Published online Apr 14, 2010. doi: 10.3748/wjg.v16.i14.1720
Revised: December 27, 2009
Accepted: January 3, 2010
Published online: April 14, 2010
AIM: To analyze whether computer-enhanced dynamic analysis of elastography movies is able to better characterize and differentiate between different degrees of liver fibrosis.
METHODS: The study design was prospective. A total of 132 consecutive patients with chronic liver diseases and healthy volunteers were examined by transabdominal ultrasound elastography. All examinations were done by two doctors.
RESULTS: Due to the limitations of the method, we obtained high-quality elastography information in only 73.48% of the patients. The κ-means clustering method was applied to assess the inter-observer diagnosis variability, which showed good variability values in accordance with the experience of ultrasound examination of every observer. Cohen’s κ test indicated a moderate agreement between the study observers (κ = 0.4728). Furthermore, we compared the way the two observers clustered the patients, using the test for comparing two proportions (t value, two-sided test). There was no statistically significant difference between the two physicians, regardless of the patients’ real status.
CONCLUSION: Transabdominal real-time elastography is certainly a very useful method in depicting liver hardness, although it is incompletely tested in large multicenter studies.
- Citation: Gheonea DI, Săftoiu A, Ciurea T, Gorunescu F, Iordache S, Popescu GL, Belciug S, Gorunescu M, Săndulescu L. Real-time sono-elastography in the diagnosis of diffuse liver diseases. World J Gastroenterol 2010; 16(14): 1720-1726
- URL: https://www.wjgnet.com/1007-9327/full/v16/i14/1720.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i14.1720
Palpation continues to be of great value in modern medicine, both practiced by doctors and as a technique for self-examination. However, palpation is limited to a few accessible organs, and the interpretation of the information sensed by the fingers is highly subjective. Recently, elastography has emerged as an option in several commercial ultrasound systems, and is starting to prove clinically valuable in many areas, particularly for example in assisting breast cancer diagnosis[1,2], or in guiding minimally invasive treatment of prostate cancer[3,4]. The technique reveals the physical properties of the tissue by characterizing the difference in hardness between diseased and surrounding tissue[5,6]. The method measures mechanically induced deformation (strain) of structures in the B mode image to quantify the elasticity of the tissue. By measuring the tissue strain induced by compression, it is possible to estimate the tissue hardness. The region of interest for the elastography calculations is selected manually and should include the targeted area and the surrounding tissues[7].
Elasticity measurements have been reported to be useful for the diagnosis and differentiation of many tumors, which are usually harder than normal surrounding tissues[8-10]. Furthermore, different solid tumors situated near the gastrointestinal tract might be also visualized by endoscopic ultrasound (EUS) elastography and potentially characterized by this technique. EUS elastography has been used in several studies for the characterization and differentiation of benign and malignant lymph nodes, with variable sensitivity, specificity, and accuracy, with better results than those obtained by conventional EUS criteria[11-13]. Furthermore, the feasibility of EUS elastography has been tested in pancreatic diseases, with very good results[14,15].
Recently, transabdominal real-time elastography was proposed as a new method for noninvasive staging of liver fibrosis[16-18]. For many years, liver biopsy was the only method to evaluate liver fibrosis and it has traditionally been considered as the gold standard[19]. However, it is a painful invasive method associated with poor patient compliance, discomfort and, in very rare cases, with serious complications[16,20]. High inter-observer variability has been reported between two pathologists when analyzing the same biopsy sample[19-21]. Therefore, recent research has been focused on the evaluation of noninvasive methods for the assessment of liver fibrosis, both by biochemical tests as well as imaging methods[22], as an alternative to liver biopsy.
The aim of this study was to analyze whether computer-enhanced dynamic analysis of elastography movies is better able to characterize and differentiate between different degrees of liver fibrosis. We previously have reported that analysis of selected elastography images is operator-dependent, therefore, our approach was to use a dynamic analysis of several frames of elastography movies that might reduce possible selection bias or artifacts.
The study design was prospective. A total of 132 consecutive patients with chronic liver diseases and healthy volunteers were examined by transabdominal ultrasound elastography during an 18-mo period in the Research Center of Gastroenterology and Hepatology, University of Medicine and Pharmacy Craiova, Romania. Patients (73 men and 59 women) were 26-74 years old. As a result of the limitations of the method, only 97 patients were considered for further elastography analysis as follows: healthy volunteers (n = 27), chronic viral B and C hepatitis (n = 26), and liver cirrhosis (n = 29), fatty alcoholic liver disease (n = 21). Chronic viral hepatitis was proven by the presence of hepatitis C virus antibodies or hepatitis B surface antigen in serum and the persistence of liver inflammation or liver parameter alterations for > 6 mo. In all the patients with chronic viral hepatitis, liver biopsy was performed 1-3 d before real-time elastography. Liver cirrhosis was proven by clear demonstration of portal hypertension signs (including esophageal and/or gastric varices) in a clinical suggestive setting. Patients with ascites were excluded from the study. Fatty alcoholic liver disease was diagnosed by ultrasound aspects of the liver and excessive alcohol intake (> 30 g daily), in the absence of viral infection and any doubt of cirrhosis. The control group consisted of healthy adult volunteers [normal levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST); negative tests for blood viral markers] who did not have a history of relevant concomitant illness or cancer. None of the healthy volunteers had an excessive daily alcohol intake (< 10 g daily).
EUS elastography equipment includes a Hitachi EUB 8500 ultrasound system with an embedded elastography module (Hitachi Medical Systems Europe Holding AG, Zug, Switzerland) and a 6.5-MHz linear probe. The same conditions of brightness, contrast, intensity, and gain of the ultrasound system were used in all examinations. However, because the numeric elastography information is displayed using a rainbow color-coded scale, with values from 1 to 256, changes in the system settings did not affect the subsequent post-processing analysis.
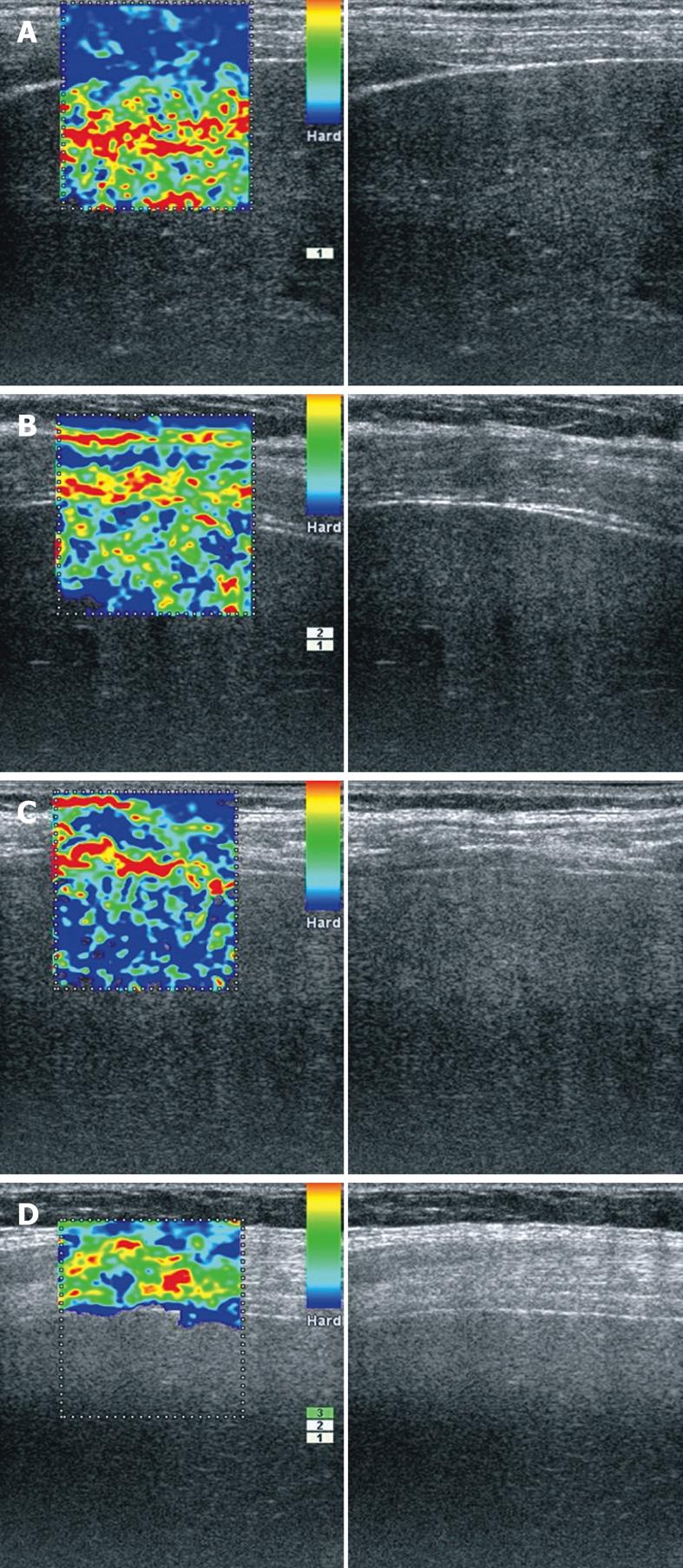
All examinations were done by two doctors with different degrees of experience in ultrasound, a proficient physician (SA) and a physician beginner in ultrasound technique (GDI), in a typical clinical setting with previous knowledge of the patient’s underlying disease. The ultrasound probe was placed in a convenient right intercostal space and elastography information was gathered during breath holding at the end-expiration phase. Three 10-s movies were recorded by each examiner for every patient, with a region of interest set to include the liver and surrounding tissues (Figure 1A-C). The movies were stored uncompressed at maximum quality for further accurate computer-enhanced analysis.
Liver biopsy was performed through the right intercostal space in the right liver lobe after transabdominal ultrasound found the most useful area. After betadine disinfection and local anesthesia, liver biopsy was performed at the previously marked site. Liver fibrosis stages were evaluated semi-quantitatively according to the Metavir scoring system[23-25]. Liver fibrosis was staged on an F0-F4 scale: F0-no fibrosis, F1-portal fibrosis without septa, F2-portal fibrosis with few septa, F3-numerous septa without cirrhosis, and F4-cirrhosis.
The elasticity of tissue was reconstructed within the region of interest and translated into a color signal that overlay the grey scale image. To visualize tissue elasticity patterns, different elasticity values were marked with different colors (on a scale of 1 to 256). The system was set-up to use a hue color map (red/green/blue), where hard tissue areas were marked with dark blue, medium hard tissue areas with cyan, intermediate tissue areas with green, medium soft tissue areas with yellow, and soft tissue areas with red. The complete spectrum from blue to red was applied to every elastography record and indicated the graduation of relative elasticity within the region of interest. The quality of tissue compression was indicated by a numeric scale from 1 to 7 within the image.
Each recorded elastography movie was subjected to a computer-enhanced dynamic analysis using a public domain Java-based image processing tool (Image J)[26] with a special plug-in developed by the IT Department of the University of Medicine and Pharmacy, Craiova. The plug-in was used to compute and dynamically analyze the individual hue histograms of each frame from an elastography movie; all programmers and statisticians being blinded to the clinical and pathological information to minimize the human bias. Shortly, every color frame was transformed into numerical form, and characterized by a single average hue histogram vector. Each individual value of the vector corresponded to the number of pixels of each color, in other words, to the number of pixels that corresponded to each elasticity level, from 1 to 256. The numeric values displayed by computer were offered to the statistical team and analyzed.
A prospective cross-sectional study was carried out on 97 samples of elastography records from chronic hepatitis patients and healthy volunteers recorded by two doctors. Using all clinical and laboratory test data, expert doctors (Săftoiu A and Gheonea DI) diagnosed the patients into four types (normal, liver steatosis, chronic hepatitis, and cirrhosis). First, an a priori power analysis determined the acceptability of the patient record sample size. Second, the κ-means clustering algorithm was applied to assess the inter-observer diagnosis variability. Basically, we used the κ-means algorithm to cluster automatically the 97 patients into four groups that corresponded to the four types of diseases; furthermore we compared these clusters with the final diagnosis. Finally, the analysis of variance, comparison of the proportions of well-diagnosed patients, Cohen’s κ statistics, the proportion of agreement between the two examiners, and Stuart-Maxwell’s statistics were performed to evaluate the appropriateness of each doctor’s diagnosis and the inter-observer reliability between expert doctors.
Furthermore, statistical analysis concerning the liver biopsy Metavir scores (F1, F2, F3) compared with the elasticity value (determined by computer-enhanced elastography movies assessment) was performed using Spearman’s correlation coefficient.
A total of 132 consecutive patients were examined by real-time elastography using the right intercostal space as an acoustic window for gathering elasticity information about the liver. Due to the limitations of the method concerning the low penetrability of elastography into the tissues (Figure 1D), we obtained high-quality elastography information in only 97 patients (73.48%). The two examiners categorized the study patients into four groups (cirrhosis, chronic hepatitis, alcoholic steatosis, healthy volunteers) in accordance with the determined clinical status. Furthermore, the results of computer-enhanced analysis of the elastography movies were compared statistically.
First, we performed a power analysis to determine the acceptability of the patient record sample size, because one of the main goals of this study was to investigate the appropriateness of each observer examination and the inter-observer reliability between expert doctors. Accordingly, a sample of 97 records were considered, which provided a statistical power of 95% (type I error rate α = 0.10).
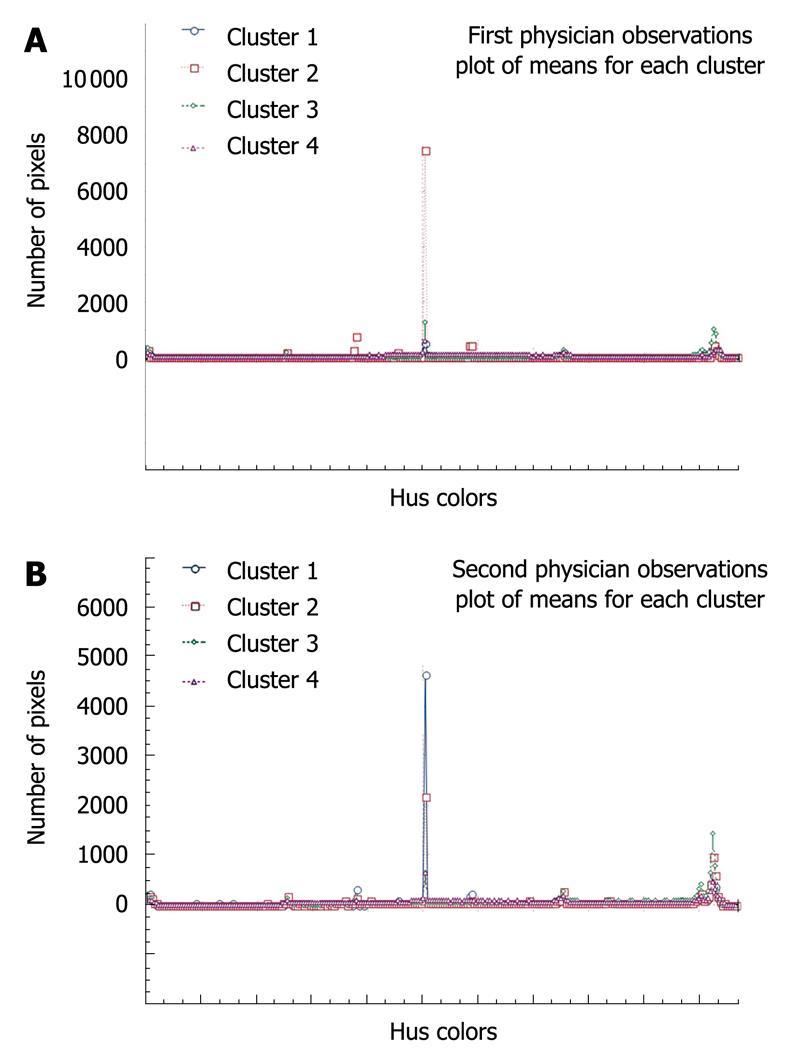
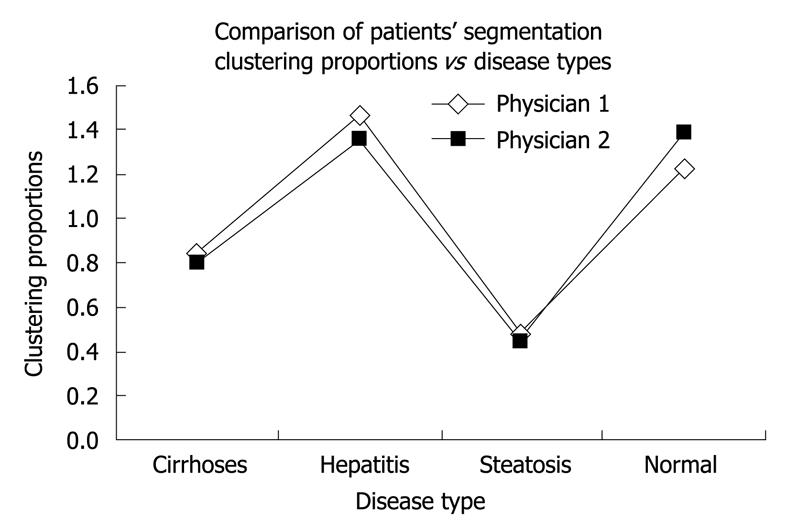
Second, the κ-means clustering method was applied to assess the inter-observer diagnosis variability. It aimed to distinguish between different groups of patients and can therefore be used to enhance knowledge of diseases and make automatic diagnosis predictions. Technically, based on each observer film computer analysis, we have used the κ-means algorithm to cluster automatically the 97 patients into four groups corresponding to the four types of status. Next, we compared the results of the two segmentations of patients, which corresponded to each doctor’s diagnosis, to investigate the possible differences between them. To evaluate the appropriateness of each examination, we used the well-known analysis of variance, which compared the within-cluster variability (small if the classification was good) to the between-cluster variability (large if the classification was good). Thus, we obtained a very good classification for Săftoiu A (P = 0.014) and a poorer classification for Gheonea DI (P = 0.15), which meant that the first decision was better than the second one (more homogeneous clusters and more different from each other). This implied that the first observer was much more experienced than the second in performing elastography. We also considered the two graphs of the means across clusters, which corresponded to each examiner (useful for visually summarizing the differences in means between clusters). As we saw from these two graphs (Figure 2A and B), there was no significant visual difference between the two doctors’ ways of clustering the patients, even if the previous analysis of variance showed a difference between them. Moreover, we considered the patients’ segmentation across the four types, which showed a similar way of clustering the patients, irrespective of examiner (Figure 3). Besides the above approaches used to assess the inter-observer variability, we applied the test for comparing two proportions (t value, two-sided test). We found that there was no statistically significant difference between the two physicians’ computer-enhanced movie analysis, regardless of disease type: cirrhosis (P = 0.54), chronic hepatitis (P = 0.85), steatosis (P = 0.81), and healthy subjects (P = 0.78).
Another way of analyzing the agreement between the two observers was application of Cohen’s κ test as a measure of association between the two measurements (categorical variables), which consisted of the real-time elastography examinations performed by the two doctors. We considered for each examiner only one examination per patient, which gave a sample of 97 records. Each examiner classified each patient into one of the following categories: steatosis, normal, hepatitis, cirrhosis, thus, the 97 records referred to the above four categories. The four categories were nominal, therefore, Cohen’s simple unweighted coefficient κ was the only form that could meaningfully be used (Table 1). According to Landis and Koch[27], κ = 0.4728 indicated a moderate agreement between the two doctors. Thus, we conclude that, of all the decisions that we would have expected to be non-concordant if nothing more than coincidence were operating, 47.28% of the decisions were in fact concordant.
| Observedκ | Standard error | 95% CI | |
| Lower limit | Upper limit | ||
| 0.4728 | 0.0762 | 0.3235 | 0.6221 |
Independently of the Cohen’s κ value, it is also possible to measure the proportion of agreement between the two observers[28] within each of the four categories separately (confidence intervals for proportions are calculated according to the Wilson efficient-score method, corrected for continuity). Table 2 shows that the greatest agreement concerned normal patients (84.85%), while the smallest was for patients with cirrhosis (33.33%). In addition, we used Stuart-Maxwell’s test for the four categories as a measure of the overall disagreement between the two doctors. The null hypothesis H0 was that the distribution of diagnosis type among the four categories was the same for the two observers. Using the corresponding χ2 statistics with 4-1 (3) degrees of freedom, the corresponding significance level (P = 0.001) showed that we could reject hypothesis H0, that is, there seemed to be a significant overall disagreement between the two doctors.
| Proportions of agreement | 95% CI | ||||
| Decision | Maximum possible | Chanceexpected | Observed | Lower limit | Upper limit |
| Normal | 0.8485 | 0.2169 | 0.5250 | 0.3634 | 0.6818 |
| Steatosis | 0.5789 | 0.1961 | 0.3953 | 0.2537 | 0.5555 |
| Hepatitis | 0.5517 | 0.1381 | 0.5000 | 0.3168 | 0.6832 |
| Cirrhosis | 0.3333 | 0.0089 | 0.3333 | 0.0177 | 0.8747 |
| Composite | 0.7882 | 0.3084 | 0.6353 | 0.5232 | 0.7350 |
Although all the correlation methods applied for determining the correspondence between real status of the examined patients (normal, liver steatosis, chronic hepatitis, cirrhosis) and elasticity assessed by real-time elastography were positive, we could not establish a good correlation between the Metavir score (chronic hepatitis patients) and the results of elastography movies analysis (results not shown).
Recently, elastography has been presented as a new ultrasound-associated technology for the assessment of tissue elasticity. Computer-enhanced dynamic analysis of liver elastography movies was the objective of our current study. Furthermore, we studied the inter-observer variability and the correspondence between elastography and clinical (final) diagnosis. This approach would also eliminate the selection bias induced by analysis of static images[7], because it takes into account the information contained in several frames of a liver elastography movie.
The first goal of our statistical analysis was to see if percutaneous elastography of the liver was able to diagnose correctly the real status of the patients. We analyzed three independent cine-loop elastography examinations recorded by two separate examiners who were blinded to each other. For each patient, we thus recorded six real-time cine-loop elastography examinations, which were further analyzed through hue histogram analysis, with averaged values for a 10-s cine loop. Although the aspects of the images suggested the correct diagnosis (Figure 1A-C), we automatically analyzed every frame of the movies recorded by the two investigators. To evaluate the appropriateness of each examination, the κ-means clustering method was performed, which showed agreement between the clinical diagnosis and the results of computer analysis of the recorded movies. The results were very good, especially for the first investigator (Săftoiu A), thus proving that experience in performing ultrasound is important in obtaining quality and accurate recordings of the study patients.
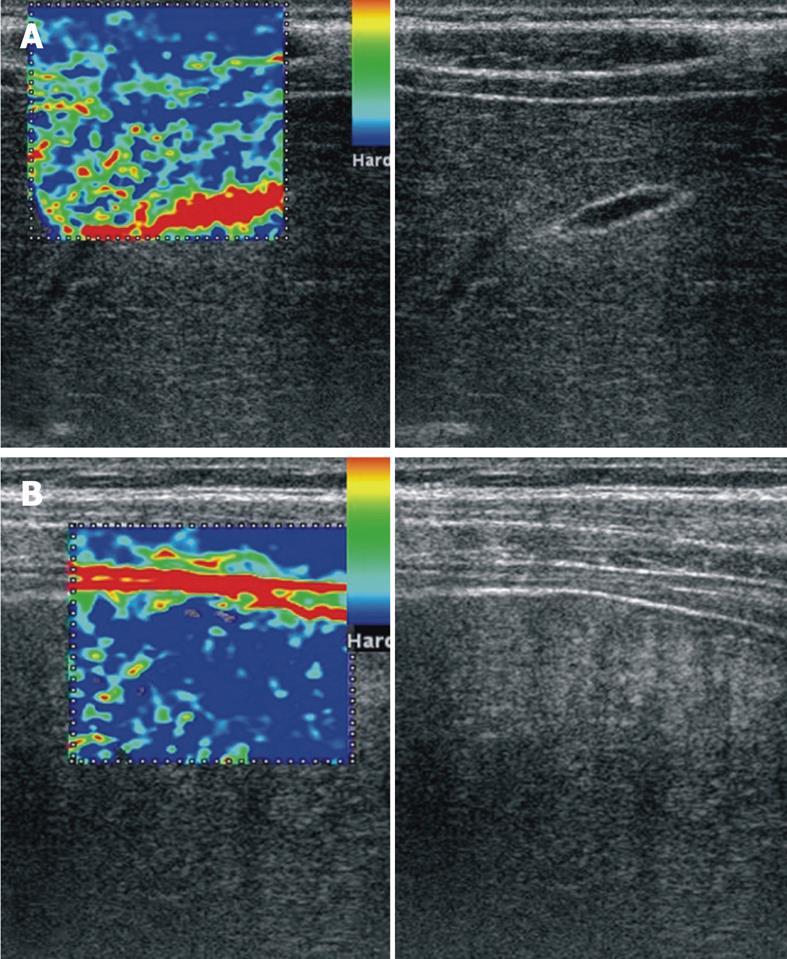
As we previously suggested[7], the region of interest for all the movies was set to include also the surrounding liver tissue (fatty tissue, intercostals muscles, diaphragm, peritoneum), which were considered to have the same elasticity in all patients despite their subsequent disease. We do not believe that inclusion of hepatic vein branches[29] would be very useful as a reference structure, because the presence of vessels induces clear artifacts in the elastography images (Figure 4A), similar to the presence of ascites (Figure 4B). This is easily understandable if we look at the colors displayed by the real-time elastography software in the region of interest. In the presence of a very highly elastic structure such as the hepatic vein or ascites, the rest of the liver would be depicted as hard, irrespective of its elasticity. However, the development of ascites is a strong indicator for the presence of cirrhosis, which makes noninvasive staging of fibrosis unnecessary.
Liver biopsy is an important diagnostic tool and helps therapeutic decision making in chronic liver diseases patients[30]. Histopathological examination was considered the most appropriate method in chronic hepatitis for assessing changes after antiviral therapy[31], and is considered mandatory for grading and staging in most patients. The question is whether liver biopsy can be regarded as the gold standard for the staging and grading[32] of diffuse liver diseases when risks of biopsy, inadequate sampling, and intra-observer and inter-observer error are taken into account[30]. Our elastography statistical results showed a very good inter-observer variability analyzed by all three methods presented above. Therefore, computer-aided diagnosis of elastography calculations can be a very useful and reproducible method in depicting the hardness of the liver.
Even so, we were not able to distinguish between intermediate degrees of liver fibrosis (F1, F2, F3) in the chronic hepatitis patients subgroup in which we performed liver biopsy. To the best of our knowledge, there is only one published study[16] that has succeeded partially in correlating the degree of fibrosis with real-time elastography calculation, especially in patients with F ≥ 2. Unfortunately, the obtained values were calculated as means of static images selected by the examiner, which could have had a significant influence on the results. Furthermore, it is not clear if the region of interest was set to include also the surrounding tissues of the liver as a reference area.
One important limitation of our approach was the examination of the liver with a linear transducer of 6.5 MHz, which might be too high to examine the right liver lobe correctly and consistently. We did not select only patients with a normal body mass index, therefore, in only 73.48% of the examinations did we obtain constant and high quality elastography information from the area of interest. The penetration of real-time elastography is limited to 3-4 cm, therefore, it is difficult to record useful elastography information inside the liver if the thoracic wall is thicker than 2-3 cm. A better option might be represented by the use of a lower frequency linear transducer, or another means of performing elastography of the liver, for example, by using EUS with the transducer placed in the stomach near the left liver lobe. Development of a pressure gauge is certainly necessary because manual application of pressure cannot be standardized. Usually, a small deformation (< 2%) of the tissues is needed, and this is very difficult to obtain, even by experienced doctors.
In conclusion, transabdominal real-time elastography is certainly a very useful method in depicting liver hardness, although it has been tested incompletely in large multicenter studies and should be compared with other noninvasive methods (blood markers, transient elastography). We also suggest an improvement of the examination methodology, which should take into account previous observations made by different authors (better transducers, improved elastography software) to establish real-time elastography as a new revolutionary method that can replace liver biopsy for assessment of different stages of fibrosis in patients with chronic hepatitis.
Chronic liver diseases are marked by the gradual destruction of liver tissue over time, which eventually causes liver cirrhosis. Cirrhosis is the seventh leading cause of death in the United States, according to the National Institute of Diabetes and Digestive and Kidney Diseases. Liver biopsy is still the gold standard in many centers for quantifying liver fibrosis.
Recently, research has focused on the evaluation of noninvasive methods for the assessment of liver fibrosis: routine hematological and biochemical tests, surrogate fibrosis markers in the blood and their algorithms, glycomics, proteomics, transient elastography, and real-time elastography.
Elasticity measurements have been reported to be useful for the diagnosis and differentiation of different diseases. Recently, transabdominal real-time elastography was proposed as a new method for noninvasive staging of liver fibrosis. The presents study clearly demonstrated that computer-enhanced dynamic analysis of elastography movies is better able to characterize and differentiate between different degrees of liver fibrosis.
Transabdominal real-time elastography is certainly a very useful method in depicting liver hardness and allows the replacement of other invasive methods, such as liver biopsy, which are associated with patient discomfort and mortality in some cases.
Transabdominal real-time elastography is an imaging technique, completely noninvasive, that visualizes the tissue strain during compression that characterizes the difference in hardness between diseased and normal tissues. Tissue compression produces strain within the tissue. The strain is smaller in harder compared with softer structures.
In the present study, the authors aimed to analyze whether computer-enhanced dynamic analysis of elastography movies is better able to characterize and differentiate between different degrees of liver fibrosis. The topic is interesting, and novel.
Peer reviewer: Livia Biancone, Professor, Cattedra di Gastroenterologia, Dipartimento di Medicina Interna, Università di “Tor Vergata”, Via Montpellier, 1 Rome 00131, Italy
S- Editor Wang YR L- Editor Kerr C E- Editor Ma WH
| 1. | Garra BS, Cespedes EI, Ophir J, Spratt SR, Zuurbier RA, Magnant CM, Pennanen MF. Elastography of breast lesions: initial clinical results. Radiology. 1997;202:79-86. |
| 2. | Hiltawsky KM, Krüger M, Starke C, Heuser L, Ermert H, Jensen A. Freehand ultrasound elastography of breast lesions: clinical results. Ultrasound Med Biol. 2001;27:1461-1469. |
| 3. | Cochlin DL, Ganatra RH, Griffiths DF. Elastography in the detection of prostatic cancer. Clin Radiol. 2002;57:1014-1020. |
| 4. | König K, Scheipers U, Pesavento A, Lorenz A, Ermert H, Senge T. Initial experiences with real-time elastography guided biopsies of the prostate. J Urol. 2005;174:115-117. |
| 5. | Frey H. [Realtime elastography. A new ultrasound procedure for the reconstruction of tissue elasticity]. Radiologe. 2003;43:850-855. |
| 6. | Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, Yamakawa M, Matsumura T. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341-350. |
| 7. | Sãftoiu A, Gheonea DI, Ciurea T. Hue histogram analysis of real-time elastography images for noninvasive assessment of liver fibrosis. AJR Am J Roentgenol. 2007;189:W232-W233. |
| 8. | Skovorda AR, Klishko AN, Gusakian DA, Maevskiĭ EI, Ermilova VD, Oranskaia GA, Sarvazian AP. [Quantitative analysis of mechanical characteristics of pathologically altered soft biological tissues]. Biofizika. 1995;40:1335-1340. |
| 9. | Hong Y, Liu X, Li Z, Zhang X, Chen M, Luo Z. Real-time ultrasound elastography in the differential diagnosis of benign and malignant thyroid nodules. J Ultrasound Med. 2009;28:861-867. |
| 10. | Miyagawa T, Tsutsumi M, Matsumura T, Kawazoe N, Ishikawa S, Shimokama T, Miyanaga N, Akaza H. Real-time elastography for the diagnosis of prostate cancer: evaluation of elastographic moving images. Jpn J Clin Oncol. 2009;39:394-398. |
| 11. | Săftoiu A, Vilmann P, Ciurea T, Popescu GL, Iordache A, Hassan H, Gorunescu F, Iordache S. Dynamic analysis of EUS used for the differentiation of benign and malignant lymph nodes. Gastrointest Endosc. 2007;66:291-300. |
| 12. | Iglesias García JJ, Lariño Noia J, Alvarez Castro A, Cigarrán B, Domínguez Muñoz JE. Second-generation endoscopic ultrasound elastography in the differential diagnosis of solid pancreatic masses. Pancreatic cancer vs. inflammatory mass in chronic pancreatitis. Rev Esp Enferm Dig. 2009;101:723-730. |
| 13. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. |
| 14. | Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971-978. |
| 15. | Săftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, Popescu GL, Iordache A, Hassan H, Iordache S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086-1094. |
| 16. | Friedrich-Rust M, Ong MF, Herrmann E, Dries V, Samaras P, Zeuzem S, Sarrazin C. Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR Am J Roentgenol. 2007;188:758-764. |
| 17. | Friedrich-Rust M, Schwarz A, Ong M, Dries V, Schirmacher P, Herrmann E, Samaras P, Bojunga J, Bohle RM, Zeuzem S. Real-time tissue elastography versus FibroScan for noninvasive assessment of liver fibrosis in chronic liver disease. Ultraschall Med. 2009;30:478-484. |
| 18. | Kanamoto M, Shimada M, Ikegami T, Uchiyama H, Imura S, Morine Y, Kanemura H, Arakawa Y, Nii A. Real time elastography for noninvasive diagnosis of liver fibrosis. J Hepatobiliary Pancreat Surg. 2009;16:463-467. |
| 19. | Lupşor M, Badea R, Stefănescu H, Grigorescu M, Sparchez Z, Serban A, Branda H, Iancu S, Maniu A. Analysis of histopathological changes that influence liver stiffness in chronic hepatitis C. Results from a cohort of 324 patients. J Gastrointestin Liver Dis. 2008;17:155-163. |
| 20. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. |
| 21. | Bedossa P, Poynard T, Naveau S, Martin ED, Agostini H, Chaput JC. Observer variation in assessment of liver biopsies of alcoholic patients. Alcohol Clin Exp Res. 1988;12:173-178. |
| 22. | Iglesias García J, Lariño Noia J, Souto R, Alvarez Castro A, Cigarrán B, Domínguez Muñoz JE. Endoscopic ultrasound (EUS) elastography of the liver. Rev Esp Enferm Dig. 2009;101:717-719. |
| 23. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. |
| 24. | Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147-1171. |
| 25. | Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology. 2002;36:S152-S160. |
| 26. | Girish V, Vijayalakshmi A. Affordable image analysis using NIH Image/ImageJ. Indian J Cancer. 2004;41:47. |
| 27. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. |
| 28. | Maxwell AE. Comparing the classification of subjects by two independent judges. Br J Psychiatry. 1970;116:651-655. |
| 29. | Ferraioli G, Gulizia R, Filice C. Real-time elastography in the assessment of liver fibrosis. AJR Am J Roentgenol. 2007;189:W170. |
| 30. | Cholongitas E, Senzolo M, Standish R, Marelli L, Quaglia A, Patch D, Dhillon AP, Burroughs AK. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol. 2006;125:710-721. |
| 31. | Lee SS. Review article: indicators and predictors of response to anti-viral therapy in chronic hepatitis C. Aliment Pharmacol Ther. 2003;17:611-621. |
| 32. | Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160-1174. |