Published online Mar 7, 2009. doi: 10.3748/wjg.15.1134
Revised: October 10, 2008
Accepted: October 17, 2008
Published online: March 7, 2009
Multivisceral surgical resection for cure was successfully performed in a 70-year-old man suffering from a primary hepatocellular carcinoma (HCC) associated with direct invasion to the stomach and pancreas. The patient presented with gastric outlet obstruction, upper abdominal pain and a history of chronic liver disease due to hepatitis B virus (HBV) infection. Upper gastrointestinal (GI) endoscopy revealed an infiltrating tumor protruding through the gastric wall and obliterating the lumen. Computer tomograghy (CT) and magnetic resonance imaging (MRI) scan demonstrated a 15-cm tumor in the left lateral segment of the liver with invasion to the stomach and pancreas. Alpha-foetoprotein (AFP) levels and liver function tests were normal. The patient underwent an en bloc left hepatectomy, total gastrectomy, distal pancreatectomy with splenectomy and radical lymphadenectomy. Pathology revealed a poorly differentiated, giant cell HCC involving the stomach and pancreas. Disease-free margins of resection were achieved. The patient’s postoperative course was uneventful. Sixteen months after surgery, he has no recurrence or distal metastasis. Direct invasion of HCC into the GI tract is rarely encountered. Complete surgical resection should be considered in selected patients with an appropriate hepatic functional reserve.
-
Citation: Korkolis DP, Aggeli C, Plataniotis GD, Gontikakis E, Zerbinis H, Papantoniou N, Xinopoulos D, Apostolikas N, Vassilopoulos PP. Successful
en bloc resection of primary hepatocellular carcinoma directly invading the stomach and pancreas. World J Gastroenterol 2009; 15(9): 1134-1137 - URL: https://www.wjgnet.com/1007-9327/full/v15/i9/1134.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1134
Hepatocellular carcinoma (HCC) is characterized by a soft consistency and extensive growth. These specific features of HCC mean that it rarely infiltrates the gastrointestinal (GI) tract directly. The incidence is reported to be 0.5% to 2% of clinical HCC cases[12]. Whether such an invasion causes massive hemorrhage or obstruction, a complete en bloc resection of these extensive HCCs can be safely performed using modern surgical techniques and sophisticated perioperative management. In this report, we describe a patient suffering from a giant, extrahepatically growing HCC associated with direct invasion to the stomach and pancreas. The tumor was successfully extirpated with a multivisceral oncologic resection. A thorough review of the literature is also presented.
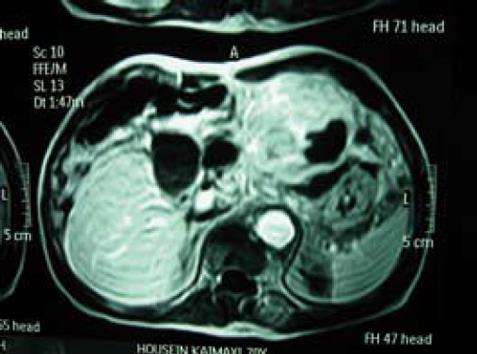
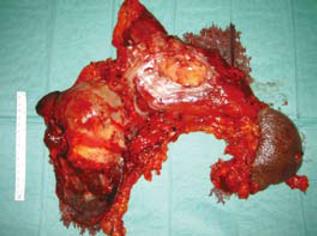
A 70-year-old Caucasian male presented with signs of gastric outlet obstruction, upper abdominal pain and a chronic hepatitis B virus (HBV) infection. Upper GI endoscopy revealed a large, infiltrating tumor protruding through the anterior wall of the body of the stomach and almost completely obliterating the gastric lumen. No esophageal varices were found. Total colonoscopy showed no abnormality. The computed tomography (CT) scan demonstrated the presence of chronic liver disease and a giant tumor of the left lobe. The magnetic resonance imaging (MRI) revealed a space-occupying lesion, 15 cm × 12 cm × 9.5 cm in size, originating from the inferior surface of segments II and III. It was mainly solid with areas of tissue necrosis, hemorrhage and cystic degeneration. A smooth fibrous capsule was covering part of its outer surface. The lesion showed extensive extrahepatic growth with invasion to the body and fundus of the stomach, as well as direct contact with the upper surface of the pancreas. A low grade HCC was suggested (Figure 1). Biochemical analysis on admission indicated that alpha-foetoprotein (AFP) level was 2.1 ng/mL (normal value, < 10 ng/mL) and CA19.9 level was 33.2 ng/mL (normal value < 34 ng/mL). Liver function test results were normal. The patient underwent an en bloc radiofrequency-assisted left hepatectomy using the RF Cooltip needle (Radionics, Valleylab, MA, USA) and vascular staplers (EndoGIA, Covidien Healthcare, USA), total gastrectomy, distal pancreatectomy with splenectomy, radical hepaticoduodenal, perigastric and celiac trunk lymphadenectomy, as well as cholecystectomy (Figure 2). Total operative time was 160 min and estimated blood loss was less than 200 mL. GI continuity was restored with a Roux-en-Y end-to-side esophagojejunal reconstruction.
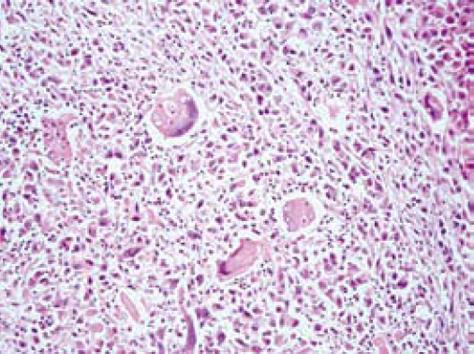
Pathology confirmed the presence of a poorly differentiated giant cell HCC developed in a liver cirrhosis. It was characterized by multinucleated giant cells and extensive areas of tissue necrosis (Figure 3). The tumor was invading the resected stomach and capsule of the body of pancreas and it was metastatic to 7 out of 54 resected lymph nodes. Disease-free margins of resection were achieved.
The patient had an uneventful course and was discharged on the 9th postoperative day. No adjuvant treatment was given but close follow-up was suggested. Sixteen months after surgery, he is doing well with no evidence of locoregional recurrence or distant metastasis.
Involvement of the GI tract by HCC is uncommon. In clinical HCC cases, the prevalence is 0.5% to 2% and in postmortem examination is discovered in about 10% of patients with HCC[2–4]. Chen et al[2], who found GI tract invasion by HCC in 8 of 396 patients (2%), were the first to report GI tract involvement by HCC during the course of the disease. The mode of metastasis was presumed to be hematogenous spread in 2 patients (to the stomach in one and the jejunum in the other) and direct invasion in 5 patients (invasion to the stomach in one and of the duodenum in four), but was undetermined in the remaining patient, in whom the stomach was involved.
In the English language literature, 30 patients with direct GI tract invasion from HCC, including the present one, have been found in 12 case reports and 4 retrospective studies[5–10] (Table 1).
| Characteristics | Data |
| Sex | |
| Male | 29 |
| Female | 1 |
| Age (yr) | |
| Mean | 65.0 (32-73) |
| Clinical manifestations | |
| Melena | 18 |
| Hematemesis | 6 |
| Epigastric pain | 4 |
| Nausea/vomiting | 4 |
| Abdominal mass | 1 |
| Positive FOBT | 6 |
| Organs involved | |
| Duodenum | 12 |
| Stomach | 9 |
| Colon | 7 |
| Duodenum and colon | 1 |
| Stomach and pancreas | 1 |
| Size of tumor (cm) | |
| Mean | 13.7 |
| Range | 4-22 |
| Esophageal varices | |
| Present | 6 |
| Absent | 15 |
| Unknown | 9 |
| Etiology | |
| HBV | 9 |
| HCV | 6 |
| Alcoholic | 6 |
| Unknown | 9 |
| Treatment | |
| Surgical therapy | |
| Curative surgery | 8 |
| Palliative surgery | 1 |
| Nonsurgical therapy | 11 |
| Supportive therapy | 10 |
The most frequent initial symptom was melena, which was documented in 18 patients[1112]. Hematemesis was recognized in only 6 of 23 patients in whom tumors involved the upper GI tract. The history of hematemesis points out the site of tissue invasion and the amount of bleeding from the tumor. In those cases, massive varicose hemorrhage from portal hypertension should be considered as an alternative source of hematemesis. Endoscopic studies are essential to determine the source of bleeding. In 19 of the 29 patients, in whom HCC involved directly the upper GI tract; initial endoscopic assessment revealed an ulcerative hemorrhagic tumor protruding into the lumen of the stomach or duodenum.
The most common site of invasion was the duodenum, followed by the stomach and colon[1314]. This is the first reported case of a direct invasion of both the stomach and pancreas from extrahepatically growing HCC that causes upper GI tract obstruction rather than hemorrhage.
The presumed mode of direct involvement to the GI tract is initiated by the adhesion of the serosal side of the adjacent organ with a bulky, exophytic tumor[26]. Some authors[68] have noted that in several patients with direct HCC invasion of the GI tract, the patient had received some form of regional therapy [transarterial chemoembolization (TACE), intra-arterial chemotherapy (IA), either alone or in combination] because of unresectability and/or had experienced abdominal surgery. Accordingly, it was postulated that possible mechanisms underlying the direct GI tract involvement by HCC, other than as part of its natural course, may be TACE or IA chemotherapy-induced tumor necrosis, resulting in the promotion of subcapsular tumor adhesion to the GI serosa. Postoperative intraabdominal adhesions and scarring may account for the proximity of the GI tract to the tumor. Although no history of abdominal surgery or regional treatment was encountered, extensive necrosis found in the resected tumor specimen might partly explain its invading behavior in the presented case.
The median survival of patients who received curative surgery, nonsurgical treatment, and supportive therapy were 9.7, 3.0, and 1.2 mo, respectively[1015]. The patients who had undergone oncologic surgery for cure survived for significantly longer compared to those receiving nonsurgical or supportive treatment, as strongly supported by the long disease-free survival of our patient. In light of the difficulty achieving relief of either bleeding or obstruction, surgical removal of such a tumor, together with involved structures, should be strongly considered.
Direct invasion of extrahepatically growing HCC to the GI tract is an unusual finding. Complete en bloc surgical resection of the tumor with negative margins may be the treatment of choice in order to control symptoms and to obtain oncologic cure in selected patients with an appropriate hepatic functional reserve.
| 1. | Yeo W, Sung JY, Ward SC, Chung SC, Lee WY, Li AK, Johnson PJ. A prospective study of upper gastrointestinal hemorrhage in patients with hepatocellular carcinoma. Dig Dis Sci. 1995;40:2516-2521. [Cited in This Article: ] |
| 2. | Chen LT, Chen CY, Jan CM, Wang WM, Lan TS, Hsieh MY, Liu GC. Gastrointestinal tract involvement in hepatocellular carcinoma: clinical, radiological and endoscopic studies. Endoscopy. 1990;22:118-123. [Cited in This Article: ] |
| 3. | Tung WY, Chau GY, Loong CC, Wu JC, Tsay SH, King KL, Huang SM, Chiu JH, Wu CW, Lui WY. Surgical resection of primary hepatocellular carcinoma extending to adjacent organ(s). Eur J Surg Oncol. 1996;22:516-520. [Cited in This Article: ] |
| 4. | Lin CP, Cheng JS, Lai KH, Lo GH, Hsu PI, Chan HH, Hsu JH, Wang YY, Pan HB, Tseng HH. Gastrointestinal metastasis in hepatocellular carcinoma: radiological and endoscopic studies of 11 cases. J Gastroenterol Hepatol. 2000;15:536-541. [Cited in This Article: ] |
| 5. | Cho A, Ryu M, Ochiai T. Successful resection, using pancreas-sparing duodenectomy, of extrahepatically growing hepatocellular carcinoma associated with direct duodenal invasion. J Hepatobiliary Pancreat Surg. 2002;9:393-396. [Cited in This Article: ] |
| 6. | Hashimoto M, Watanabe G, Matsuda M, Yamamoto T, Tsutsumi K, Tsurumaru M. Case report: gastrointestinal bleeding from a hepatocellular carcinoma invading the transverse colon. J Gastroenterol Hepatol. 1996;11:765-767. [Cited in This Article: ] |
| 7. | Nicoll AJ, Ireton HJ, Crotty B. Gastrointestinal bleeding from hepatocellular carcinoma invading the stomach. J Gastroenterol Hepatol. 1994;9:533-535. [Cited in This Article: ] |
| 8. | Maruyama A, Murabayashi K, Hayashi M, Nakano H, Isaji S, Uehara S, Kusuda T, Miyahara S, Kondo A, Yabana T. Hepatocellular carcinoma complicated by gastrointestinal hemorrhage caused by direct tumor invasion of stomach. J Hepatobiliary Pancreat Surg. 1999;6:90-93. [Cited in This Article: ] |
| 9. | Hatano E, Ikai I, Shimizu M, Maetani Y, Konda Y, Chiba T, Terajima H, Yamamoto N, Yamamoto Y, Shimahara Y. Resection for hepatocellular carcinoma with duodenal invasion: report of a case. Hepatogastroenterology. 2003;50:1034-1036. [Cited in This Article: ] |
| 10. | Fujii K, Nagino M, Kamiya J, Uesaka K, Sano T, Yuasa N, Oda K, Nimura Y. Complete resection of hepatocellular carcinoma with direct invasion to the stomach remnant. J Hepatobiliary Pancreat Surg. 2004;11:441-444. [Cited in This Article: ] |
| 11. | Srivastava DN, Gandhi D, Julka PK, Tandon RK. Gastrointestinal hemorrhage in hepatocellular carcinoma: management with transheptic arterioembolization. Abdom Imaging. 2000;25:380-384. [Cited in This Article: ] |
| 12. | Okusaka T, Okada S, Ishii H, Nagahama H, Yoshimori M, Yamasaki S, Takayasu K, Kakizoe T, Ochiai A, Shimoda T. Hepatocellular carcinoma with gastrointestinal hemorrhage caused by direct tumor invasion to the duodenum. Jpn J Clin Oncol. 1997;27:343-345. [Cited in This Article: ] |
| 13. | Tanaka A, Takeda R, Yamamoto H, Utsunomiya H, Okamura R, Kataoka M, Mukaihara S, Yamaoka Y. Extrahepatic large hepatocellular carcinoma with peritoneal dissemination: multimodal treatment, including four surgical operations. J Hepatobiliary Pancreat Surg. 2000;7:339-344. [Cited in This Article: ] |
| 14. | Humbert P, Sarmiento J, Boix J, Planas R, Quintero E, Franquet T, Villagrasa M. Hepatocellular carcinoma presenting with bleeding due to duodenal perforation by the tumor. Endoscopy. 1987;19:37-38. [Cited in This Article: ] |
| 15. | Chen CY, Lu CL, Pan CC, Chiang JH, Chang FY, Lee SD. Lower gastrointestinal bleeding from a hepatocellular carcinoma invading the colon. J Clin Gastroenterol. 1997;25:373-375. [Cited in This Article: ] |