INTRODUCTION
Lymphomas in the gastrointestinal (GI) tract may be ulcerative, superficial, polypoid, or diffuse, and may also have other less common characteristics[1]. Multiple lymphomatous polyposis (MLP) is characterized by the formation of multiple mucosal polyps[2]. Polypoid lesions of MLP are usually widely spread to several regions of the GI tract including the esophagus, stomach, duodenum, and intestine. Histological findings tend to lead to the classification of most MLPs as mantle cell lymphomas[3-5]. Therefore, MLP may be one of the poor prognostic lymphomas in the GI tract, even though several regimens of systemic chemotherapy have been adapted for its treatment.
We report a rare and interesting case of MLP whose histology was a marginal zone B-cell mucosa-associated lymphoid tissue (MALT) lymphoma. A range of polypoid lesions was observed from duodenal bulb to the terminal ileum by various image examinations. The present case failed to respond to treatment to eradicate H pylori, but was successfully managed using a CHOP regimen combined with rituximab.
CASE REPORT
A 50-year old woman underwent an abdominal CT scan during an educational admission for diabetes mellitus at another hospital. CT scan revealed a diffuse thickening of small intestinal wall and swollen paraaortic lymph nodes, but no abnormal mass in her pancreas. Gallium citrate scintigraphy showed abnormal accumulation in the small intestine. Esophagogastroduodenoscopy (EGD) showed multiple polypoid lesions in the duodenum, and histological finding revealed non-specific inflammatory changes including infiltration of lymphoid cells. All these findings indicated a lymphatic proliferative disease. To obtain a differential diagnosis, she underwent endoscopic partial mucosal resection to perform the Southern blot analysis for immunoglobulin (Ig) genes but results were negative for changes in Ig-heavy chain JH. Then the patient was admitted to our hospital for further examination and treatment.
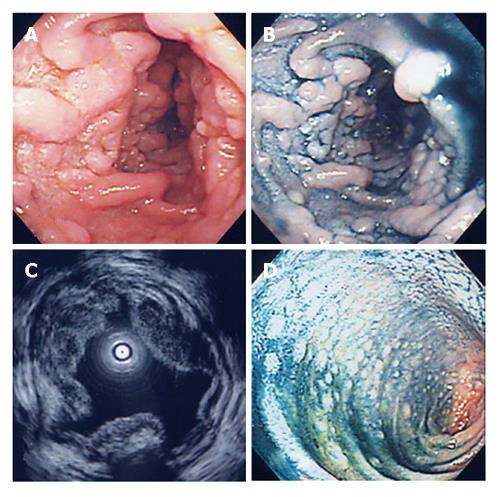
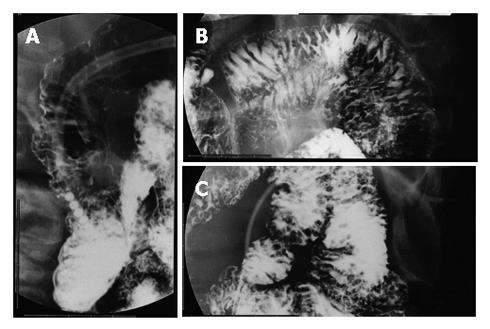
On admission, she had no clinical symptoms. Physical examinations revealed no superficial lymphadenopathy and no abnormal abdominal mass. Laboratory findings showed only mild liver dysfunction, mild elevation of lactate dehydrogenase and total cholesterol. No atypical lymphocytes were seen in peripheral blood, and the serum soluble interleukin-2 level was within normal limits. EGD showed multiple yellowish granular polypoid lesions sequentially spreading from the bulb to the distal tract over descending portion of the duodenum (Figure 1A and B). On the other hand, such polypoid lesions were not detected in the stomach except for atrophic mucosa. Cultures were positive for H pylori. Endoscopic ultrasonography revealed hypoechoic thickening from the first to the second sonographic layer, indicating that it was almost confined to the mucosal layer (Figure 1C). Fluoroscopic examination of the small intestine aiming to estimate the extent of lesion showed many round defects from the duodenal bulb to the end of jejunum (Figure 2), suggesting that the lesion was extending from the duodenum to the jejunum and ileum. Lower endoscopic findings showed whitish small polypoid lesions in the terminal ileum, but no obvious lesion in the colon and rectum (Figure 1D). Abdominal CT scan showed thickening of the duodenal and small intestinal walls. Abdominal MRI images also showed swollen paraaortic lymph nodes (approximately 1 cm in diameter) and an abnormal mass at the left side of supra-mesenteric artery (7 cm × 3 cm) which was probably composed of swollen mesenteric lymph nodes.
Figure 1 EGD (A), dye contrast EGD (B), EUS (C), and lower endoscopy (D) showing features of the lesions.
Figure 2 Fluoroscopic examinations showing many small round shaped defects in the second portion of the duodenum (A), jejunum (B), and ileum (C).
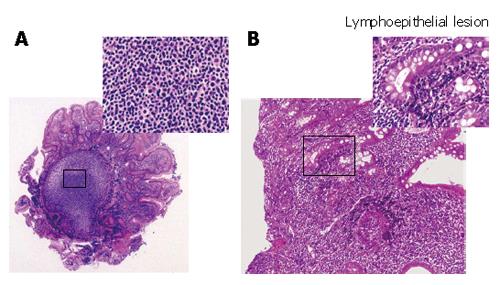
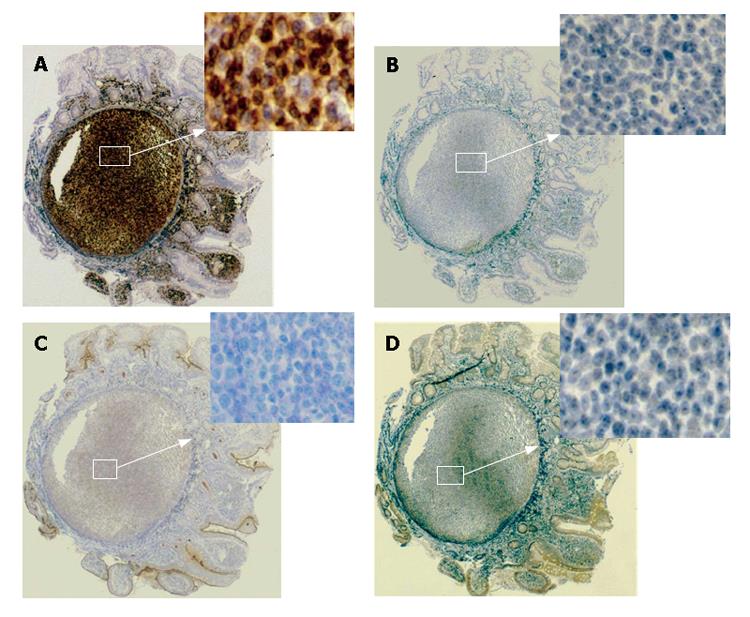
Histological findings of the specimens obtained from the lesions of duodenum and ileum showed follicular colonization dominantly composed of many small lymphocytes and a few large lymphocytes (Figure 3A), as well as a lymphoepithelial lesion, one of the typical features of mucosa-associated lymphoid tissue (MALT) lymphoma (Figure 3B). Immunohistochemical examinations showed that a large number of lymphoma cells were positively stained for bcl-2 and CD20 (the B-cell marker), but negative for UCHL-1 (T-cell marker). On the other hand, the tumor cells were negatively stained for CD5, CD10, and Cyclin D1 (Figure 4). All these findings including the immunohistochemical profiles led us to diagnose this lesion as a marginal zone B-cell lymphoma of the MALT type according to the WHO classification[6] but not as a mantle cell lymphoma, forming MLP sequentially spreading from the duodenal bulb to the terminal ileum. According to Lugano’s classification[7], its staging was clinically diagnosed as stage II.
Figure 3 Follicular colonization (A) and many small lymphocytes and a few large lymphocytes in the duodenal tissues (B) in the biopsied specimens (HE, × 200)
Figure 4 Immunohistological profiles of the lesions revealing positive staining for bcl-2 (A) and negative staining for CD5 (B), CD10 (C), and CyclinD1 (D).
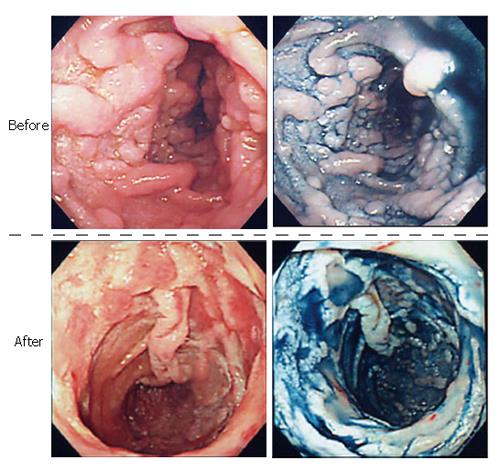
Eradication therapy was selected as the first treatment modality for H pylori infection (lansoprazole 60 mg/d, clarithromycin 800 mg/d, and amoxicillin 1500 mg/d, for one week), since it was less invasive for the patient. Follow-up EGD findings after H pylori eradication showed no improvement of these lesions. We selected the systemic chemotherapy as the second treatment modality for H pylori infection because the lymphoma may spread to the duodenum and other areas, even though the histopathological findings did not reveal a high potential for it to become malignant. The patient then received a standard CHOP (cyclophosphamide: 750 mg/m2, doxorubicin hydrochloride: 50 mg/m2, vincristine sulfate: 1.4 mg/m2, and predonisolone: 100 mg) regimen in combination with rituximab: 375 mg/m2 regimen, followed by a further CHOP treatment at their half dose. Ten days after the first therapeutic course, EGD findings showed that each polypoid lesion of the duodenum became smaller, and their tops collapsed and showed evidence for erosion. Following 2 courses of CHOP plus rituximab regimen, the lesions and abdominal lymph nodes almost completely resolved (Figure 5). A 5-year follow-up with EGD and histological examination showed no recurrence of the disease.
Figure 5 Endoscopic features of the lesions before and after the treatment.
DISCUSSION
The present case of lymphatic proliferative disease in the GI tract was diagnosed by endoscopy, radiographic imaging, and genetic profiling. The clinical features indicated a MLP type of lymphoma in the duodenum and small intestine. Actually, many reports have demonstrated the consensus that the majority of MLPs are histologically mantle cell type lymphomas, and a few cases of follicular lymphoma or T-cell lymphoma have been reported[8-12]. In the present case, however, histological examination showed characteristic findings such as lymphoepithelial lesions and immunohistological profiles (positive CD20 and bcl-2; negative UCHL-1, CD5, CD10, and CyclinD1). These findings motivated us to diagnose the case as a B-cell MALT lymphoma, which was discriminated from a mantle cell or follicular type lymphoma. On the other hand, the differential diagnosis was considered as an immunoproliferative small intestinal disease (IPSID), a subtype of MALT lymphoma[13]. However, this case was clinically different from the typical features of IPSID, because the dominant region for IPSID is duodenum and upper jejunum and it usually causes malabsorption syndrome or protein-losing gastroenteropathy. Thus, the present case was more consistent with a MALT lymphoma forming MLP rather than IPSID. To our knowledge, only 4 cases of MALT lymphoma forming MLP have been reported[14-16], indicating that our present case is actually rare. In regard to the origin of this lymphoma, we could not completely deny the suspicion that a lymph node might be the origin of the disease because of the swollen mesenteric lymph nodes. However, the involvement of other lymph nodes was unremarkable although the polypoid lesions were widely distributed in the GI tract. Furthermore, no atypical lymphocytes were shown in peripheral blood, and this case was compatible with the definition about primary GI tract lymphoma described by Lewin and Herrmann[17,18]. Thus, it was more likely that the primary lesion of the present lymphoma was in the GI tract.
Recently, treatment to eradicate H pylori has become a standard management for primary gastric MALT lymphoma[19-21]. Certainly, there are various reports showing that eradication of H pylori is effective for duodenal MALT lymphoma[22,23]. Nakamura et al[24] reported that primary duodenal lymphoma might have different characteristics between locations at the bulb and descending portion. Lymphomas originating from duodenal bulb might have similar characteristics with gastric MALT lymphomas, suggesting that H pylori eradication therapy is often effective. Unfortunately, the lesions observed over the descending portion might be less associated with H pylori infection, indicating that H pylori eradication therapy cannot be expected to be totally effective. On the other hand, the efficacy of H pylori eradication therapy for the small intestine or colorectal lymphoma is not known, though a few responsive cases have been reported[25,26]. Since H pylori eradication therapy was ineffective in the present case, we used a more potent therapy due to the potential of the lymphoma to spread more extensively and its possible transformation of MALT lymphoma cells into diffuse large B cell lymphoma cells in the presence of a few large cells in the specimens. In general, surgical resection, systemic chemotherapy, and radiation therapy are performed in the treatment of malignant lymphoma in the GI tract[27]. Since curative surgical resection or radiation therapy was not suitable in our case because the lesion was already diffuse and widespread, we selected a systemic chemotherapy as a second line treatment. Combination therapy of a CHOP regimen, the most common regimen in combination with rituximab (a molecular targeting agent for this particular follicular type lymphoma), has been recently used in the treatment of GI tract lymphoma[28,29]. This combination therapy contributed to a significant improvement in the present case. We performed careful follow-up examinations such as laboratory analyses, EGD examination, and various abdominal images at every six months interval. EGD examination revealed no obvious recurrence of MLP and there was no evidence for recurrent lesions or clinical symptoms during the 5-year follow-up.
In conclusion, we reported a rare case of MALT lymphoma in the small intestine which was histologically diagnosed as a marginal zone B-cell MALT lymphoma. CHOP regimen in combination with rituximab is an effective therapy for MALT lymphomas in the GI tract.
Supported in part by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Science and Culture in Japan
S- Editor Liu Y L- Editor Wang XL E- Editor Zhou T