Published online Jul 7, 2007. doi: 10.3748/wjg.v13.i25.3517
Revised: April 20, 2007
Accepted: May 12, 2007
Published online: July 7, 2007
AIM: To evaluate the effects of adenovirus-mediated gene transfer of RhoA siRNA and RhoC siRNA on proliferation and invasion of SGC7901 cells by Rho/ PI3K/Akt pathway.
METHODS: Plasmid of RhoA siRNA and RhoC siRNA were constructed and transfected into SGC7901 cells. siRNA and LY294002 (PI3K inhibitor) were designed as the control group. The mRNA and protein expressions of RhoA and RhoC were respectively detected with RT-PCR and western blotting. In order to find out the changes of proliferation and invasion power of SGC7901 cell lines, we analyzed the data by MTT, Boyden chamber and evaluated apoptosis of cell with flow cytometry. We treated BALB /C nude mice with RhoA and RhoC-siRNA, and tumor control rate (%) in nude mice was calculated.
RESULTS: RhoA and RhoC siRNA transfections specifically down-regulated the corresponding mRNA and protein levels in SGC7901 Cells.The experiment of permeated artificial basal membrane showed that the invasion power of SGC7901 cell lines are on the decline after treatment of Ad-RhoA and RhoC-siRNA (12.64 ±3.27 vs 87.38 ± 17.38, P < 0.05). The values of 490 nm wavelength light absorption were different in the five groups. The number of alive cells in the group of RhoA and RhoC-siRNA was lower than others in the 6th d (0.71 ± 0.01 vs 3.82 ± 0,11 P < 0.05). The apoptosis rate of transfected RhoA and RhoC-siRNA group with FACS were 19.07% ± 1.78 and there were significant differences between treated and control groups (19.07 ± 1.78% vs 1.23 ± 0.11%, P < 0.01). The tumor transplantation experiment in BALB/C nude mice showed intratumoral injection of RhoA or RhoC siRNA can inhibit tumor growth.
CONCLUSION: RhoA and RhoC siRNA gene therapy mediated by adenovirus may be useful for inhibiting growth and invasion of SGC7901 through a PI3K/Akt pathway. These results provide a novel therapeutic target in preventing gastric cancer cell invasion and metastasis.
- Citation: Sun HW, Tong SL, He J, Wang Q, Zou L, Ma SJ, Tan HY, Luo JF, Wu HX. RhoA and RhoC -siRNA inhibit the proliferation and invasiveness activity of human gastric carcinoma by Rho/PI3K/Akt pathway. World J Gastroenterol 2007; 13(25): 3517-3522
- URL: https://www.wjgnet.com/1007-9327/full/v13/i25/3517.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i25.3517
Gastric cancer is a common malignant tumor in China. In the tissue of gastric cancer, the expression RhoA and RhoC gene has been reported to be significantly correlated with carcinogenesis, development and infiltration metastasis of gastric cancer[1]. However, the molecular aspects of carcinogenesis and metastasis of gastric carcinoma remains elusive. It is important to attempt to inhibit tumor growth in such a manner as to block the inhibition of the apoptotic signaling pathway in tumor cells[2-4]. RhoA is over expressed during tumorigenesis, and some reports showed that the overexpression of RhoA and RhoC is involved proliferation and invasion in gastric cancer[5,6].
The RhoA and RhoC proteins comprise an important subset of the Rho GTPase family that has been implicated in invasive gastric carcinomas, and is related to the PI3K/Akt signal pathway. To dissect the specific contributions of RhoA and RhoC in the behavior of invasive gastric carcinoma cells, we used RhoA and RhoC gene interference to inhibit proliferation and invasion of SGC7901 cells of gastric carcinoma[7,8]. We have used small interfering RNA (siRNA) approaches to interfere the expression of the RhoA and RhoC isoforms in the SGC7901 cells. Using these cell lines, we were able to define distinct functions for these Rho isoforms and to uncover unexpected relationships between them.
To investigate the inhibitory effects and biological functions of RhoA and RhoC siRNA on gastric cancer cells, our experiment will also study the effects of RhoA-siRNAs and RhoC-siRNA gene on proliferation and invasion of SGC7901 cells in vivo and in vitro. Our report suggests that the tumor suppressive properties of RhoA-siRNAs and RhoC-siRNA genes are related, in part, to its ability to down-regulate the PI3K/Akt pathway, inhibit proliferation and invasion and facilitate apoptosis.
The extraction kit of Achromycin and Plasmid are purchased from WuHan Boster company. Anti-HA, annexin V and fluorescein isothiocyanate (FITC) were purchased from Becton Dickinson Company. Anti-RhoA,B and C rabbit polyclonal antibody was purchased from Sigma. Anti-Flag antibody and kin Rho binding domain was purchased from Sigma. CX4hyg/EcoVR was kindly supplied by Wu professor (Tongji medical University, China). RPMI 1640 medium and Dulbecco's modified Eagle's medium (DMEM) were purchased from Sigma. The other chemicals and reagents were purchased from WuHan ZhongShan Chemical Co. The antibodies for rabbit anti-humanβ-actin and sheep anti-rabbit two antibodies were purchased from Becton Dickinson Company (USA).
SGC7901 cell lines and 293 cells (encasing cells of adenovirus vector) were provided by WuHan University Cell Center. The complete medium (RPMI1640) contained 100 U/mL penicillin, 100 mg/L streptomycin and 10% calf serum. Cells were digested and transferred in culture with 0.25% parenzyme and 0.02% EDTA in the incubator and incubated at 37°C in 5% CO2 and saturated humidity.
The RhoA and RhoC coding sequences were scanned to identify AA (n19)TT sequences. Candidate sequences were compared with RhoA and RhoC cDNA sequences and their specificity was verified in the non-redundant human DNA database using a BLAST algorithm (accession through NCBI). A control siRNA was also tested. It was selected because it exhibited no cellular toxicity. The sequences selected for the sense and antisense strands are for anti-RhoA siRNA, sense 5'-GACAUGCUUGCUCAUAGUCTT-3', antisense 3'-TTCUGUACGAACGAG-UAUCAG-5'; for anti RhoC siRNA, sense 5'GACCUGCCUCCUCAUCGUCTT-3', antisense 3'-TTCUGGACGGAGGAGUAGCAG-5'; for the control siRNA, sense 5'-CAGU-CAGGAGGAUCCAAAGTG-3', antisense 3'-TTGUCAGUCCUCCUAGGUUUC-5'.
Synthetic oligonucleotides prepared by Sigma, were annealed to form a short double-stranded RNA with a 3'dithymidine overhang. Hybridization was performed in a buffer containing 2 mmol/L sodium acetate, 100 mmol/L potassium acetate, and 30 mmol/L Hepes buffer, pH 7.4. Total RNA was extracted from adherent cells using RNeasy columns. Reverse transcription semi-quantitative PCR was performed using 1 μg of total RNA in the one-step RT-PCR reaction kit as follows: 15 and 20 cycles of 94°C for 30 s, 55°C for 30 s and 60°C for 60 s.
SGC7901 and 293 line cells were incubated in culture medium that contained 10% calf serum, and were transfected in a routine method. 293 line cells were transfected with Ad- RhoA and RhoC-siRNA, and virus amplification was performed. Virus titer was measured with plaque-forming assay. SGC7901 line cells were detected after being transfected for 24 h, total RNA was extracted with Trizol. RT-PCR was measured with one-step method. The tumor cells were incubated in serum-free culture medium and supernatant was collected. Total proteins were taken from every sample, and we performed electrophoresis on polyacrylamide gels containing 10% SDS gel substrate, which were eluted, poached, incubated and bleached individually.
In the experiment, enzymolysis was displayed in black strap on the white background. These images were analyzed with quantity One 411 software in the Oylmpus image analysator, the calculation formula was: Ratio activity of MMP = (strap density-background density/specimen proteinum concentration) × strap area (mm2/mg per mL).
The SGC7901 cell lines were divided into five groups: 1st group: RhoA-siRNA and RhoC-siRNA, 2nd group: RhoA-siRNA, 3rd group: RhoC-siRNA, 4th group: siRNA and 5th group: LY294002 (PI3K inhibitor). All virus titers were 50MOI).
Western blot analysis was performed with 50 μg of the cell lysate from the transfected cells and anti-RhoA and RhoC mice monoclonal antibody (Sigma Co). RhoA and RhoC proteins in the cells were extracted and detected with the Bio-Rad DC protein kit. In accordance with the kit descriptions, we have adjusted the concentration of the proteins. Polyacrylamide gel (SDS-PAGE) electrophoresis was performed with 10% gel, transmembrane and immunity display was done later. Membranes were immunoblotted overnight with mice anti-RhoA and RhoC monoclonal antibodies diluted 1:500. Primary antibody binding was detected by enhanced chemiluminescence using horseradish peroxidase coupled with anti-mice or anti-goat antibody (1:10000) for 40 min at room temperature. After film exposure with X-ray, the pictures were scanned and preserved as documents, and the output of gray scale were analyzed with image software.
To perform the Boyden chamber member, we put 2.5 × 104 SGC7901 cells lines into the Boyden chamber cabin, cells were incubated for 48 h both before after transfection. These cell lines were incubated in the cell incubator for 24 h, and fixation and staining were done. The five fields of vision were taken up under light microscope, and the cell number under the membrane was counted. There were three specimens every time, and repeated 1 time.
After every group of cells had been selected and cultivated, they were inoculated into 96 pores plate in 1 × 104/well. The MTT was routinely performed for 6 d, and repeated 3 times. The output of OD was measured on 490 nm wavelength and a curve was drawn.
After transient transfection of plasmid for 24 h, the cell were collected and washed 3 times with PBS, fixed in 4°C of cool alcohol overnight. The cells were centrifuged and once collected, washed before detection with flow cytometry. The cells were dyed with 1% iodation aethyl-troche for 30 min. The rate of apoptosis was analyzed by Cellquit software.
1 × 107 SGC7901 cells were inoculated into the back of BALB/C nude mice, after 21 d, the long-diameter (a) and short-diameter (b) were measured with a sliding caliper. The volume was measured with Formula (V = 1/2a × b2). When the tumors were as large as 50-70 mm3, the nude mice were divided into 5 groups: (1) RhoA and RhoC-siRNA, (2) RhoA-siRNA, (3) RhoC-siRNA LY294002, (4) siRNA and (5) 0.9% NS and were injected intratumoral every 3 d. The RhoA and RhoC-siRNA concentrations were 80 nM. We sacrificed the mice on d 20 after beginning intratumoral injections and removed the tumors. Tumor control rate (%) was calculated with Formula:
Tumor vol control rate (%) = (1 - average vol of treated tumor/average vol of control tumor) × 100%.
Tumor weight control rate (%) = (1 - average weight of treated tumor/average weight of control tumor) × 100%.
The experimental data was expressed in mean ± SEM. The data was analyzed with analysis of variance, and treated with SPSS10.0 software package. The blot membranes were scanned and analyzed with NIH image software. The statistical significance of differences between groups was calculated by applying t-test, and P < 0.05 was taken as significant.
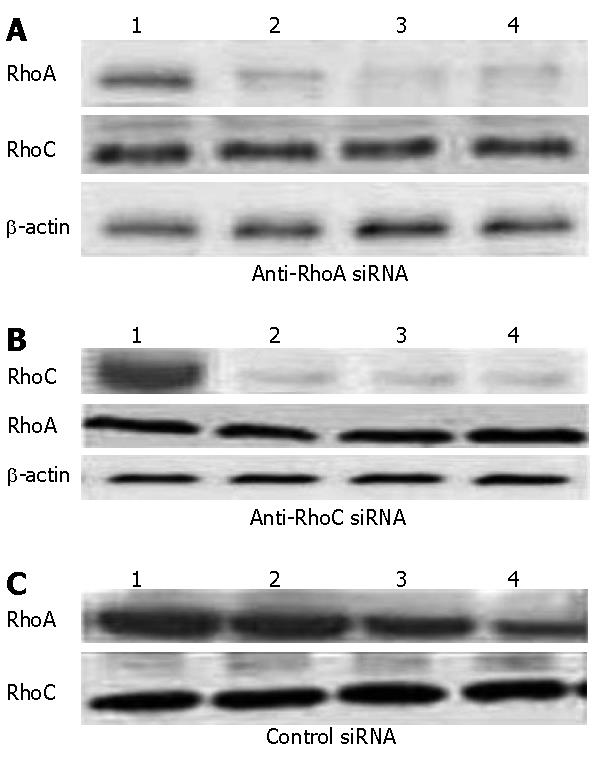
In order to assess the ability and specificity of the siRNA inhibit RhoA and RhoC gene, western blot analysis revealed that when RhoA or RhoC-siRNA was transfected, expression of the corresponding target proteins was decreased by at least 90% (Figure 1); but when the incubation was prolonged for another 24 h without any further addition of siRNA, expression levels began to increase, mainly because of the short life span of siRNA in these cells.
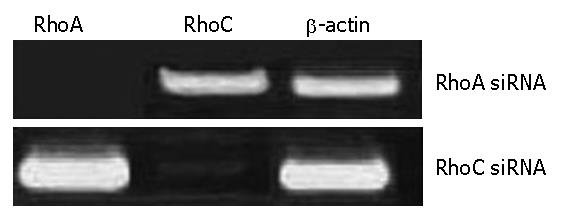
The RhoA and RhoC gene was detected by RT-PCR after recombination adenovirus-mediated gene was transfected RhoA-siRNAs and RhoC-siRNA into SGC7901.The virus titers were 0.75 × 108 pfu/mL after amplification of Ad- RhoA-siRNAs and RhoC-siRNA virus. The results of RT-PCR demonstrated there were positive mRNA straps on the map of SGC7901 transfected with RhoA-siRNAs and RhoC-siRNA (Figure 2).
RhoA and RhoC- siRNA transfections specifically down-regulate the corresponding mRNA and protein levels in SGC7901 cells. We analyzed the efficacy of siRNA-mediated inhibition of RhoA and RhoC synthesis by both reverse transcriptase-polymerase chain reaction (RT-PCR) and western blotting.The corresponding mRNAs and proteins of RhoA and RhoC were downregulated 24 h later; two transfections were more effective than one. This down-regulation is specific, because RhoA-siRNA did not modify RhoC mRNA expression, and RhoC-siRNA did not modify RhoA mRNA expression. Furthermore, β-actin mRNA levels were not modified by exposure to either of these siRNA. Finally, an unrelated control siRNA also failed to modify RhoA or RhoC mRNA expression when transfected two or even three times at 24 h intervals.
The Rho proteins were detected with western blot, according to divided groups, we analyzed three groups expression of RhoA and RhoC proteins. We used the gray scale as a value to calculate the blot membranes and analyzed with NIH image software.
The shape of cells that permeated artificial basal membrane was dyed. The results showed that the invasion power of SGC7901 cells were on the decline after treatment of Ad- RhoA and RhoC-siRNA, and there were significances between the group of RhoA & C-siRNA and others (P < 0.05, Table 1).
| RhoA & C-siRNA | RhoA -siRNA | RhoC -siRNA | siRNA | LY294002 | |
| Invading cells | 12.64 ± 3.27 | 38.26 ± 14.75 | 35.27 ± 10.94 | 87.38 ± 17.38 | 37.01 ± 12.38 |
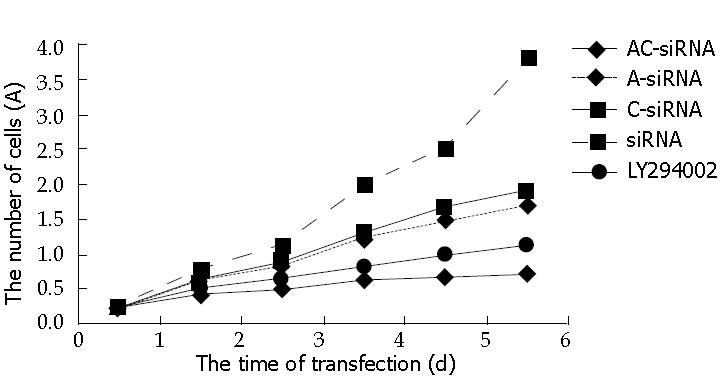
The values of 490 nm wavelength light absorption were different in the five groups. Double tail T-test showed: The number of alive cells in the group of RhoA and RhoC -siRNA was lower than others in the 6th d (P < 0.05), and there was no significance between RhoA-siRNA group and RhoC siRNA group (P > 0.05), but there was significance between RhoA and RhoC-siRNA group and siRNA or LY294002. These data demonstrated that RhoA and RhoC -siRNA can inhibit the growth of tumor (Figure 3). The inhibitory power for combined RhoA and RhoC -siRNA was stronger than RhoA or RhoC-siRNA separately. Also, the growth curve of MTT demonstrated that SGC7901 transfected with RhoA and RhoC-siRNA is more effective than the PI3K inhibitor LY294002.
The apoptosis rate of groups transfected by RhoA and RhoC-siRNA with FACS were (19.07 % ± 1.78%). The LY294002 and control groups were (9.44% ± 1.28%) and (1.23% ± 0.11%). There were significant differences between treated and control groups (P < 0.01), and the LY294002 and control groups (P < 0.05) (Table 2).
| Group | Apoptosis rate (%) | P |
| 1 RhoA,C-siRNA | 19.07 ± 1.78 | Compared with any group (P < 0.01 or P < 0.05) |
| 2 RhoA-siRNA | 7.13 ± 0.21 | Compared with 4 or 5 group (P < 0.05), compared with 3 group (P > 0.05) |
| 3 RhoC-siRNA | 8.11 ± 0.17 | Compared with 4 or 5 group (P < 0.05) |
| 4 siRNA | 1.23 ± 0.11 | Compared with any group (P < 0.01 or P < 0.05) |
| 5 LY294002 | 9.44 ± 1.28 | Compared with 4 group (P < 0.01) |
Transplantation tumor experiments in BALB/C nude mice showed that intratumoral injection of Anti-RhoA or Anti-RhoC siRNA inhibits tumor growth (Table 3).
| Group | Volume after 2 wk (mm3) | t | P | Weight after 2 wk (g) | t | P | Rate of inhibition (%) | |
| Volume | Weight | |||||||
| RhoA & C-siRNA | 37.7 ± 5.65 | 7.216 | 0.001 | 0.245 ± 0.116 | 4.121 | 0.013 | 51.7 | 56.8 |
| RhoA-siRNA | 67.7 ± 2.12 | 4.776 | 0.003 | 0.311 ± 0.123 | 3.011 | 0.013 | 27.2 | 21.3 |
| RhoC-siRNA | 65.7 ± 5.78 | 4.346 | 0.003 | 0.323 ± 0.121 | 3.012 | 0.013 | 25.7 | 25.8 |
| siRNA | 97.7 ± 3.33 | 0.555 ± 0.132 | 0.013 | 7.1 | 2.3 | |||
| LY294002 | 49.7 ± 4.21 | 6.116 | 0.003 | 0.297 ± 0.161 | 3.453 | 0.013 | 29.3 | 23.4 |
1 × 107 SGC7901 cells were inoculated into on the back of BALB/C nude mice, after 21 d, the long-diameter (a) and short-diameter (b) were measured with sliding caliper. The volume was measured with Formula (V = 1/2a × b2). When the tumors were as large as 50-70 mm3, the nude mice were divided into 4 groups: RhoA and RhoC-siRNA, LY294002, siRNA and 0.9% NS were intratumoral injected every 3 d. The concentrations were 80 nmol/L.
Expression of RhoA and RhoC were found in the tissue of gastric cancer. Both RhoA and RhoC genes are known to play a very important role in growth and metastasis, and there have been reports that RhoA and RhoC are related to advanced gastric cancer[9,10].
The Rho-related members, RhoA and RhoC share high sequence identity. Rho family proteins are prominent members of the well-known Ras super family of small GTPases that can cycle between inactive GDP-bound state and active GTP-bound state and that exhibit intrinsic GTPase activities. These GTPases have been implicated in the progression of tumors from a broad range of cellular origins, and analyses at both the RNA and protein level have correlated their increased expression with tumor progression. Several Rho GTPases have been shown to regulate diverse signal transduction pathways and are involved in a variety of biological processes, including cell morphology, motility, proliferation, and apoptosis[11-13].
It has been known that gastric carcinogenesis is a multi-step process with morphological progression involving multiple genetic and epigenetic events. The results of studies about RhoA and RhoC showed that RhoA and RhoC is over expressed in gastric cancers and suggest that Rho-protein dependent cell signaling may be important for gastric carcinogenesis transformation; it therefore might be anticipated that inhibition of Rho-protein synthesis could provide an effective means for inhibiting cancer cell proliferation and invasiveness[14,15].
Rho protein up-regulation or overexpression correlates with poor prognosis in gastric cancer patients[16,17]. Rho kinases (ROCKs) are protein serine/threonine kinases and are down-stream effector molecules of Rho. Rho GTPases, including RhoA and RhoC are highly expressed in a variety of human cancers, including gastric cancer. RhoA-GDPases became activated RhoA- GTPases in cancerous cells[18,19]. Rho-kinase (also termed ROCK) is involved in the activity of cancer via invasion and metastasis through Rho/ROCK pathway[20,21].
Deletion or down-regulation of tumor related genes plays an important role in the multiple steps of tumorigenesis and progression of gastric carcinoma. We used gastric carcinoma cells and RNA interference (iRNA) approach to analyze the functions of RhoA and RhoC, two Rho isoforms that share high sequence identity. What we have discovered indicates that RhoA impedes the migration and invasion of gastric carcinoma cells, a function distinct from RhoC[22,23].
The regulation of RhoA and RhoC activation and the mechanisms by which these GTPases influence cell functions differently are two key issues that need to be addressed. The fact that the RhoA and RhoC sequences differ significantly only in their COOH-termini (25) arguing that the opposing effects of these two isoforms on invasion resides in these COOH sequences[24]. The siRNA cells we have generated should be quite useful for assessing this hypothesis. For example, expression of COOH-terminal mutants of RhoC in the RhoC siRNA cells should facilitate the identification of specific RhoC amino acids that contribute to an invasive phenotype[13,25].
The present study suggests that inhibitor expression of RhoA and RhoC is common and can inhibit tumor progression in some carcinomas. However, RNA interference (iRNA) of RhoA and RhoC in progression of gastric cancer was not reported[26]. We have shown the successful application of iRNA to assess the functions of RhoA and RhoC in invasive breast carcinoma cells[27].
As a result, RNA interference of RhoA and RhoC demonstrated that they are able to inhibit the function of Rho and Rock protein. The results of numerous studies support the hypothesis that inhibition of Rho-dependent cell signaling could provide an effective means for the prevention of invasion and metastasis[28,29].
According to some reports, the RhoA siRNA have been related to cancer cell aggressiveness. The RhoA siRNA can inhibit both proliferation and invasion of tumor cells, and RhoC siRNA maybe be expected to decrease secretion of angiogenic factors, leading to reduced angiogenesis. We combined RhoA siRNA with RhoC siRNA to result in synergism functions in our studies.
LY294002, as an inhibitor of the PI3K/Akt pathway, can exert anti-carcinoma effects on invasion and metastasis of the cells through PI3K/Akt pathway. We used LY294002 as a positive control[30]. Our data demonstrate that RhoA and RhoC siRNAs are more effective than the classical Rho- dependent signaling inhibitors at blocking cancer cell aggression. Indeed, The mechanisms responsible for this resistance to LY294002 is not yet known, but can perhaps be explained by the fact that the product of the Ki-ras proto-oncogene remains prenylated in FTI-treated but not in GGTI-treated cells. Compared to RhoA or RhoC siRNA, probably reflects the former lack of specificity, since GGTI also inhibits the prenylation of several other proteins[31].
Boyden chamber[32] has been regarded as method of evaluation of invasion power of cells in vitro. The number of invasive SGC7901 cells was significantly inhibited after being infected with a RhoA siRNA combined with RhoC siRNA. These results imply that gene therapy mediated by adenovirus may be useful for inhibiting growth and inducing anti-invasion of SGC7901 cells.
In summary, combined RhoA and RhoC siRNA transfections can down-regulate the corresponding mRNA and protein levels in SGC7901 cells. RhoA and RhoC siRNA gene therapy mediated by adenovirus may be useful for inhibiting growth, anti-invasion and metastasis of SGC7901 cells through the PI3K/Akt pathway. These results provide a novel therapeutic target in preventing cancer cell invasion and metastasis.
We thank Professor Shi-Lun Tong, He Jie, Li Zou, Shu-Jing Ma and Department of Gastrointestinal Surgery of Renmin Hospital of Wuhan University for its excellent assistance throughout this investigation.
S- Editor Liu Y L- Editor Alpini GD E- Editor Lu W
| 1. | Lin MT, Lin BR, Chang CC, Chu CY, Su HJ, Chen ST, Jeng YM, Kuo ML. IL-6 induces AGS gastric cancer cell invasion via activation of the c-Src/RhoA/ROCK signaling pathway. Int J Cancer. 2007;120:2600-2608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Xu J, Langefeld CD, Zheng SL, Gillanders EM, Chang BL, Isaacs SD, Williams AH, Wiley KE, Dimitrov L, Meyers DA. Interaction effect of PTEN and CDKN1B chromosomal regions on prostate cancer linkage. Hum Genet. 2004;115:255-262. [PubMed] [Cited in This Article: ] |
| 3. | Suto T, Sugai T, Habano W, Uesugi N, Kanno S, Saito K, Nakamura S. Allelotype analysis of the PTEN, Smad4 and DCC genes in biliary tract cancer. Anticancer Res. 2002;22:1529-1536. [PubMed] [Cited in This Article: ] |
| 4. | Liu N, Bi F, Pan Y, Sun L, Xue Y, Shi Y, Yao X, Zheng Y, Fan D. Reversal of the malignant phenotype of gastric cancer cells by inhibition of RhoA expression and activity. Clin Cancer Res. 2004;10:6239-6247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Castiblanco GA, Pires NY, Wistuba OI, Riquelme SE, Andrade ML, Corvalán RA. Pathogenic role of PTEN tumor suppressor gene in ovarian cancer associated to endometriosis. Rev Med Chil. 2006;134:271-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Bilbao C, Rodríguez G, Ramírez R, Falcón O, León L, Chirino R, Rivero JF, Falcón O, Díaz-Chico BN, Díaz-Chico JC. The relationship between microsatellite instability and PTEN gene mutations in endometrial cancer. Int J Cancer. 2006;119:563-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Wang ZN, Xu HM, Jiang L, Zhou X, Lu C, Zhang X. Positive association of RhoC gene overexpression with tumour invasion and lymphatic metastasis in gastric carcinoma. Chin Med J (Engl). 2005;118:502-504. [PubMed] [Cited in This Article: ] |
| 8. | Kondo T, Sentani K, Oue N, Yoshida K, Nakayama H, Yasui W. Expression of RHOC is associated with metastasis of gastric carcinomas. Pathobiology. 2004;71:19-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Pan Q, Bao LW, Teknos TN, Merajver SD. Targeted disruption of protein kinase C epsilon reduces cell invasion and motility through inactivation of RhoA and RhoC GTPases in head and neck squamous cell carcinoma. Cancer Res. 2006;66:9379-9384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Faried A, Faried LS, Kimura H, Nakajima M, Sohda M, Miyazaki T, Kato H, Usman N, Kuwano H. RhoA and RhoC proteins promote both cell proliferation and cell invasion of human oesophageal squamous cell carcinoma cell lines in vitro and in vivo. Eur J Cancer. 2006;42:1455-1465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Bellovin DI, Simpson KJ, Danilov T, Maynard E, Rimm DL, Oettgen P, Mercurio AM. Reciprocal regulation of RhoA and RhoC characterizes the EMT and identifies RhoC as a prognostic marker of colon carcinoma. Oncogene. 2006;25:6959-6967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Erschbamer MK, Hofstetter CP, Olson L. RhoA, RhoB, RhoC, Rac1, Cdc42, and Tc10 mRNA levels in spinal cord, sensory ganglia, and corticospinal tract neurons and long-lasting specific changes following spinal cord injury. J Comp Neurol. 2005;484:224-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Pillé JY, Denoyelle C, Varet J, Bertrand JR, Soria J, Opolon P, Lu H, Pritchard LL, Vannier JP, Malvy C. Anti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Mol Ther. 2005;11:267-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Simpson KJ, Dugan AS, Mercurio AM. Functional analysis of the contribution of RhoA and RhoC GTPases to invasive breast carcinoma. Cancer Res. 2004;64:8694-8701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 2004;301:43-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 359] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 16. | Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3598] [Cited by in F6Publishing: 3571] [Article Influence: 162.3] [Reference Citation Analysis (0)] |
| 17. | Kandpal RP. Rho GTPase activating proteins in cancer phenotypes. Curr Protein Pept Sci. 2006;7:355-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Meili R, Sasaki AT, Firtel RA. Rho Rocks PTEN. Nat Cell Biol. 2005;7:334-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Zhang Y, Zhang B. D4-GDI, a Rho GTPase regulator, promotes breast cancer cell invasiveness. Cancer Res. 2006;66:5592-5598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Walker K, Olson MF. Targeting Ras and Rho GTPases as opportunities for cancer therapeutics. Curr Opin Genet Dev. 2005;15:62-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Malliri A, Collard JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Horiuchi A, Imai T, Wang C, Ohira S, Feng Y, Nikaido T, Konishi I. Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab Invest. 2003;83:861-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 179] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Arthur WT, Ellerbroek SM, Der CJ, Burridge K, Wennerberg K. XPLN, a guanine nucleotide exchange factor for RhoA and RhoB, but not RhoC. J Biol Chem. 2002;277:42964-42972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Yao H, Dashner EJ, van Golen CM, van Golen KL. RhoC GTPase is required for PC-3 prostate cancer cell invasion but not motility. Oncogene. 2006;25:2285-2296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Lin M, DiVito MM, Merajver SD, Boyanapalli M, van Golen KL. Regulation of pancreatic cancer cell migration and invasion by RhoC GTPase and caveolin-1. Mol Cancer. 2005;4:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Kleer CG, Zhang Y, Pan Q, Gallagher G, Wu M, Wu ZF, Merajver SD. WISP3 and RhoC guanosine triphosphatase cooperate in the development of inflammatory breast cancer. Breast Cancer Res. 2004;6:R110-R115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Ikoma T, Takahashi T, Nagano S, Li YM, Ohno Y, Ando K, Fujiwara T, Fujiwara H, Kosai K. A definitive role of RhoC in metastasis of orthotopic lung cancer in mice. Clin Cancer Res. 2004;10:1192-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Shinto E, Tsuda H, Matsubara O, Mochizuki H. Significance of RhoC expression in terms of invasion and metastasis of colorectal cancer. Nihon Rinsho. 2003;61 Suppl 7:215-219. [PubMed] [Cited in This Article: ] |
| 29. | van Golen KL, Bao L, DiVito MM, Wu Z, Prendergast GC, Merajver SD. Reversion of RhoC GTPase-induced inflammatory breast cancer phenotype by treatment with a farnesyl transferase inhibitor. Mol Cancer Ther. 2002;1:575-583. [PubMed] [Cited in This Article: ] |
| 30. | van Golen KL, Bao LW, Pan Q, Miller FR, Wu ZF, Merajver SD. Mitogen activated protein kinase pathway is involved in RhoC GTPase induced motility, invasion and angiogenesis in inflammatory breast cancer. Clin Exp Metastasis. 2002;19:301-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | van Golen KL, Wu ZF, Qiao XT, Bao LW, Merajver SD. RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 2000;60:5832-5838. [PubMed] [Cited in This Article: ] |
| 32. | Schichor C, Kerkau S, Visted T, Martini R, Bjerkvig R, Tonn JC, Goldbrunner R. The brain slice chamber, a novel variation of the Boyden Chamber Assay, allows time-dependent quantification of glioma invasion into mammalian brain in vitro. J Neurooncol. 2005;73:9-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |