Published online Feb 7, 2009. doi: 10.3748/wjg.15.570
Revised: December 25, 2008
Accepted: December 31, 2008
Published online: February 7, 2009
AIM: To determine the inhibitory effect of Astragalus memebranaceushas on gastric cancer cell supernatant-induced apoptosis of human peritoneal mesothelial cells.
METHODS: Human peritoneal mesothelial cell (HPMC) line HMrSV5 was co-incubated with gastric cancer cell supernatant (MKN45) and/or Astragalus memebranaceushas. Morphological changes in gastric cancer cells were observed under phase-contrast microscope. Quantitative cell damage was determined by MTT assay. Apoptosis was determined under transmission electron microscope and quantified by detecting acridine orange/ethidium bromide-stained (AO/EB) condensed nuclei under fluorescent microscope or by flow cytometry. Expressions of Bcl-2 and Bax were evaluated with immunostaining.
RESULTS: Morphological changes and exfoliation occurred and naked areas appeared in cultured HMrSV5 cells 24 h after they were treated with gastric cancer cell supernatant. Cell supernatant from MKN45 gastric cancer cells induced apoptosis of HMrSV5 cells in a time-dependent manner. Obvious morphological changes were observed in cell apoptosis, such as condensation of chromatin, nuclear fragmentations and apoptotic bodies. Astragalus memebranaceus could partly suppress these changes and regulate the expressions of Bcl-2 and Bax in HMrSV5 cells.
CONCLUSION: Gastric cancer cells induce apoptosis of HPMCs through the supernatant. Astragalus memebranaceushas inhibits this phenomenon and can be used an adjuvant chemothera-peutic agent in gastric cancer therapy.
- Citation: Na D, Liu FN, Miao ZF, Du ZM, Xu HM. Astragalus extract inhibits destruction of gastric cancer cells to mesothelial cells by anti-apoptosis. World J Gastroenterol 2009; 15(5): 570-577
- URL: https://www.wjgnet.com/1007-9327/full/v15/i5/570.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.570
Peritoneal dissemination is one of the most important factors for human gastric cancer and stands in the way of its successful surgical treatment[1]. It appears in the terminal stage and significantly worsens the prognosis of gastric carcinoma. However, the mechanisms underlying this propensity for metastasis are not yet clearly understood.
Peritoneal carcinomatosis can be considered a series of events that form a peritoneal metastatic cascade. Peritoneal stromal tissue appears to be a friendly host for tumor proliferation, providing a rich source of growth factors and chemokines involved in tumor metastasis. At present, our understanding of the molecular mediators that orchestrate this cascade is poor[2]. It has been reported that mesothelial cells prevent cancer invasion and undergo morphologoc changes in response to the factors released by cancer cells[3]. Before invading tumor cells and gaining firm adherence to the submesothelial monolayer, mesothelial cells must penetrate the mesothelial monolayer. Buck et al[4] have illustrated the protective effect of mesothelium in a rat model. When Walker 256 tumor cells are injected into the peritoneal cavity of rats, in which the parietal peritoneum has been stripped of its mesothelial lining leaving the basement membrane intact, rapid implantation occurs in denuded areas[4]. However, in un-operated rats with an intact mesothelium, peritoneal implantation is a rare event. Findings suggest that, prior to gastric cancer cell adhesion to the peritoneum, mesothelial cells become hemispherical, exfoliation occurs, and the naked areas of submesothelial connective tissue are exposed to the peritoneal cavity[56]. Invasion of the mesothelial monolayer appears to occur by tumor-induced mesothelial apoptosis[78]. In this study, we examine the effects of factors released by gastric cancer cells on peritoneal mesothelial cell apoptosis in vitro.
Astragalus memebranaceus is a traditional Chinese herbal medicine used in treatment of common cold, diarrhea, fatigue, anorexia and cardiac diseases[9–11]. It also has been used as an immunomodulating agent in treatment of immunodeficiency diseases, to alleviate the adverse effects of chemotherapeutic drugs[12–14], such as significantly reduced myelosuppression in cancer patients[1516]. The active pharmacological constituents of radix Astragalus memebranaceus include various polysaccharides, saponins and flavonoida as well as L-arginine or L-canavanine[17]. Moreover, studies also showed that Astragalus polysaccharides have effects on colon cancer, mammary tumor, urological tumor, gastric cancer, etc.[1819]. In recent years, it has been proposed that Astragalus may possess anti-apoptosis potential in peritoneal mesothelial cells[2021]. In spite of this, the anti-apoptotic effects of Astragalus saponin extract on human peritoneal mesothelial cells during peritoneal carcinomatosishas have not been extensively studied. This study was to observe the anti-apoptosis effects of Astragalus saponin extract on human peritoneal meso-thelial cells during peritoneal gastric cancer metastasis.
Astragalus injection was obtained from Chiatai Qingchunbao Pharmaceutical Co. Ltd (China). 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazoliumbromide (MTT) and acridine orange/ethidium bromide (AO/EB) were obtained from Fluka (USA). Propidium iodide (PI) was obtained from Biosharp (USA). Bcl-2, Bax and actin primary antibodies, as well as second antibodies goat anti-mouse IgG were obtained from Santa Cruz Biotechnology Inc (CA, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal calf serum (FCS) were obtained from GIBCOBRL (Grand Island, NY, USA). Other laboratory reagents were obtained from Sigma (USA). Phase-contrast microscope (Japan, Nikon), fluorescence microscopy (Japan, Olympus), transmission electron microscopy (Japan, Hitachi H-6001), flow cytometry (FACScalibur; Becton Dickson, USA), were also used in this study.
Human peritoneal mesothelial cell line HMrSV5 was kindly provided by Professor You-Ming Peng, Second Hospital of Zhongnan University (Changsha, China) and Professor Pierre RONCO, Hospital TENON (Paris, France). Human gastric carcinoma metastatic liver cell line, MKN-45, and normal gastric epithelial cell line, GES-1, were obtained from Beijing Cancer Research Institute, China. The cells were placed into 75 cm2 tissue culture flasks and grown at 37°C, under a humidified atmosphere containing 50 mL/L CO2 and 950 mL/L air, in DMEM supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin and 100 &mgr;g/mL streptomycin, 2 mmol/L L-glutamine, 20 mmol/L hydroxyethyl piperazine ethanesulfonic acid (HEPES). The medium was changed every two or three days. The cells were dislodged using 0.25% trypsin and 0.02mol/L EDTA in PBS for subculture.
Serum-free conditioned media (SF-CM) from gastric cancer cells or normal gastric epithelial cells were prepared as previously described[1]. Briefly, 5.0 × 105 cells were seeded into 100 mm tissue culture dishes with 10 mL DMEM supplemented with 10% FCS and incubated at 37°C for 3 d. To obtain SF-CM, the cells were washed twice with PBS and then incubated for 2 d with 3 mL of serum-free DMEM. SF-CM were eluted and centrifuged at 1000 ×g for 5 min, passed through filters (pore size, 0.45 &mgr;m) and stored at -20°C until use.
Human peritoneal mesothelial cell line HMrSV5 was cultured to sub-confluence in a 50 cm2 dish with 10% FCS containing DMEM. The medium was changed to 4 test solutions: (1) serum free DMEM, (2) SF-CM from gastric cancer cell line MKN45, (3) SF-CM from gastric cancer cell MKN45 + 200 &mgr;g/mL Astragalus injection, (4) DMEM + 200 &mgr;g/mL Astragalus injection.
Mesothelial cells in 24-well chambers were exposed to test solutions for 24 h, gently washed with PBS, and then examined under a phase-contrast microscope for the size, shape, and integrity of cell membrane, cytoplasm, and nuclei.
After incubation in test solutions for 24 h, the cells were trypsinized and then fixed in PBS (pH 7.3) containing ice-cold 2.5% glutaraldehyde. The specimens were rinsed with PBS, post-fixed in 1% osmium tetroxide containing 0.1% potassium ferricyanide, dehydrated through a graded series of ethanol (30%-90%), and embedded in Epon. The specimens were cut into semi-thin (300 nm) sections with a Reichart ultra-cut, stained with 0.5% toluidine blue, and examined under a light microscope. The ultra-thin sections (65 nm) were stained with 2% uranyl acetate and Reynold’s lead citrate, and examined under a transmission electron microscope (× 5000 or × 8000 magnification).
Tests were performed to assess mesothelial cell viability after treatment with SF-CM for gastric cancer cell MKN45 and Astragalus injection. After exposure to the control or test solutions for a specific period (observed at 12 h, 24 h, 48 h), the cells in 96 well plates were incubated with 50 &mgr;g/mL MTT at a dilution of 1:10 based on the volume of culture medium, for 3 h at 37°C. At the end of incubation, MTT solution was removed, 150 &mgr;L DMSO was added to each well and stirred to dissolve the dark-blue formazon crystals formed. The proportion of viable cells was determined by measuring the optical density of each sample at 480 nm with a spectrophotometer. The cells were exposed three times to each solution for the same period of time. The means for the groups of cultures were compared. Control wells with no Astragalus injection and SF-CM were added. The same test was performed to assess the MKN45 viability after treatment with Astragalus injection.
After incubation in test solutions for 12, 24 and 48 h, the cells were harvested by trypsinization, resuspended in PBS at a concentration of 1 × 106/mL and fixed in 2 mL methanol for 30 min at 4°C. After mesothelial cells were fixed, the mixture was incubated in 0.5 mL propid iumiodide (PI) solution (0.05 mg/mL in 3.8 mol/L Na citrate) and 0.5 mL RNAse A (0.5 mg/mL) at room temperature for 30 min. Finally, the cells were resuspended in 1 mL PBS and analyzed by flow cytometry according to the manufacturer’s instructions. The cells in the subdiploid peak were considered apoptotic.
Human peritoneal mesothelial cells were cultured to sub-confluence in a 24-chamber slide with 10% FCS containing DMEM. The medium was changed to (1) serum free DMEM, (2) SF-CM from gastric cancer cell MKN45, (3) SF-CM from MKN45 + 200 &mgr;g/mL Astragalus injection, (4) DMEM + 200 &mgr;g/mL Astragalus injection, and (5) SF-CM from normal gastric epithelial cell line GES-1, respectively. After incubation for 48 h, apoptosis was quantified with fluorescent staining method by detecting AO/EB condensed nuclei with fluorescent microscopy. AO/EB staining could identify alive, early and late apoptotic cells and necrotic cells. Sub-confluent cells (70%-80% confluent) in 24 well uncoated plates were exposed to apoptotic stimuli for 48 h. Mesothelial cells in 24 well plates were gently washed with PBS and immediately treated with acridine orange (100 &mgr;g/mL) for 5 min and ethidium bromide (100 &mgr;g/mL) for 5 min. Each well was then examined under an epifluorescence microscope. An excitation wavelength of 455 nm was used to evaluate apoptosis under a fluorescence microscope. Apoptosis was defined by morphological criteria. Cells containing normal nuclear chromatin exhibited green nuclear staining. Cells containing fragmented nuclear chromatin exhibited orange to red nuclear staining.
Human peritoneal mesothelial cells were cultured to sub-confluence in a 50 cm2 dish with 10% FCS containing DMEM. The media were then changed to test solutions for 24 h. The cells were lysed in an ice-cold lysing solution containing 154 mmol/L NaCl, 1 mmol/L EDTA, 1% octylphenoxy polyethoxyethanol, 1 mmol/L phenylmethanesulfonyl fluoride, 1 mg/mL pepstatin, 1 mg/mL aprotinin, and 0.25% nadeoxycholate. Samples were rotated for 15 min at 4°C and then centrifuged at 12 000 r/min for 5 min at 4°C. The supernatant was recovered, and protein concentration was measured by bicinchoninic acid assay (Bio-Rad), with bovine serum albumin as the standard. Samples were incubated for 5 min at 95°C in a loading buffer (12 mmol/L Tris-HCl, pH 6.8, with 25% glycerol, 2% sodium dodecyl sulfate, 14.4 mmol/L 2-mercaptoethanol, and 0.1% bromophenol blue), and 50 mg of protein was loaded on different percentages different percentages of SDS-polyacrylamide gels (exclusion limits) corresponding to the molecular weight of target proteins. After electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane by electro-blotting. The membrane was blocked in 1% BSA, 0.05%Tween and PBS solution overnight at 4°C. Mouse antihuman monoclonal antibodies to Bcl-2 and Bax were used as primary antibodies. Horseradish peroxidase-labelled goat anti-mouse IgG was used as a secondary antibody. Blots were developed by incubation in a chemiluminescence substrate and exposed to X-ray films.
All data were expressed as mean ± SE. Student’s t-test was used to compare drug effects. P < 0.05 was considered statistically significant.
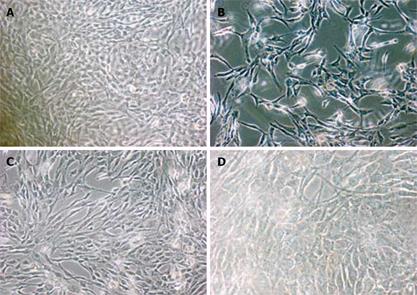
A typical polygonal cobblestone was found in untreated mesothelial cells in SF-CM (Figure 1A), but morphological changes occurred in treated mesothelial cells in SF-CM. The most obvious morphological changes in mesothelial cells was exfoliation and naked areas (Figure 1B). Astragalus injection could partly suppress such morphological changes (Figure 1C).
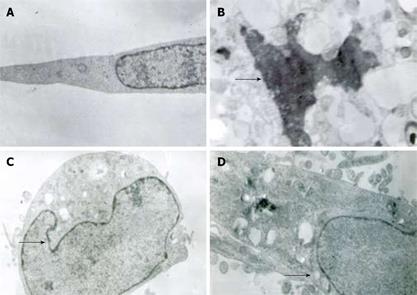
Twenty-four hours after treatment of MKN45, the apoptotic features (such as condensation of nuclear chromatin, wrinkling of nuclear membrane, dilation of endoplasmic reticulum, and relatively normal structure of mitochondria) were verified by electron microscopy (Figure 2). Under transmission electron microscope, the membrane of nuclei was complete, and the chromatin concentrated into masses on the boundary of the membrane or formation of arch (Figure 2C). Phenomena of budding and formation of apoptotic bodies were also observed (Figure 2D).
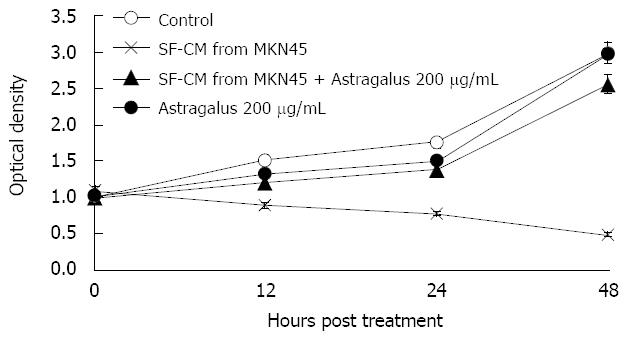
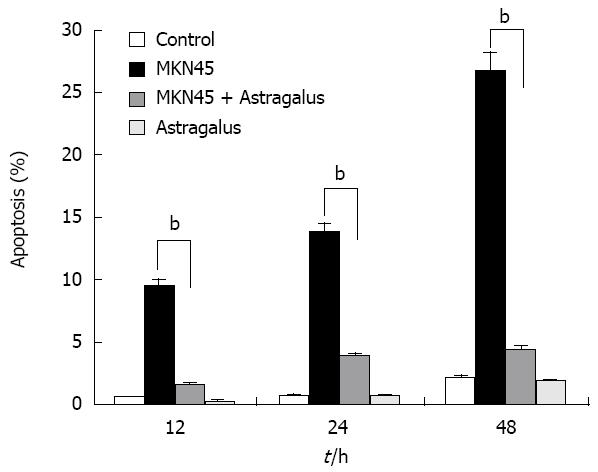
Anti-apoptotic effects of Astragalus injection were observed in SF-CM and apoptosis of gastric cancer cells shared the common intracellular signaling pathways. To evaluate the potential anti-apoptosis effects of combined SF-CM and Astragalus injection on mesothelial cells, we examined the cytotoxic effect of SF-CM and Astragalus injection on human mesothelial cell line HMrSV5. SF-CM could effectively inducing apoptosis of mesothelial cells in a time-dependent manner (Figure 3). Astragalus injection could effectively suppress mesothelial cell apoptosis in SF-CM at 0, 12, 24 and 48 h, respectively.
To evaluate the potential effects of Astragalus injection on MKN45 cells, we examined the cytotoxic effect of Astragalus injection on MKN45 cells. No significant effect of Astragalus injection on MKN45 cell viability was observed (P = 0.995, Table 1).
| Group | Optical density |
| Control (MKN45) | 2.096 ± 0.834 |
| MKN45 + Astragalus 200 &mgr;g/mL | 2.183 ± 0.844 |
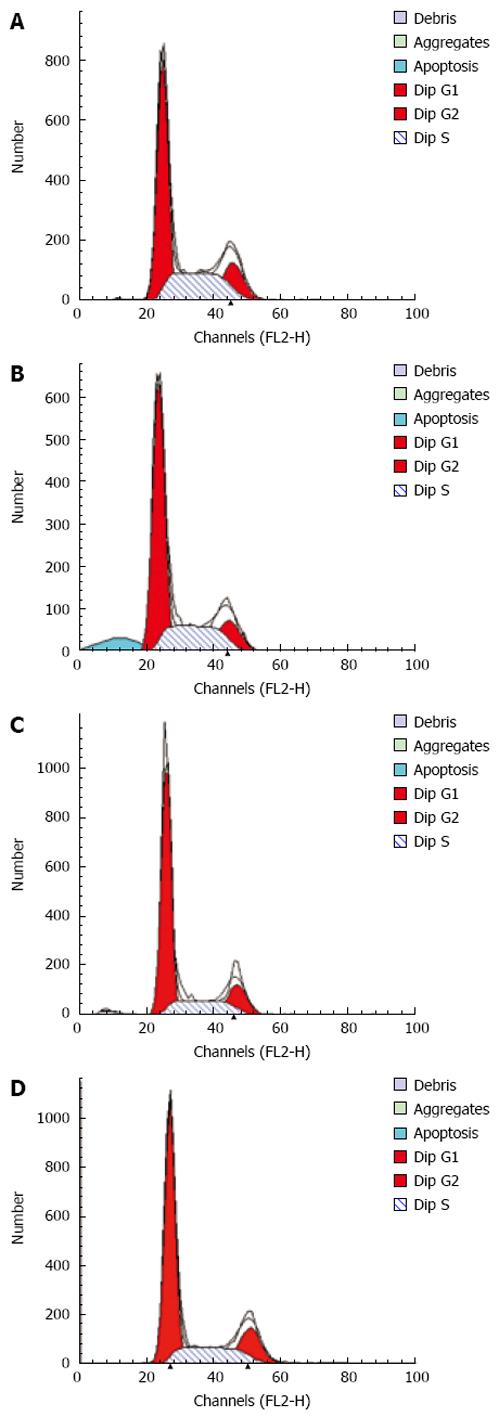
After incubation for 12 h, flow cytometry showed that the apoptosis peak occurred ahead the diploid peak when mesothelial cells were incubated with SF-CM for MKN45 (9.51% ± 0.71%). In contrast, the apoptosis peak value was not increased in the serum free DMEM group (0.70% ± 0.16%). The apoptosis peak value of was 1.65% ± 0.09% in the serum free DMEM group after treatment with Astragalus injection. The percentage of apoptosis was less than 0.5% (0.3% ± 0.07%) in the serum free DMEM group after treatment with DMEM + 200 &mgr;g/mL Astragalus injection. The data demonstrate that Astragalus injection could augment anti-apoptosis effects on MKN45 cells (Figure 4) and could effectively suppress mesothelial cell apoptosis induced by MKN45 as detected by MTT (Figure 5).
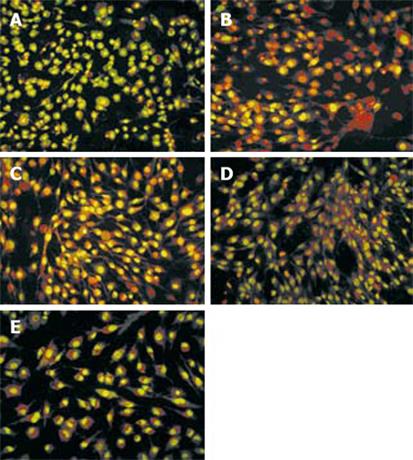
After mesothelial cells were treated with SF-CM from gastric cancer cell line MKN45 for 48 h, marked morphological changes in cell apoptosis occurred, such as condensation of chromatin, nuclear fragmentation and apoptotic bodies (Figure 6). Compared with the other three groups, the number of apoptotic cells significantly increased after treatment with SF-CM from gastric cancer cell line MKN45.
The number of apoptotic cells decreased significantly, while the number of green cells increased (Figure 6B and C). Astragalus injection inhibited the apoptosis of mesothelial cells induced by gastric cancer cells, while normal gastric epithelial cell line GES-1 could not induce apoptosis of mesothelial cells (Figure 6E). The number of apoptotic cells decreased after treatment with Astragalus injection (Figure 6B and C).
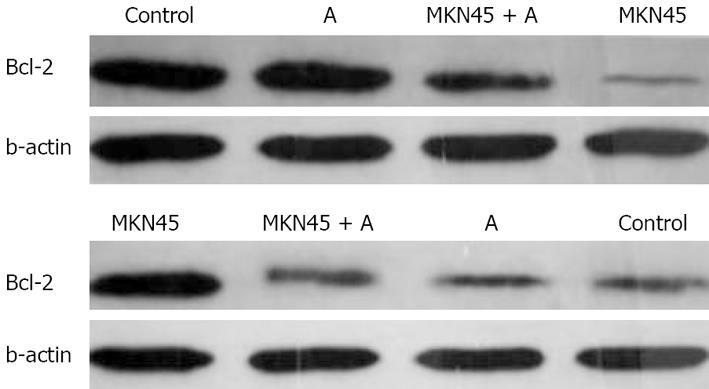
We further delineated the underlying mechanisms of Astragalus injection by which apoptosis of mesothelial cell apoptosis is induced. We examined the effects of Astragalus injection on the expression of Bcl-2 and Bax. Bcl-2 expression was increased and Bax expression was reduced in mesothelial cells 24 h after treatment with Astragalus injection (Figure 7).
It has been reported that mseothelial cells can prevent cancer invasion and undergo morphologic changes in response to factors released by cancer cells[22]. This phenomenon can be explained by the “seed and soil” theory: metastases occur when some tumor cells only live and grow in a congenial environment. Peritoneum might be a congenial environment for scirrhoid gastric cancer cells. Mesothelial cells can prevent cancer cell infiltration into sub-mesothelial connective tissue. It was reported that abdominal cavity cancer cells release early inflammatory factors and induce apoptosis of peritoneal mesothelial cells[2324]. When mesothelial cells become hemispherical, exfoliation occurs, and naked areas of sub-mesothelial connective tissue are exposed to the peritoneal cavity, and this injured peritoneum is a congenial environment for peritoneal metastasis[25].
In the present study, gastric cancer cell supernatant could significantly affect mesothelial cell viability, whereas normal gastric epithelial cell line GES-1 could not. Under contrast phase microscopy, mesothelial cells became hemispherical and exfoliation occurred when serum-free conditioned media were added into cultured mesothelial cells. Furthermore, cytoplasmic atrophy, nuclear shrinkage, and formation of extracellular and/or intracellular apoptotic bodies were observed under a transmission electron microscope. Astragalus injection could partly suppress these morphological changes. Moreover, apoptosis was also quantified by fluorescence microscopy and flow cytometry, showing that factors released by gastric cancer cells in the abdominal cavity induce apoptosis of mesothelial cells, cause exfoliation, and eventually result in metastasis. These may be the mechanisms by which cancer cells adhere to sub-mesothelial connective tissue. More tests will be conducted to better characterize the apoptotic signaling induced by gastric cancer cells.
Our study demonstrated that Astragalus injection could inhibit apoptosis of human peritoneal mesothelial cells induced by gastric cancer cell supernatant, suggesting that morphological changes in human peritoneal mesothelial cells are significantly decreased when human peritoneal mesothelial cells are exposed to both Astragalus injection and gastric cancer cells. Moreover, Astragalus injection could regulate the expression of Bcl-2 and Bax.
Bcl-2, an inhibitor of the mitochondrial apoptosis pathway, exerts its action by blocking proapoptotic counterparts, which in turn prevents release of cytochrome C and activation of caspases[2627]. The results of this study show that treatment of mesothelial cells with Astragalus injection significantly increased Bcl-2 expression in mesothelial cells, indicating that Astragalus injection exerts its antiapoptotic effects by maintaining the expression of Bcl-2 (Figure 6).
Bax is a death promoter, which is neutralized by heterodimerization with Bcl-2. When Bax translocates into the outer mitochondrial membrane, a leakage of cytochrome C from the mitochondria into the cytosol occurs[28]. Caspases-9 and -3 are activated sequentially, leading to breakdown of chromosomal DNA. In the present study, MKN45 significantly increased the Bax expression in mesothelial cells, while Astragalus injection partly suppressed the expression of Bax, indicating that there is a great possibility that Astragalus injection-mediated antiapoptosis of mesothelial cells is due to the regulation of Bcl-2 and Bax activation. Hence, identification of target compounds is imminent.
In general, investigation of the application of Astragalus injection in protecting human mesothelial cells is lacking. Astragalus injection has only been used in peritoneal dialysis therapy[29]. Studies on Astragalus injection were mainly concentrated on its immuno-modulating functions. Recently, Astragalus injection has been shown to have anticancer activity. In this study, we demonstrated the apoptotic effects of gastric cancer cell supernatant on human peritoneal mesothelial cells during peritoneal gastric cancer metastasis.
In conclusion, Astragalus injection can suppress apoptosis of mesothelial cells and can thus be used in treatment of gastric cancer.
Peritoneal carcinomatosis is a common form of disease progression in gastrointestinal cancer. Gastric cancer-induced apoptosis of peritoneal mesothelial cells has been assumed to be an important part in gastric cancer peritoneal metastatic cascade. Astragalus extract, an active component isolated from Astragalus Root, has recently been reported to have anti-apoptosis activities.
In general, investigations on the application of Astragalus injection in protecting human mesothelial cells are lack. Astragalus injection has only been used in peritoneal dialysis therapy. Studies on Astragalus injection were mainly concentrated on its immunomodulating functions. Recently, Astragalus injection has been shown to have anticancer activity. The objective of this study was to determine the inhibitory effect of Astragalus memebranaceushas on apoptosis of human peritoneal mesothelial cells induced by gastric cancer cell supernatant.
The inhibitory effects of Astragalus injection on apoptosis of human peritoneal mesothelial cells during peritoneal gastric cancer metastasis were investigated in this study.
Astragalus injection could suppress apoptosis of mesothelial cells and can thus be used in treatment of gastric cancer.
Human peritoneal mesothelial cells (HPMC) are sentinel cells that can sense and respond to signals within their microenvironment. Calycosin is a vasorelaxant with no competitive Ca2+ release.
This is a very excellent work. The authors demonstrated the anti-apoptosis effects of Astragalus on human peritoneal mesothelial cells during peritoneal gastric cancer metastasis.
| 1. | Yashiro M, Chung YS, Nishimura S, Inoue T, Sowa M. Fibrosis in the peritoneum induced by scirrhous gastric cancer cells may act as “soil” for peritoneal dissemination. Cancer. 1996;77:1668-1675. [Cited in This Article: ] |
| 2. | Jayne D. Molecular biology of peritoneal carcinomatosis. Cancer Treat Res. 2007;134:21-33. [Cited in This Article: ] |
| 3. | Kiyasu Y, Kaneshima S, Koga S. Morphogenesis of peritoneal metastasis in human gastric cancer. Cancer Res. 1981;41:1236-1239. [Cited in This Article: ] |
| 4. | Buck RC. Walker 256 tumor implantation in normal and injured peritoneum studied by electron microscopy, scanning electron microscopy, and autoradiography. Cancer Res. 1973;33:3181-3188. [Cited in This Article: ] |
| 5. | Heath RM, Jayne DG, O’Leary R, Morrison EE, Guillou PJ. Tumour-induced apoptosis in human mesothelial cells: a mechanism of peritoneal invasion by Fas Ligand/Fas interaction. Br J Cancer. 2004;90:1437-1442. [Cited in This Article: ] |
| 6. | Chen JY, Chi CW, Chen HL, Wan CP, Yang WC, Yang AH. TNF-alpha renders human peritoneal mesothelial cells sensitive to anti-Fas antibody-induced apoptosis. Nephrol Dial Transplant. 2003;18:1741-1747. [Cited in This Article: ] |
| 7. | Jayne DG. The molecular biology of peritoneal carcinoma-tosis from gastrointestinal cancer. Ann Acad Med Singapore. 2003;32:219-225. [Cited in This Article: ] |
| 8. | Alkhamesi NA, Ziprin P, Pfistermuller K, Peck DH, Darzi AW. ICAM-1 mediated peritoneal carcinomatosis, a target for therapeutic intervention. Clin Exp Metastasis. 2005;22:449-459. [Cited in This Article: ] |
| 9. | Sun Y, Jin L, Wang T, Xue J, Liu G, Li X, You J, Li S, Xu Y. Polysaccharides from Astragalus membranaceus promote phagocytosis and superoxide anion (O2-) production by coelomocytes from sea cucumber Apostichopus japonicus in vitro. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147:293-298. [Cited in This Article: ] |
| 10. | Wang PC, Zhang ZY, Zhang J, Tong TJ. Two isomers of HDTIC isolated from Astragali Radix decrease the expression of p16 in 2BS cells. Chin Med J (Engl). 2008;121:231-235. [Cited in This Article: ] |
| 11. | Yuan C, Pan X, Gong Y, Xia A, Wu G, Tang J, Han X. Effects of Astragalus polysaccharides (APS) on the expression of immune response genes in head kidney, gill and spleen of the common carp, Cyprinus carpio L. Int Immunopharmacol. 2008;8:51-58. [Cited in This Article: ] |
| 12. | World Health Organization (WHO). Medicinal Plants in China. WHO Regional Publications: Manila 1989; . [Cited in This Article: ] |
| 13. | Zee-Cheng RK. Shi-quan-da-bu-tang (ten significant tonic decoction), SQT. A potent Chinese biological response modifier in cancer immunotherapy, potentiation and detoxification of anticancer drugs. Methods Find Exp Clin Pharmacol. 1992;14:725-736. [Cited in This Article: ] |
| 14. | Ma XQ, Shi Q, Duan JA, Dong TT, Tsim KW. Chemical analysis of Radix Astragali (Huangqi) in China: a comparison with its adulterants and seasonal variations. J Agric Food Chem. 2002;50:4861-4866. [Cited in This Article: ] |
| 15. | Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 2004;320:1103-1111. [Cited in This Article: ] |
| 16. | Duan P, Wang ZM. [Clinical study on effect of Astragalus in efficacy enhancing and toxicity reducing of chemotherapy in patients of malignant tumor]. Zhongguo Zhongxiyi Jiehe Zazhi. 2002;22:515-517. [Cited in This Article: ] |
| 17. | Rittenhouse JR, Lui PD, Lau BH. Chinese medicinal herbs reverse macrophage suppression induced by urological tumors. J Urol. 1991;146:486-490. [Cited in This Article: ] |
| 18. | Tin MM, Cho CH, Chan K, James AE, Ko JK. Astragalus saponins induce growth inhibition and apoptosis in human colon cancer cells and tumor xenograft. Carcinogenesis. 2007;28:1347-1355. [Cited in This Article: ] |
| 19. | Cui R, He J, Wang B, Zhang F, Chen G, Yin S, Shen H. Suppressive effect of Astragalus membranaceus Bunge on chemical hepatocarcinogenesis in rats. Cancer Chemother Pharmacol. 2003;51:75-80. [Cited in This Article: ] |
| 20. | Zhang ZC, Li SJ, Yang YZ, Chen RZ, Ge JB, Chen HZ. Effect of astragaloside on cardiomyocyte apoptosis in murine coxsackievirus B3 myocarditis. J Asian Nat Prod Res. 2007;9:145-151. [Cited in This Article: ] |
| 21. | Li RJ, Qiu SD, Chen HX, Tian H, Wang HX. The immuno-therapeutic effects of Astragalus polysaccharide in type 1 diabetic mice. Biol Pharm Bull. 2007;30:470-476. [Cited in This Article: ] |
| 22. | Yashiro M, Chung YS, Inoue T, Nishimura S, Matsuoka T, Fujihara T, Sowa M. Hepatocyte growth factor (HGF) produced by peritoneal fibroblasts may affect mesothelial cell morphology and promote peritoneal dissemination. Int J Cancer. 1996;67:289-293. [Cited in This Article: ] |
| 23. | Chegini N. The role of growth factors in peritoneal healing: transforming growth factor beta (TGF-beta). Eur J Surg Suppl. 1997;67:17-23. [Cited in This Article: ] |
| 24. | Iwamoto I, Imada A. [Effects of growth factors on proliferation of cultured human peritoneal mesothelial cells]. Nippon Jinzo Gakkai Shi. 1992;34:1201-1208. [Cited in This Article: ] |
| 25. | Heath RM, Jayne DG, O’Leary R, Morrison EE, Guillou PJ. Tumour-induced apoptosis in human mesothelial cells: a mechanism of peritoneal invasion by Fas Ligand/Fas interaction. Br J Cancer. 2004;90:1437-1442. [Cited in This Article: ] |
| 26. | Fang CC, Yen CJ, Tsai TJ, Chen RH, Lee PH, Tomino Y. Antibiotics induce apoptosis of human peritoneal mesothelial cells. Nephrology (Carlton). 2003;8:142-149. [Cited in This Article: ] |
| 27. | Leard LE, Broaddus VC. Mesothelial cell proliferation and apoptosis. Respirology. 2004;9:292-299. [Cited in This Article: ] |