Published online Oct 14, 2008. doi: 10.3748/wjg.14.5880
Revised: August 18, 2008
Accepted: August 25, 2008
Published online: October 14, 2008
AIM: To investigate the cumulative development incidence and predictive factors for idiopathic pulmonary fibrosis in hepatitis C virus (HCV) positive patients.
METHODS: We studied 6150 HCV infected patients who were between 40-70 years old (HCV-group). Another 2050 patients with hepatitis B virus (HBV) were selected as control (HBV-group). The mean observation period was 8.0 ± 5.9 years in HCV-group and 6.3 ± 5.5 years in HBV-group. The primary goal is the development of idiopathic pulmonary fibrosis (IPF) in both groups. The cumulative appearance rate of IPF and independent factors associated with the incidence rate of IPF were calculated using the Kaplan-Meier method and the Cox proportional hazard model. All of the studies were performed retrospectively by collecting and analyzing data from the patient records in our hospital.
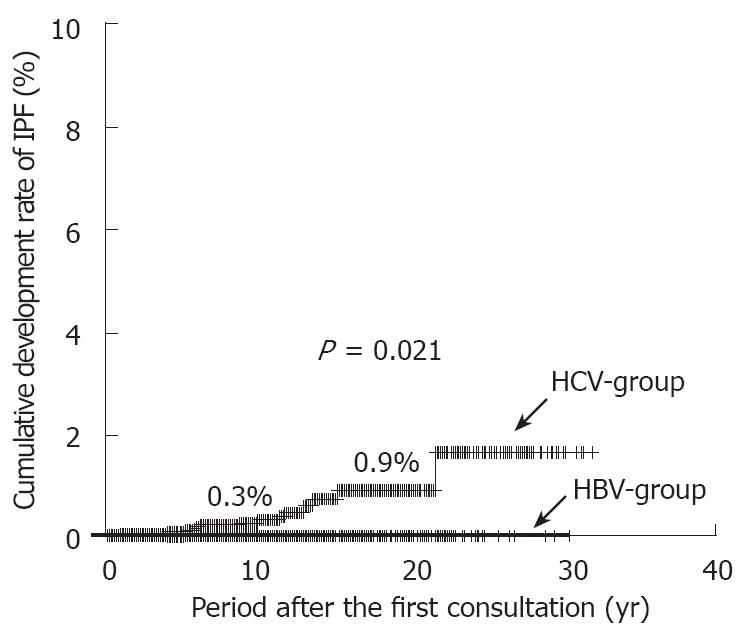
RESULTS: Fifteen patients in HCV-group developed IPF. On the other hand, none of the patients developed IPF in HBV-group. In HCV-group, the cumulative rates of IPF development were 0.3% at 10th year and 0.9% at 20th year. The IPF development rate in HCV-group was higher than that in HBV-group (P = 0.021). The IPF development rate in patients with HCV or HBV was high with statistical significance in the following cases: (1) patients ≥ 55 years (P < 0.001); (2) patients who had smoking index (package per day × year) of ≥ 20 (P = 0.002); (3) patients with liver cirrhosis (P = 0.042).
CONCLUSION: Our results indicate that age, smoking and liver cirrhosis enhance the development of IPF in HCV positive patients.
- Citation: Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Hirakawa M, Saito S, Ikeda K, Kumada H. Hepatitis C virus enhances incidence of idiopathic pulmonary fibrosis. World J Gastroenterol 2008; 14(38): 5880-5886
- URL: https://www.wjgnet.com/1007-9327/full/v14/i38/5880.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5880
| Number | Criteria |
| Major criteria 1 | Exclusion of other known caused of interstitial lung disease, such as certain drug toxicities, environmental exposures, and connective tissue diseases |
| Major criteria 2 | Abnormal pulmonary function studies that include evidence of restriction (reduced breathing vital capacity) and impaired gas exchange (increased AaPO2 with rest or after exercising or decreased diffusion lung capacity) |
| Major criteria 3 | Bibasilar reticular abnormalities with minimal ground glass opacities on conventional chest radiographs or high-resolution computed tomography scans |
| Major criteria 4 | Histological lung examination or bronchoalveolar lavage showing no features to support an alternative diagnosis |
| Minor criteria 1 | Age > 50 yr |
| Minor criteria 2 | Insidious onset of otherwise unexplained dyspnea on exertion |
| Minor criteria 3 | Duration of illness ≥ 3 mo |
| Minor criteria 4 | Bibasilar, inspiratory crackles (dry or “Velcro” type in quality) |
| Total | HBV-group | HCV-group | P | |
| Number (n) | 8200 | 2050 | 6150 | |
| Age (yr) | 51.8 ± 9.0 | 51.7 ± 8.7 | 51.8 ± 9.1 | 1 |
| Sex (male, %) | 77.8% (6380) | 77.8% (1595) | 77.8% (4785) | 1 |
| Liver cirrhosis2 | 20.2% (1659) | 18.5% (379) | 20.8% (1280) | < 0.001 |
| Total alcohol intake of > 200 kg3 | 23.0% (1490/6465) | 17.4% (253/1450) | 24.7% (1237/5015) | < 0.001 |
| Smoking index of > 203 | 27.9% (1680/6032) | 23.5% (293/1246) | 29.0% (1387/4786) | < 0.001 |
| AST (IU/L) | 75.9 ± 124.5 | 82.9 ± 138.2 | 73.8 ± 120.3 | < 0.001 |
| ALT (IU/L) | 104.2 ± 107.5 | 124.4 ± 119.9 | 98.5 ± 103.6 | < 0.001 |
| Total bilirubin (mg/dL) | 0.83 ± 0.94 | 0.98 ± 0.85 | 0.81 ± 0.77 | < 0.001 |
| γGTP (IU/L) | 74.0 ± 106.2 | 77.1 ± 128.7 | 73.2 ± 99.8 | 0.951 |
| Platelet count (x 104/mm3) | 19.3 ± 18.7 | 19.1 ± 14.4 | 19.4 ± 19.7 | 0.725 |
| Factor | Univariate analysis | Multivariate analysis1 | ||||||
| Category | Hazard ratio | 95% CI | P | Category | Hazard ratio | 95% CI | P | |
| Age (yr) | < 55/≥ 55 | 1/11.78 | 3.52-39.37 | < 0.001 | < 55/≥ 55 | 1/12.52 | 3.52-44.59 | < 0.001 |
| Smoking index1 | < 20/≥ 20 | 1/4.56 | 1.52-13.61 | 0.007 | < 20/≥ 20 | 1/5.90 | 1.95-17.82 | 0.002 |
| Liver staging (fibrosis) | Non-LC/LC | 1/3.67 | 1.29-10.48 | 0.015 | Non-LC/LC | 1/3.00 | 1.04-8.64 | 0.042 |
| Sex | Male/Female | 1/0.45 | 0.13-1.62 | 0.223 | ||||
| Platelet (x 104/mm3) | < 15/≥ 15 | 1/0.47 | 0.10-2.23 | 0.341 | ||||
| γGTP (IU/L) | < 110/≥ 110 | 1/1.95 | 0.41-9.31 | 0.405 | ||||
| Total alcohol intake1 | < 200/≥ 200 | 1/1.50 | 0.50-4.48 | 0.467 | ||||
| AST (IU/L) | < 76/≥ 76 | 1/1.16 | 0.30-4.53 | 0.834 | ||||
| ALT (IU/L) | < 100/≥ 100 | 1/1.05 | 0.29-3.74 | 0.946 | ||||
| Case | Age (yr)1 | Sex | Liver disease1 | Smoking index2 | Age at the time of IPF onset | Period after IPF development2 | Alive or death2 | Cause of death |
| 1 | 49 | M | LC | 25 | 57 | 5.7 | Death | HCC |
| 2 | 50 | M | CH | 38 | 62 | 12.3 | Death | IPF |
| 3 | 52 | M | CH | 0 | 73 | 3.8 | Death | IPF |
| 4 | 57 | M | LC | 28 | 68 | 8.7 | Death | d-LC |
| 5 | 58 | M | LC | 22 | 68 | 1.1 | Death | IPF |
| 6 | 61 | M | LC | 0 | 71 | 6 | Death | IPF |
| 7 | 61 | F | LC | 30 | 66 | 3.2 | Death | IPF |
| 8 | 62 | M | LC | 0 | 72 | 10.2 | Death | HCC |
| 9 | 62 | F | LC | 5 | 66 | 3.5 | Death | d-LC |
| 10 | 63 | M | CH | 26 | 75 | 10.6 | Alive | |
| 11 | 63 | M | CH | 34 | 64 | 12.1 | Death | HCC |
| 12 | 64 | M | CH | 40 | 69 | 10.1 | Alive | |
| 13 | 66 | F | CH | 24 | 79 | 1.8 | Alive | |
| 14 | 69 | M | LC | 0 | 70 | 2.3 | Death | HCC |
| 15 | 70 | M | LC | 42 | 76 | 4.1 | Death | HCC |
Hepatitis C virus (HCV) is one of the more common causes of chronic liver disease in world. Chronic hepatitis C is an insidiously progressive form of liver disease that relentlessly but silently progresses to cirrhosis in 20%-50% of cases over a period of 10-30 years[1-3]. In addition, HCV is a major risk for hepatocellular carcinoma (HCC)[4-8]. Yearly incidence of HCC in patients with HCV-related cirrhosis is estimated at 5%-10%, and it is one of the major causes of death, especially in Japan.
Chronic HCV infection has been associated with a variety of extrahepatic complications such as essential mixed cryoglobulinemia[9-11], porphyria cutanea tarda[9], membranoproliferative glomerulonephritis[12,13], autoimmune thyroiditis[14-16], sialadenitis[17], and cardiomyopathy[18]. A few previous studies have presented conflicting results, with some suggesting that an incidence of anti-HCV antibody positivity in patients with idiopathic pulmonary fibrosis (IPF) is significantly higher than that in patients without IPF both in Italy and Japan[19,20]. Others found that incidence of anti-HCV antibody positivity is not high compared to controlled patients[21]. These discrepancies might depend on factors such as geographical differences of race and/or ethnicity or differences in the false positive rate of anti-HCV testing.
In any case, there is little or no information on the yearly cumulative incidence and risk factors on the development rate of IPF in patients with HCV. In our hospital, we evaluate a large number of patients with HCV-related hepatitis, and often find HCC among our patients. Interestingly, we also find a small proportion of patients with HCV-related hepatitis who develop IPF.
With this background in mind, the present retrospective cohort study was initiated to investigate the cumulative incidence and risk factors of IPF among HCV-infected patients.
The number of patients who were diagnosed with chronic HCV infection between April 1975 and March 2006 in the Department of Hepatology, Toranomon Hospital, Tokyo, Japan was 11 500. Of these, 6150 patients met the following criteria: (1) age of 40-70 years; (2) features of chronic hepatitis or cirrhosis diagnosed by laparoscopy, liver biopsy, ultrasonography clinical features, and/or laboratory tests; (3) positive for anti-HCV antibody and HCV-RNA; (4) no history of treatment with antiviral agents; (5) negative for hepatitis B surface antigens (HBsAg), antinuclear antibodies, or antimitochondrial antibodies in serum, as determined by radioimmunoassay or spot hybridization; (6) no evidence of HCC nodules as shown by ultrasonography and/or computed tomography; (7) no underlying systemic disease, such as systemic lupus erythmatosus, rheumatic arthritis; (8) no cough or dyspnea after exercising; (9) no history of chronic lung disease. Patients with any of the following criteria were excluded from the study: (1) alpha-fetoprotein of 400 ng/mL or higher; (2) advanced and decompensated stage of cirrhosis with encephalopathy, icterus, or refractory ascites; (3) a short follow-up period of 6 mo or less; (4) development of HCC within 6 mo after the initiation of follow-up. These 6150 patients were regarded as in HCV-group. The 2050 patients that did not have the HCV marker and have the HBsAg marker were selected as a control and they comprised the hepatitis B virus (HBV)-group. The patients in HBV-group meet all of the above criteria but 3. Control patients were matched 1:3 with HCV positive patients for age and sex. We compared the differences of the cumulative development rate of IPF in both the HC-group and the HBV-group. Next, we assessed predictive factors for IPF in patients with hepatitis C. All of the studies were performed retrospectively by collecting and analyzing data from the patient records.
IPF was diagnosed by respiratory specialist based on the presence of at the least three of the following four diagnostic major criteria, as well as all of the following four minor criteria, as shown in Table 1. Diagnostic criteria of IPF were recommended by American Thoracic Society/European Respiratory Society[22]. We excluded hepatopulmonary syndrome by conventional chest radiographs or high-resolution computed tomography scans, electrocardiogram, and/or ultrasonic cardiography.
Anti-HCV was detected using a second-generation enzyme-linked immunosorbent assay (ELISA II) (Abbott Laboratories, North Chicago, IL). HCV-RNA was determined by the Amplicor method (Cobas Amplicor HCV Monitor Test, v2.0, Roche Molecular Systems, Inc., NJ). HBsAg was tested by radioimmunoassay (Abbott Laboratories, Detroit, MI). The used serum samples were stored -80°C at the first consultation. Diagnosis of HCV infection was based on detection of serum HCV antibody and positive RNA. The study started in April 1975. At that time, HCV testing was not available. Thus, the patients diagnosed as HCV-infected were tested after HCV testing became available.
Status of liver cirrhosis was mainly determined on the basis of peritoneoscopy and/or liver biopsy. Six thousand eight hundred and twenty six out of 8200 were diagnosed by peritoneoscopy and/or liver biopsy. Liver biopsy specimens were obtained using a modified Vim Silverman needle with an internal diameter of 2 mm (Tohoku University style, Kakinuma Factory, Tokyo, Japan), fixed in 10% formalin, and stained with hematoxylin-eosin, Masson’s trichrome, silver impregnation, and periodic acid-Schiff after diastase digestion. The size of specimens for examination was more than six portal areas. Baseline liver histology of chronic hepatitis was classified according to the extent of fibrosis, into four stages in progression order: stage 1, periportal expansion; stage 2, portoportal septa; stage 3, portocentral linkage or bridging fibrosis; stage 4, liver cirrhosis[23]. Remaining patients were diagnosed by clinical features, laboratory tests, and ultrasonographic findings. Ultrasonography was performed with a high-resolution, real-time scanner (model SSD-2000; Aloka Co., Ltd, Tokyo Japan. Logic 700 MR; GE-Yokokawa Medical Systems, Tokyo, Japan). The diagnosis of liver cirrhosis was defined as having a score of > 8 in ultrasonographical scoring system based on liver surface, liver parenchyma, hepatic vessel and spleen size as reported by Lin et al[24].
Patients were followed-up monthly to tri-monthly after the first medical examination in our hospital. Physical examination and biochemical tests were conducted at each examination together with regular check up using abdominal CT or US imaging in each patient. When a patient had any symptoms in relation to IPF (dry cough, dyspnea), we further explored the possibility of that patient having IPF. Three hundred thirty-four patients were lost to follow-up. Because the appearance of IPF and death was not identified in these 334 patients, they were considered as censored data in statistical analysis[25]. Moreover, patients treated with anti-viral agents were regarded as withdrawals at the time of starting antiviral agents.
Nonparametric procedures were employed for the analysis of background features of the patients, including the Mann-Whitney U test and χ2 method. The cumulative appearance rate of IPF was calculated from the period of the first medical examination at our hospital to the appearance of IPF, using the Kaplan-Meier method. Differences in the development of IPF were tested using the log rank test. Independent factors associated with the incidence rate of IPF were analyzed by the Cox proportional hazard model. The following nine variables were analyzed for potential covariates for incidence of IPF at the time of first medical examination at our hospital: age, sex, state of liver disease (chronic hepatitis or liver cirrhosis), smoking index, total alcohol intake, platelet count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase. P < 0.05 in two-tailed test was considered significant. Data analysis was performed using the SPSS computer program package (SPSS 11.5 for Windows, SPSS, Chicago, IL).
Table 2 shows the characteristics of the 8200 patients with HCV or HBV. There were no significant differences between the two groups with regard to sex ratio or age. However, there were significant differences in histopathological stage of the liver, AST, ALT, total bilirubin, total intake of alcohol, and smoking index. The 1594 (77.8%) out of 2050 in HBV-group and 5232 (85.1%) of 6150 in HCV-group were diagnosed by laparoscopy and/or liver biopsy. The breakdown of histological staging in HCV-group was as follows: stage 1, 2707; stage 2, 1188; stage 3, 300; stage 4 (liver cirrhosis), 1037. The outbreak of histological staging in HBV-group was as follows: stage 1, 705; stage 2, 439; stage 3, 157; stage 4 (liver cirrhosis), 293.
On relationship between liver histology and IPF, liver biopsies were done in ten of 15 patients with IPF before diagnosis of IPF. The period before diagnosis of IPF was 10.2 ± 4.1 years. There was no evidence of plasma cells to indicate possible autoimmune hepatitis, which as a systemic disease could be associated with IPF.
In the HCV-group, 15 patients developed IPF during a mean observation period of 8 years. The cumulative rate of newly diagnosed IPF was 0.3% at the end of the 10th year, and 0.9% at the 20th year (Figure 1). On the other hand, none of the patients developed IPF during a mean observation period of 6.3 years in the HBV-group. The cumulative rate of newly diagnosed IPF in the HCV-group was higher than that in the HBV-group (P = 0.021).
We then investigated the factors, except for virus marker, associated with the incidence of IPF in all the 8200 patients with HBV or HCV (Table 3). Univariate analysis identified the following three factors that influenced incidence of IPF: age (P < 0.001), state of liver disease (P = 0.007), and smoking index (P = 0.015).These three parameters were entered into multivariate Cox proportional hazard analysis. The IPF development rate of HCV positive patients was high with statistical significance in the following cases: (1) patients ≥ 55 years (P < 0.001); (2) patients who had smoking index (package per day × year) of ≥ 20 (P = 0.002), (3) patients who had liver cirrhosis (P = 0.042).
Next, we examined the factors associated with the incidence of IPF in all the 6150 patients with HCV multivariate Cox proportional hazard analysis. The IPF development rate of HCV positive patients was high with statistical significance in the following cases: (1) patients ≥ 55 years (OR: 14.24; 95% CI = 3.39-59.74, P < 0.001); (2) patients who had smoking index (package per day × year) of ≥ 20 (OR: 5.43; 95% CI = 1.74-16.88, P = 0.003); (3) patients who had liver cirrhosis (OR: 3.82; 95% CI = 1.23-11.86, P = 0.02).
Table 4 summarizes the characteristics of 15 patients who developed IPF in the HCV-group. Thirteen patients (12 men and 3 women; median age at the time of onset of IPF = 70 years, range = 57 to 79 years) with IPF were diagnosed by well-trained respiratory specialists.
During the observation period, 12 of the 15 patients with IPF in the HCV-group died. Seven patients died of liver-related disease (HCC, decompensated liver cirrhosis, rupture of esophageal varices) and five patients died of IPF. Liver-related death accounted for 58% (7/12) of all deaths and HCC was the major cause of liver-related deaths. IPF-related death accounted for 42% (5/12) of all deaths. On the other hand, of 6002 HCV positive patients without IPF, 1905 died of various diseases during the observation period. Of 1905 patients who died, 44 patients died of lung-related disease, such as acute pneumonitis or pulmonary tuberculosis. The incidence of death based on lung-related disease in patients with IPF was significantly higher than that in patients without IPF (P < 0.001).
We have described the development incidence of IPF in patients with HCV in the present study. The present study was limited by a retrospective cohort trial and age in patients. We selected patients with ages of 40-70 years at the first consultation. The reason is as follows: (1) onset of IPF is rare in young people with < 40 years; (2) the number of patients with > 70 years at the first consultation is few. Another limitation of the study was that HBsAg positive patients were selected as controlled group. Though there were no significant differences between the two groups with regard to sex ratio and age, there were significant differences in stage of the liver, AST, ALT, total bilirubin, total intake of alcohol, and smoking index. In Japan, HBV infection is usually acquired perinatally or in early childhood. Moreover, patients infected with HBV often have family history of HBV infection. Thus, patients infected with HBV might tend to avoid drinking alcohol or smoking on the merits of family advice. Next, AST, ALT, and total bilirubin levels were significantly high in HBV-group. These results may show that patients with HBV tend to have acute exacerbation of the liver. Another limitation is that we further explored the possibility that patient having IPF when a patient had any symptoms in relation to IPF (dry cough, dyspnea). However, Toranomon hospital was opened for attending patients who were officers or officials. Therefore, most of enrolled patients are officers, officials, or office workers. They have generally undergone chest checkup by the use of X-ray every year during incumbency and after retirement. This means that onset of IPF was checked constantly by respiratory specialists. Therefore, even if the diagnosis of IPF was late in cases in which hepatologists had no experience in the Department of Respiratory Disease, chest checkup by the use of X ray every year could assist on diagnosing IPF.
Among features of the present study are prolonged observation study and large number of the study population. The attending patients were followed closely base on the following reasons: (1) our hospital was opened for attending patients who were officers or officials; therefore, most of enrolled patients are officers, officials, or office workers; (2) our hospital is located in the center of the Tokyo metropolitan area in Japan, so it is convenient for patients to go to the our hospital. The present study shows several findings with regard to IPF in HCV positive patients. First, the IPF development rate in HC-group was higher than that in HB-group. Our retrospective study is the first to determine the annual incidence of IPF among patients with HCV at 0.03%-0.04%. The morbidity for IPF was estimated to be 0.003% to 0.004% in the general population in Japan[26]. Little is known about the relationship between the incidence of IPF and HCV. Conflicting studies on the incidence of HCV infection in patients with IPF have been published. Ueda et al[19] and Meliconi et al[20] reported a higher prevalence of HCV-antibody in patients with IPF compared with the general population. However, Irving et al[21] could not confirm the hypothesis that HCV may be a cause of IPF. The controversial results of the different research groups may be explained by the geographical differences of race and other factors. An accurate assessment of the exact risk could only come from a large cohort study. Thus, the present cohort study shows that prevalence of IPF tends to be slightly higher in the HC group compared to those in HB group in Japan. We believe this epidemiological study first elucidates the annual incidence of IPF among patients with HCV; the annual appearance rate was 0.03%-0.04%.
Second, the IPF development rate of HCV positive patients was high with statistical significance in the following cases: (1) patients ≥ 55 years; (2) patients who had smoking index of ≥ 20; (3) patients who had liver cirrhosis. Our results indicate that aging, liver cirrhosis and smoking enhance the development of IPF in patients with chronic hepatitis C infection. Idilman et al[27] have reported that HCV infection might be associated with an occult pulmonary inflammatory reaction manifested by an increased number of polymorphonuclear neutrophils in bronchoalveolar lavage fluid. Aging, liver cirrhosis, and smoking might enhance an occult pulmonary inflammatory reaction.
Third, the incidence rate of lung-related death of HCV positive patients with IPF was higher than that without IPF. IPF-related death corresponded to one-third of all deaths in patients with IPF. It can progress rapidly after such exacerbation and often proves fatal, despite treatment with oral corticosteroids and intravenous high-dose corticosteroid therapy. The fact that patients with IPF have high possibility dying from acute exacerbation due to IPF during the follow-up shows the need to provide a high level of care to patients with IPF. In general, hepatologist regard the daily management of patients with HCV. When HCV patients complain dry cough and dyspnea, hepatologists should check the complication of IPF.
In the present study, hepatopulmonary syndrome was excluded by conventional chest radiographs or high-resolution computed tomography scans, electrocardiogram, and/or ultrasonic cardiography. However, hepatopulmonary syndrome tends to complicate in advanced liver disease. Therefore, it is still possible that some of these patients with IPF had complication of hepatopulmonary syndrome.
Despite extensive research, IPF remains a disease of unknown etiology with a poor prognosis after acute exacerbation. Idilman et al[27] have reported that an increased bronchoalveolar lavage neutrophil count in individuals with HCV induced chronic active hepatitis was identified. This finding suggests that HCV may have the potential to induce an alveolitis leading to fibrotic changes in the lung. In the formation of IPF in HCV positive patients, there are other mechanisms such as accumulation to lung tissue of immunoglobulin and/or immune complex or direct involvement of HCV-RNA. These mechanisms may be mutually related.
First mechanism could be explained by the following. Gut-derived antigens and antibodies from the bowel via portal circulation or other antigens and antibodies were not segregated in sufficient amounts in patients with severe liver dysfunction. Immune complexes formed by these antigens and antibodies were passed into the systemic circulation and finally, these immune complexes are accumulated in the glomeruli or lung. Owing to this mechanism, there could be a high prevalence of immunoglobulin deposition in glomeruli of patients with mesangial proliferative glomerulonephritis and membranoproliferative glomerulonephritis[28]. We also examined the lung in a few patients using immunofluorescence microscopy. However, we did not detect immunoglobulin in formalin-fixed lung tissue. These results might indicate that serum immunoglobulin play a minor role in IPF. However, there might be possibility of showing low sensitivities due to use the formalin-fixed tissue. On the other hand, Koike et al[29] reported that transgenic mice carrying the HCV envelope gene revealed an exocrinopathy resembling Sjogren syndrome. Similar to this, HCV might directly cause IPF. More studies are needed to confirm the mechanism producing IPF in HCV positive patients.
In conclusion, our retrospective study is the first to determine the annual incidence of IPF among patients with HCV at 0.03%-0.04%. Our results indicate that age, liver cirrhosis and smoking enhance the development of IPF in patients with chronic hepatitis C infection.
Idiopathic pulmonary fibrosis (IPF) is present in patients with chronic hepatitis C virus (HCV) infection. In any case, there is little or no information on the yearly cumulative incidence and risk factors on the development rate of IPF in patients with HCV.
A few previous studies have presented conflicting results with some suggesting that an incidence of anti-HCV antibody positivity in patients with IPF is significantly higher than that in patients without IPF both in Italy and Japan, whereas others found that an incidence of anti-HCV antibody positivity is not high compared to controlled patients.
The morbidity for IPF was estimated to be 0.003% to 0.004% in the general population in Japan. This retrospective study is the first to determine the annual incidence of IPF among patients with HCV at 0.03%-0.04%. The results indicate that age, liver cirrhosis and smoking enhance the development of IPF in patients with chronic hepatitis C infection.
The fact that patients with IPF have a high possibility of dying from acute exacerbation due to IPF during the follow-up shows the need to provide a high level of care to patients with IPF. In general, hepatologists regard the daily management of patients with HCV. When HCV patients complain dry cough and dyspnea, hepatologists should check the complication of IPF.
IPF was diagnosed by respiratory specialist based on the diagnostic criteria recommended by American Thoracic Society/European Respiratory Society. Smoking index was defined as (package/d) × year, indicating the sum of before and after first consultation; period after IPF development, period (year) between onset of IPF and final consultation.
The manuscript is well written and the study is well designed. Authors investigated the cumulative development incidence and predictive factors for idiopathic pulmonary fibrosis in HCV positive patients.
Peer reviewer: Sammy Saab, PhD, UCLA, 200 Medical Plaza, Suite 214, Los Angeles 90095, United States
S- Editor Li DL L- Editor Li M E- Editor Zhang WB
| 1. | Kobayashi T, Kawa S, Tokoo M, Oguchi H, Kiyosawa K, Furuta S, Kanai M, Homma T. Comparative study of CA-50 (time-resolved fluoroimmunoassay), Span-1, and CA19-9 in the diagnosis of pancreatic cancer. Scand J Gastroenterol. 1991;26:787-797. [Cited in This Article: ] |
| 2. | Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, Alexander WJ, Hu PY, Miller JK, Gerber MA, Sampliner RE. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med. 1992;327:1899-1905. [Cited in This Article: ] |
| 3. | van Rossum TG, Vulto AG, de Man RA, Brouwer JT, Schalm SW. Review article: glycyrrhizin as a potential treatment for chronic hepatitis C. Aliment Pharmacol Ther. 1998;12:199-205. [Cited in This Article: ] |
| 4. | Colombo M, Kuo G, Choo QL, Donato MF, Del Ninno E, Tommasini MA, Dioguardi N, Houghton M. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet. 1989;2:1006-1008. [Cited in This Article: ] |
| 5. | Hasan F, Jeffers LJ, De Medina M, Reddy KR, Parker T, Schiff ER, Houghton M, Choo QL, Kuo G. Hepatitis C-associated hepatocellular carcinoma. Hepatology. 1990;12:589-591. [Cited in This Article: ] |
| 6. | Kew MC, Houghton M, Choo QL, Kuo G. Hepatitis C virus antibodies in southern African blacks with hepatocellular carcinoma. Lancet. 1990;335:873-874. [Cited in This Article: ] |
| 7. | Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797-1801. [Cited in This Article: ] |
| 8. | Ikeda K, Saitoh S, Koida I, Arase Y, Tsubota A, Chayama K, Kumada H, Kawanishi M. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993;18:47-53. [Cited in This Article: ] |
| 9. | Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. Review of extrahepatic manifestations. Ann Intern Med. 1995;123:615-620. [Cited in This Article: ] |
| 10. | Zignego AL, Ferri C, Giannini C, Monti M, La Civita L, Careccia G, Longombardo G, Lombardini F, Bombardieri S, Gentilini P. Hepatitis C virus genotype analysis in patients with type II mixed cryoglobulinemia. Ann Intern Med. 1996;124:31-34. [Cited in This Article: ] |
| 11. | Ferri C, Monti M, La Civita L, Longombardo G, Greco F, Pasero G, Gentilini P, Bombardieri S, Zignego AL. Infection of peripheral blood mononuclear cells by hepatitis C virus in mixed cryoglobulinemia. Blood. 1993;82:3701-3704. [Cited in This Article: ] |
| 12. | Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, Couser WG, Corey L, Wener MH, Alpers CE. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med. 1993;328:465-470. [Cited in This Article: ] |
| 13. | Yamabe H, Johnson RJ, Gretch DR, Osawa H, Inuma H, Sasaki T, Kaizuka M, Tamura N, Tsunoda S, Fujita Y. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection responsive to interferon-alpha. Am J Kidney Dis. 1995;25:67-69. [Cited in This Article: ] |
| 14. | Pawlotsky JM, Roudot-Thoraval F, Simmonds P, Mellor J, Ben Yahia MB, Andre C, Voisin MC, Intrator L, Zafrani ES, Duval J. Extrahepatic immunologic manifestations in chronic hepatitis C and hepatitis C virus serotypes. Ann Intern Med. 1995;122:169-173. [Cited in This Article: ] |
| 15. | Tran A, Quaranta JF, Benzaken S, Thiers V, Chau HT, Hastier P, Regnier D, Dreyfus G, Pradier C, Sadoul JL. High prevalence of thyroid autoantibodies in a prospective series of patients with chronic hepatitis C before interferon therapy. Hepatology. 1993;18:253-257. [Cited in This Article: ] |
| 16. | Boadas J, Rodriguez-Espinosa J, Enriquez J, Miralles F, Martinez-Cerezo FJ, Gonzalez P, Madoz P, Vilardell F. Prevalence of thyroid autoantibodies is not increased in blood donors with hepatitis C virus infection. J Hepatol. 1995;22:611-615. [Cited in This Article: ] |
| 17. | Haddad J, Deny P, Munz-Gotheil C, Ambrosini JC, Trinchet JC, Pateron D, Mal F, Callard P, Beaugrand M. Lymphocytic sialadenitis of Sjogren's syndrome associated with chronic hepatitis C virus liver disease. Lancet. 1992;339:321-323. [Cited in This Article: ] |
| 18. | Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490-1495. [Cited in This Article: ] |
| 19. | Ueda T, Ohta K, Suzuki N, Yamaguchi M, Hirai K, Horiuchi T, Watanabe J, Miyamoto T, Ito K. Idiopathic pulmonary fibrosis and high prevalence of serum antibodies to hepatitis C virus. Am Rev Respir Dis. 1992;146:266-268. [Cited in This Article: ] |
| 20. | Meliconi R, Andreone P, Fasano L, Galli S, Pacilli A, Miniero R, Fabbri M, Solforosi L, Bernardi M. Incidence of hepatitis C virus infection in Italian patients with idiopathic pulmonary fibrosis. Thorax. 1996;51:315-317. [Cited in This Article: ] |
| 21. | Irving WL, Day S, Johnston ID. Idiopathic pulmonary fibrosis and hepatitis C virus infection. Am Rev Respir Dis. 1993;148:1683-1684. [Cited in This Article: ] |
| 22. | American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277-304. [Cited in This Article: ] |
| 23. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [Cited in This Article: ] |
| 24. | Lin DY, Sheen IS, Chiu CT, Lin SM, Kuo YC, Liaw YF. Ultrasonographic changes of early liver cirrhosis in chronic hepatitis B: a longitudinal study. J Clin Ultrasound. 1993;21:303-308. [Cited in This Article: ] |
| 25. | Fleming TR, Harrington DP, O'Brien PC. Designs for group sequential tests. Control Clin Trials. 1984;5:348-361. [Cited in This Article: ] |
| 26. | Iwai K, Mori T, Yamada N, Yamaguchi M, Hosoda Y. Idiopathic pulmonary fibrosis. Epidemiologic approaches to occupational exposure. Am J Respir Crit Care Med. 1994;150:670-675. [Cited in This Article: ] |
| 27. | Idilman R, Cetinkaya H, Savas I, Aslan N, Sak SD, Bastemir M, Sarioqlu M, Soykan I, Bozdayi M, Colantoni A. Bronchoalveolar lavage fluid analysis in individuals with chronic hepatitis C. J Med Virol. 2002;66:34-39. [Cited in This Article: ] |
| 28. | Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kobayashi M. Glomerulonephritis in autopsy cases with hepatitis C virus infection. Intern Med. 1998;37:836-840. [Cited in This Article: ] |
| 29. | Koike K, Moriya K, Ishibashi K, Yotsuyanagi H, Shintani Y, Fujie H, Kurokawa K, Matsuura Y, Miyamura T. Sialadenitis histologically resembling Sjogren syndrome in mice transgenic for hepatitis C virus envelope genes. Proc Natl Acad Sci USA. 1997;94:233-236. [Cited in This Article: ] |