, CD is apparently more tenacious than UC[43]. Therefore, the staple is refined white rice for UC and unrefined brown rice for CD. About 30 kcal/kg per day is provided in the current diet, while total calories were not individualized in the conventional diet. Dietary analysis is needed for dietary guidance in current practice (Table 7). In addition to diet, common health practices[53] are encouraged.
| Conventional | Current | |
| Diet | Low-residue diet | Semi-lactoovovegetarian diet |
| Menu | Common for UC and CD | SVD for UC (staple: white rice) |
| SVD for RUC (staple: white rice 70%, brown rice 30%) | ||
| SVD for CD (staple: brown rice) | ||
| Refrainment | Fiber-rich diet | Minced or processed meat, bread, fast foods, sweets, cola/soda, juices |
| Dairy products | Refrainment | Egg, milk: no refrainment |
| Refrain from cheese/butter/margarine | ||
| Calories | 2000 kcal/d | About 30 kcal/kg standard body weight/d |
| Dietary analysis | Absent | Food-frequency questionnaire |
| Dietary guidance | Absent | Guidance by doctor and registered dietitian |
SVD is initiated after about 1 wk of fasting, on the same day as infliximab infusion. By this time, symptoms such as diarrhea, abdominal pain, and fever subside, and patients want to eat. SVD that contained a moderate amount of dietary fiber was not detrimental but useful for induction of remission of CD. It improved hypoalbuminemia, hypocholesterolemia, and anemia during hospitalization. Although clinical remission could be obtained by one or two infusions of infliximab, we believe that a certain period of time is needed for recovery of morphological changes in the intestine. Therefore, three infusions of infliximab in 6 wk[47] were given, not at the outpatient clinic but during hospitalization. This period of hospitalization was also useful for patients to become familiar with the SVD. Compliance with the SVD in outpatients in this study was about 75%, which indicates that an SVD could be applicable to the majority of CD patients. An SVD is completely natural and safe. There is no need to fear side effects, as with steroid hormones or immunosuppressants such as infliximab, azathioprine, 6-mercaptopurine, or methotrexate.
Fifteen of 16 patients who continued the SVD were free from relapse in our study. In addition, more than half of the patients in remission showed normal CRP levels. CRP is a sensitive indicator for predicting relapse[61]. Judging from CRP levels, it seems that more than half of the patients who continue the SVD will be free from relapse as long as they maintain the diet. However, the rest of the patients in remission with elevated CRP might relapse in the long term, which seems a limitation of the SVD. Our study clearly shows that the dietary pattern influences the relapse rate in CD. Therefore, trials of the preventive effect of medications in CD should be designed in consideration of diet.
There have been trials for prevention of relapse in CD with diets or supplements. Excellent results with an exclusion diet of intolerant foods[62], an unrefined-carbohydrate, fiber-rich diet[63], or fish oil supplement[64] have not been reproduced in other studies[65-67]. Therefore, none of the dietary modifications has been widely accepted. Currently, manipulation of gut microflora with probiotics and/or prebiotics has emerged as an attractive therapeutic modality in IBD[11,15,33,34]. The rationale of an SVD, i.e. enhancement of beneficial bacteria in the gut, is the same as that of probiotics and prebiotics. There is a limitation to mere addition of probiotics, prebiotics or food stuffs and exclusion of potentially untoward food stuffs[65-68]. We believe that, not partial, but comprehensive food control is needed for patients who are genetically predisposed to IBD. Therefore, our SVD encourages consumption of grains, vegetables, and fruits, while limiting intake of animal foods that tend to decrease beneficial bacteria[31,32] and other foods reported to be risk factors for IBD[20-30]. However, no food item is prohibited.
Although we designed our SVD with gut bacterial flora in mind, both plant-only (vegan) and plant-based (lacto-ovo-vegetarian, semi-vegetarian) vegetarians are shown to have low rates of cancer, cardiovascular disease, obesity, and total mortality[51,69]. Plant-based diets are recommended for prevention of cancer and other lifestyle-related chronic diseases[70]. Therefore, SVD will not only be effective for gut inflammation, but also promote the general health of IBD patients.
Since we are aware that an SVD is effective for relapse prevention, we are concerned with rapid, safe and reliable induction of remission. This is now attainable by infliximab[47]. Therefore, we use infliximab as the first choice of therapy in all newly diagnosed active CD cases. So far, there has been no failure in induction of remission with infliximab (Chiba et al, article in preparation). Therefore, since the advent of infliximab and SVD, we feel that CD has been much more controllable than before.
In conclusion, this study shows that an SVD is safe and has a preventive effect against relapse of CD. Normal CRP levels are maintained in more than a half of the patients with an SVD. This supports our notion that IBD is a lifestyle-related disease that is mediated mainly by a westernized diet. The concept that IBD is a lifestyle-related disease is lacking in present practice. We believe that without introduction of this concept, a major breakthrough in the prevention of relapse in CD is not attainable. Whether gut microflora are indeed enriched in beneficial bacteria by an SVD needs to be clarified. Our new findings require verification in large, randomized, controlled clinical trials.
Crohn’s disease (CD) is a chronic disease of the intestine. The peak incidence is between the ages of 15 and 25 years. Remission is induced by drip infusion or elemental diet (amino-acid-based) without meals, by drugs such as prednisolone and infliximab, by resection of the involved intestine, or by a combination of these. The biggest problem in practice in CD is frequent relapse and difficulty in maintaining remission. At 1 year, the relapse rate is around 60%-70% and the remission rate is 25%-42%. A low-residue diet has been conventionally recommended without evidence of improving remission rates. Because meals inevitably cause relapse, the use of elemental diet is popular in countries like Japan.
Although the etiology of CD is unknown, there is some evidence to indicate that it is a lifestyle-related disease that is mediated mainly by a westernized diet: increased consumption of animal protein, animal fat, and sugar, with decreased consumption of grains. Therefore, the authors designed a semi-vegetarian diet (SVD) which hopefully increases beneficial (preventive) bacteria in the intestine. In this study, the authors examined whether the SVD had a preventive effect against relapse in high-risk patients with CD who had recently achieved remission.
The SVD was highly effective in preventing relapse in CD. Remission rate with the SVD was 100% at 1 year and 92% at 2 years. This is the best result in relapse prevention. The concentration of C-reactive protein, an indicator of inflammation, was normal at the final visit in more than half of the patients on the SVD, which indicated that more than half of the patients who continue the SVD will be free from relapse as long as they maintain the diet.
SVDs can be provided anywhere in the world. Ulcerative colitis (UC) is another chronic inflammatory bowel disease that displays a less harsh clinical course than CD. An SVD can also be applied to UC.
The SVD in this study was a lacto-ovo-vegetarian diet in which eggs and milk were used. Fish was served once a week and meat once every 2 wk, both at about half of the average amount.
This is an excellent paper with clear scientific data in a clinical area of extreme importance.
Peer reviewers: Alexander G Heriot, MA, MD, FRCS, FRACS, Associate Professor, Department of Surgical Oncology, Peter MacCallum Cancer Centre, 1 St Andrews Place, Melbourne, VIC 3002, Australia; Dr. Thomas Wild, MD, Department of Surgery, Paracelsus Medical University, Feldgasse 88, Kapellerfeld, 2201, Austria
S- Editor Wang JL L- Editor Kerr C E- Editor Zheng XM
| 1. | Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390-407. |
| 2. | Cho JH, Weaver CT. The genetics of inflammatory bowel disease. Gastroenterology. 2007;133:1327-1339. |
| 3. | Bernstein CN, Shanahan F. Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut. 2008;57:1185-1191. |
| 4. | Whelan G. Inflammatory bowel disease: epidemiology. Bockus Gastroenterology. 5th ed. Vol 2. Philadelphia: WB Saunders 1995; 1318-1325. |
| 5. | Janssens AC, van Duijn CM. Genome-based prediction of common diseases: advances and prospects. Hum Mol Genet. 2008;17:R166-R173. |
| 6. | Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1-V16. |
| 7. | Travis SP, Stange EF, Lémann M, Oresland T, Chowers Y, Forbes A, D'Haens G, Kitis G, Cortot A, Prantera C. European evidence based consensus on the diagnosis and management of Crohn's disease: current management. Gut. 2006;55 Suppl 1:i16-i35. |
| 8. | Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:940-987. |
| 9. | Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn's disease in adults. Am J Gastroenterol. 2009;104:465-483; quiz 464, 484. |
| 10. | Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernández-Sueiro JL, Balish E, Hammer RE. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359-2364. |
| 11. | Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620-1633. |
| 12. | Giaffer MH, Holdsworth CD, Duerden BI. The assessment of faecal flora in patients with inflammatory bowel disease by a simplified bacteriological technique. J Med Microbiol. 1991;35:238-243. |
| 13. | Fabia R, Ar'Rajab A, Johansson ML, Andersson R, Willén R, Jeppsson B, Molin G, Bengmark S. Impairment of bacterial flora in human ulcerative colitis and experimental colitis in the rat. Digestion. 1993;54:248-255. |
| 14. | Favier C, Neut C, Mizon C, Cortot A, Colombel JF, Mizon J. Fecal beta-D-galactosidase production and Bifidobacteria are decreased in Crohn's disease. Dig Dis Sci. 1997;42:817-822. |
| 15. | Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53:1-4. |
| 16. | Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, van Blankenstein M. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut. 1996;39:690-697. |
| 17. | Report of the Working Group on Arteriosclerosis of the National Heart, Lung, and Blood Institute. Arteriosclerosis, vol. 2. USDHHS, PHS, NIH. Philadelphia: WB Saunders 1981; . |
| 18. | Popkin BM. The nutrition transition in low-income countries: an emerging crisis. Nutr Rev. 1994;52:285-298. |
| 19. | Shoda R, Matsueda K, Yamato S, Umeda N. Epidemiologic analysis of Crohn disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am J Clin Nutr. 1996;63:741-745. |
| 20. | Thornton JR, Emmett PM, Heaton KW. Diet and Crohn's disease: characteristics of the pre-illness diet. Br Med J. 1979;2:762-764. |
| 21. | Tragnone A, Valpiani D, Miglio F, Elmi G, Bazzocchi G, Pipitone E, Lanfranchi GA. Dietary habits as risk factors for inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1995;7:47-51. |
| 22. | Riordan AM, Ruxton CH, Hunter JO. A review of associations between Crohn's disease and consumption of sugars. Eur J Clin Nutr. 1998;52:229-238. |
| 23. | Sakamoto N, Kono S, Wakai K, Fukuda Y, Satomi M, Shimoyama T, Inaba Y, Miyake Y, Sasaki S, Okamoto K. Dietary risk factors for inflammatory bowel disease: a multicenter case-control study in Japan. Inflamm Bowel Dis. 2005;11:154-163. |
| 24. | Persson PG, Ahlbom A, Hellers G. Diet and inflammatory bowel disease: a case-control study. Epidemiology. 1992;3:47-52. |
| 25. | Russel MG, Engels LG, Muris JW, Limonard CB, Volovics A, Brummer RJ, Stockbrügger RW. Modern life' in the epidemiology of inflammatory bowel disease: a case-control study with special emphasis on nutritional factors. Eur J Gastroenterol Hepatol. 1998;10:243-249. |
| 26. | Amre DK, D'Souza S, Morgan K, Seidman G, Lambrette P, Grimard G, Israel D, Mack D, Ghadirian P, Deslandres C. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn's disease in children. Am J Gastroenterol. 2007;102:2016-2025. |
| 27. | Dietary and other risk factors of ulcerative colitis. A case-control study in Japan. Epidemiology Group of the Research Committee of Inflammatory Bowel Disease in Japan. J Clin Gastroenterol. 1994;19:166-171. |
| 28. | Morita N, Minoda T, Munekiyo M, Watanabe Y, Muto T, Yokoyama T, Tanaka H, Kawamura T, Morioka S, Hashimoto T. Case-control study of ulcerative colitis in Japan. Annual report of Research Committee on Epidemiology of Intractable Diseases, the Ministry of Health and Welfare of Japan. Nagoya: The Department of Preventive Medicine, School of Medicine, Nagoya University 1996; 153-158 (Abstract in English). |
| 29. | Morita N, Ohnaka O, Ando S, Watanabe Y, Takazoe M, Takagi K, Yokoyama T, Tanaka H, Kawamura T, Ohno Y. Case-control study of Crohn's disease in Japan. Annual report of Research Committee on Epidemiology of Intractable Diseases, the Ministry of Health and Welfare of Japan. Nagoya: The Department of Preventive Medicine, School of Medicine, Nagoya University 1997; 58-64 (Abstract in English). |
| 30. | D'Souza S, Levy E, Mack D, Israel D, Lambrette P, Ghadirian P, Deslandres C, Morgan K, Seidman EG, Amre DK. Dietary patterns and risk for Crohn's disease in children. Inflamm Bowel Dis. 2008;14:367-373. |
| 31. | Hentges DJ, Maier BR, Burton GC, Flynn MA, Tsutakawa RK. Effect of a high-beef diet on the fecal bacterial flora of humans. Cancer Res. 1977;37:568-571. |
| 32. | Benno Y, Suzuki K, Suzuki K, Narisawa K, Bruce WR, Mitsuoka T. Comparison of the fecal microflora in rural Japanese and urban Canadians. Microbiol Immunol. 1986;30:521-532. |
| 33. | Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305-309. |
| 34. | Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202-1209. |
| 35. | Chiba M, Akashi T, Ando H, Matsuhashi T, Kato J. Three Japanese cases of inflammatory bowel disease associated with left-sided colonic diverticulosis: its implication. Inflamm Bowel Dis. 2005;11:952-954. |
| 36. | Chiba M, Sugawara T, Tsuda H, Abe T, Tokairin T, Kashima Y. Esophageal ulcer of Crohn's disease: disappearance in 1 week with infliximab. Inflamm Bowel Dis. 2009;15:1121-1122. |
| 37. | Kirsner JB, Wall AJ. The medical treatment of ulcerative colitis and Crohn’s disease of the colon. Inflammatory bowel disease. Philadelphia: Lea and Febiger 1975; 279-293. |
| 38. | Hirakawa H, Fukuda Y, Tanida N, Hosomi M, Shimoyama T. Home elemental enteral hyperalimentation (HEEH) for the maintenance of remission in patients with Crohn's disease. Gastroenterol Jpn. 1993;28:379-384. |
| 39. | Takagi S, Utsunomiya K, Kuriyama S, Yokoyama H, Takahashi S, Iwabuchi M, Takahashi H, Takahashi S, Kinouchi Y, Hiwatashi N. Effectiveness of an 'half elemental diet' as maintenance therapy for Crohn's disease: A randomized-controlled trial. Aliment Pharmacol Ther. 2006;24:1333-1340. |
| 40. | Ahn YJ, Sakanaka S, Kim MJ, Kawamura T, Fujisawa T, Mitsuoka T. Effect of green tea extract on growth of intestinal bacteria. Microb Ecol Health Dis. 1990;3:335-338. |
| 41. | Benno Y, Endo K, Miyoshi H, Okuda T, Koishi H, Mitsuoka T. Effect of rice fiber on human fecal microflora. Microbiol Immunol. 1989;33:435-440. |
| 42. | Van Loo J, Cummings J, Delzenne N, Englyst H, Franck A, Hopkins M, Kok N, Macfarlane G, Newton D, Quigley M. Functional food properties of non-digestible oligosaccharides: a consensus report from the ENDO project (DGXII AIRII-CT94-1095). Br J Nutr. 1999;81:121-132. |
| 43. | Picco MF, Bayless TM. Prognostic consideration in idiopathic inflammatory bowel disease. Inflammatory bowel disease. 5th ed. Philadelphia: WB Saunders 2000; 765-780. |
| 44. | Sahmoud T, Hoctin-Boes G, Modigliani R, Bitoun A, Colombel JF, Soule JC, Florent C, Gendre JP, Lerebours E, Sylvester R. Identifying patients with a high risk of relapse in quiescent Crohn's disease. The GETAID Group. The Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut. 1995;37:811-818. |
| 45. | Yao T, Matsui T, Hiwatashi N. Crohn's disease in Japan: diagnostic criteria and epidemiology. Dis Colon Rectum. 2000;43:S85-S93. |
| 46. | Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn's Disease Activity Index (CDAI). Gastroenterology. 1979;77:843-846. |
| 47. | Sandborn WJ, Hanauer SB. Infliximab in the treatment of Crohn's disease: a user's guide for clinicians. Am J Gastroenterol. 2002;97:2962-2972. |
| 48. | Rutgeerts P, Hiele M, Geboes K, Peeters M, Penninckx F, Aerts R, Kerremans R. Controlled trial of metronidazole treatment for prevention of Crohn's recurrence after ileal resection. Gastroenterology. 1995;108:1617-1621. |
| 49. | Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243-249. |
| 50. | Study Group of Nutritional Element Analysis of Food. Table of nutritional element analysis of Japanese food. 5th ed. Tokyo: Ishiyaku Publishers 2001; 12-13. |
| 51. | Dwyer J. Convergence of plant-rich and plant-only diets. Am J Clin Nutr. 1999;70:620S-622S. |
| 52. | Ministry of Health, Labor, and Welfare, Japan. Dietary Reference Intakes for Japanese. Accessed October 1. Tokyo: Ishiyaku Publishers 2009; Available from: http://www.mhlw.go.jp/houdou/2004/11/d1/h1122-2b.pdf. |
| 53. | Breslow L, Enstrom JE. Persistence of health habits and their relationship to mortality. Prev Med. 1980;9:469-483. |
| 54. | Cammà C, Giunta M, Rosselli M, Cottone M. Mesalamine in the maintenance treatment of Crohn's disease: a meta-analysis adjusted for confounding variables. Gastroenterology. 1997;113:1465-1473. |
| 55. | Candy S, Wright J, Gerber M, Adams G, Gerig M, Goodman R. A controlled double blind study of azathioprine in the management of Crohn's disease. Gut. 1995;37:674-678. |
| 56. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. |
| 57. | Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, Liu G, Travers S, Heuschkel R, Markowitz J. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007;132:863-873; quiz 1165-1166. |
| 58. | Sandborn WJ, Löfberg R, Feagan BG, Hanauer SB, Campieri M, Greenberg GR. Budesonide for maintenance of remission in patients with Crohn's disease in medically induced remission: a predetermined pooled analysis of four randomized, double-blind, placebo-controlled trials. Am J Gastroenterol. 2005;100:1780-1787. |
| 59. | Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pesci M, Harrison J, Plevy SE. Infliximab prevents Crohn's disease recurrence after ileal resection. Gastroenterology. 2009;136:441-450.e1; quiz 716. |
| 60. | Van Assche G, Magdelaine-Beuzelin C, D'Haens G, Baert F, Noman M, Vermeire S, Ternant D, Watier H, Paintaud G, Rutgeerts P. Withdrawal of immunosuppression in Crohn's disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. 2008;134:1861-1868. |
| 61. | Boirivant M, Leoni M, Tariciotti D, Fais S, Squarcia O, Pallone F. The clinical significance of serum C reactive protein levels in Crohn's disease. Results of a prospective longitudinal study. J Clin Gastroenterol. 1988;10:401-405. |
| 62. | Jones VA, Dickinson RJ, Workman E, Wilson AJ, Freeman AH, Hunter JO. Crohn's disease: maintenance of remission by diet. Lancet. 1985;2:177-180. |
| 63. | Heaton KW, Thornton JR, Emmett PM. Treatment of Crohn's disease with an unrefined-carbohydrate, fibre-rich diet. Br Med J. 1979;2:764-766. |
| 64. | Belluzzi A, Brignola C, Campieri M, Pera A, Boschi S, Miglioli M. Effect of an enteric-coated fish-oil preparation on relapses in Crohn's disease. N Engl J Med. 1996;334:1557-1560. |
| 65. | Pearson M, Teahon K, Levi AJ, Bjarnason I. Food intolerance and Crohn's disease. Gut. 1993;34:783-787. |
| 66. | Ritchie JK, Wadsworth J, Lennard-Jones JE, Rogers E. Controlled multicentre therapeutic trial of an unrefined carbohydrate, fibre rich diet in Crohn's disease. Br Med J (Clin Res Ed). 1987;295:517-520. |
| 67. | Lorenz-Meyer H, Bauer P, Nicolay C, Schulz B, Purrmann J, Fleig WE, Scheurlen C, Koop I, Pudel V, Carr L. Omega-3 fatty acids and low carbohydrate diet for maintenance of remission in Crohn's disease. A randomized controlled multicenter trial. Study Group Members (German Crohn's Disease Study Group). Scand J Gastroenterol. 1996;31:778-785. |
| 68. | Rahimi R, Nikfar S, Rahimi F, Elahi B, Derakhshani S, Vafaie M, Abdollahi M. A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn's disease. Dig Dis Sci. 2008;53:2524-2531. |
| 69. | Willett WC. Convergence of philosophy and science: the third international congress on vegetarian nutrition. Am J Clin Nutr. 1999;70:434S-438S. |
| 70. | American Institute for Cancer Research. Diet and health recommendations for cancer prevention. Accessed October 1. Tokyo: Ishiyaku Publishers 2009; Available from: http://www.aicr.org. |