Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Sep 21, 2007; 13(35): 4707-4715
Published online Sep 21, 2007. doi: 10.3748/wjg.v13.i35.4707
Published online Sep 21, 2007. doi: 10.3748/wjg.v13.i35.4707
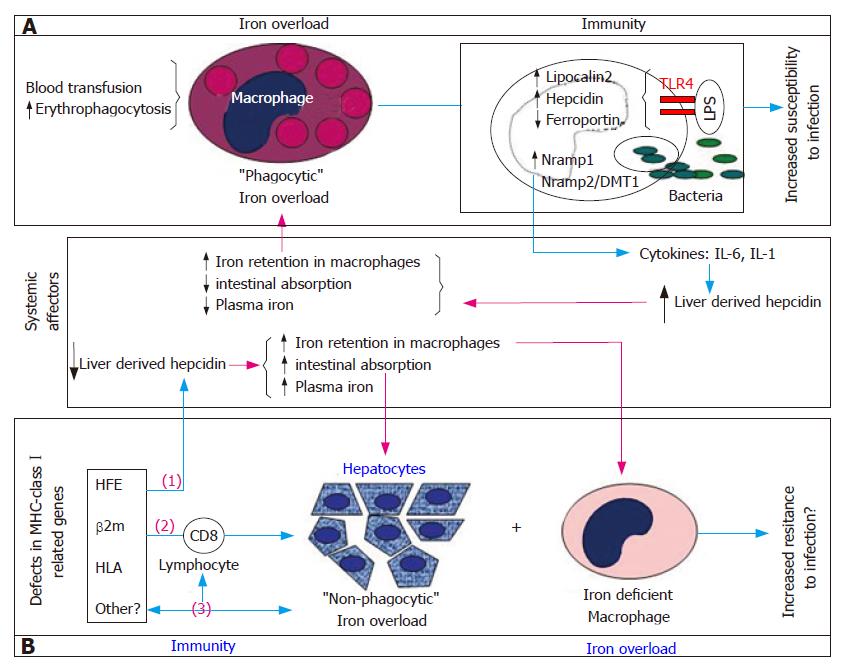
Figure 1 A model illustration of the two topics addressed in this review: how innate immunity is affected and affects “phagocytic” iron overload (Box A) and the evidence that defective immune system models, namely natural and experimental defects in MHC-classIrelated genes (HFE, β2-microglobulin and HLA) are associated with the spontaneous development of “non-phagocytic” or parenchymal iron overload (Box B).
A: Macrophages “overwhelmed” by continuous iron overloading as seen in conditions of chronic transfusion and increased erythrophagocytosis are compromised in their function of surveillance of bacterial growth resulting in increased host susceptibility to infection. As part of the natural response to bacterial infection macrophages modulate the expression of a number of genes involved in the regulation of cellular iron metabolism. By activating the cytokine cascade they induce up-regulation of liver hepcidin synthesis with further increase in macrophage iron retention and perpetuation of this cycle; B: A “non-phagocytic” or parenchymal iron overload is observed in hepatocytes of several defective immune system models associated with a relative iron deficiency in macrophages, recapitulating the findings observed in the human model of Hereditary Hemochromatosis. The mechanisms underlying the spontaneous development of iron overload in these models are not yet understood. Three possible (not mutually exclusive) mechanisms are represented in this figure: (1) the fact that both Hfe and β2m knockout mouse models lack the liver hepcidin response to iron overload[90,97,98] may support the notion of a liver-derived, systemically induced iron overload. The involvement of liver hepcidin in the development of iron overload in mice deficient in the MHC-classImolecules H2Kb and Db (HLA) was never tested; (2) the iron overload seen in the β2m-/-Rag1-/- and Hfe-/-Rag1-/- mouse models support the involvement of lymphocytes themselves as modifiers of the severe parenchymal iron overload phenotype seen in those mice[39]; (3) finally, the recent demonstration of a strong link between the MHC-classIregion and the genetic transmission of low CD8+ T lymphocyte numbers in humans and the association of total lymphocyte numbers with a more severe iron overload phenotype in patients with Hereditary Hemochromatosis[78,82,83] opens a new possibility that another, still unidentified MHC-classIassociated gene(s) may simultaneously regulate CD8+ T lymphocyte numbers and influence the development of parenchymal iron overload[84].
- Citation: Porto G, Sousa MD. Iron overload and immunity. World J Gastroenterol 2007; 13(35): 4707-4715
- URL: https://www.wjgnet.com/1007-9327/full/v13/i35/4707.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i35.4707