Published online Jun 21, 2007. doi: 10.3748/wjg.v13.i23.3189
Revised: April 10, 2007
Accepted: April 16, 2007
Published online: June 21, 2007
AIM: To distinguish subtypes of gastric signet ring cell (SRC) carcinoma by investigating the expression of gastric and intestinal phenotypic markers, and to study the significance of phenotypic classification in predicting tumor progression and outcome.
METHODS: Immunohistochemistry was performed in 66 cases of SRC carcinoma with MUC2, VILLIN, CDX2, Li-cadherin antibodies as intestinal phenotype markers and MUC5AC, HGM, MUC6 antibodies as gastric phenotype markers, and the relationship was analyzed between the phenotypic expression pattern and clinicopathologic parameters, as well as the 3-year survival rate.
RESULTS: Expression of intestinal phenotypic markers was positively associated with tumor size, wall invasion, vascular invasion, lymph node metastasis and tumor-node-metastasis (TNM) stage. Cases expressing one or more intestinal markers had a significant lower survival rate than cases expressing none of the intestinal markers.
CONCLUSION: The SRC carcinomas expressing intestinal phenotype markers exhibited a high pro-liferative potential, bad biological behaviors and poor prognosis. Examination of phenotype expression may be useful in distinguishing histological type and in predicting the prognosis of gastric SRC carcinoma.
- Citation: Tian MM, Zhao AL, Li ZW, Li JY. Phenotypic classification of gastric signet ring cell carcinoma and its relationship with clinicopathologic parameters and prognosis. World J Gastroenterol 2007; 13(23): 3189-3198
- URL: https://www.wjgnet.com/1007-9327/full/v13/i23/3189.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i23.3189
Gastric cancer is the fourth most common cancer and the second cause of cancer-related death worldwide[1]. It has been reported that 3.4%-29% of patients with gastric cancer had signet ring cell (SRC) type histology[2-7].
Comparing with other types of gastric cancer, SRC carcinomas have a tendency to appear in young and female patients[2,6,7]. Although most researchers believed that gastric SRC carcinoma is characterized by poor differentiation, strong invasive tendency and poor prognosis, the clinicopathologic parameters of this type of malignancy are still controversial[2,6-8]. Hyung et al[8] reported that patients with early gastric SRC carcinoma have higher survival rates than those with other types of early gastric cancer. Similar result can be seen in Kim et al’s[2] study showing that the 5-year survival rate of SRC gastric carcinoma is higher than other types. In contrast, Yokota et al[6] and Theuer et al[7] respectively found that the 5-year survival rate of gastric SRC carcinoma is significantly lower than other types and the prognosis for gastric carcinomas with SRC components was worse than those without SRC components. The differences in these results suggest that the biological behaviors and prognosis of gastric SRC carcinoma need further investigations.
Gastric carcinomas have been classified into two main subtypes by Lauren et al[9]: intestinal and diffuse types. The intestinal type of gastric cancer is thought to arise from the metaplastic epithelium, whereas the diffuse type is thought to arise from the proper mucosa that is not metaplastic. Gastric SRC carcinoma belonged to diffuse type in Lauren's classification. However, recent reports have shown that a considerable proportion of gastric SRC carcinoma express intestinal phenotype markers such as CDX2 and MUC2[10,11]. Some studies also showed that gastric SRC carcinomas expressing intestinal phenotype had bigger tumor size and deeper wall invasion[10,12,13]. These findings gave us clues that there might be different subtypes of gastric SRC carcinoma with distinct clinico-pathologic parameters and prognosis.
To investigate the phenotypic expression in gastric SRC carcinomas, we chose CDX2, MUC2, VILLIN and Li-cadherin as intestinal phenotype markers. Among them, CDX2 is intestine-specific caudal family homeobox transcription factor which regulate intestinal development and differentiation in mouse model. CDX2 is expressed in epithelial cells of intestine in human adult and ectopically expressed in intestinal metaplasia and intestinal type gastric carcinomas[14]. MUC2 is characteristically expressed in goblet cells of native intestinal epithelium and intestinal metastasis of gastric mucosa, but not normal gastric epithelium[15]. VILLIN is a cytoskeletal protein characterizing the core microfilament bundle of the microvillus of intestinal epithelium[16]. Li-cadherin is a novel member of the cadherin family of cell adhesion molecules which specifically expresses in the epithelia of the liver and intestine of rats. Human Li-cadherin specifically expresses in intestinal epithelium[17]. We chose MUC5AC, MUC6 and HGM as gastric phenotype markers which were commonly used to identify gastric phenotype. MUC5AC and HGM glycoprotein localizes in the cytoplasm of foveolar and mucopeptic neck epithelial cells of gastric mucosa, while MUC6 glycoprotein localize in the cytoplasm of mucopeptic neck cells and pyloric glands of the gastric mucosa[13,18].
We performed an immunohistochemical study in 66 cases of gastric SRC carcinoma, and analyzed the relationship between the phenotypic expression pattern and clinicopathologic parameters, the 3-year survival rate as well as the existence of intestinal metaplasia in the surrounding mucosa. In addition, Ki67, CD44v6, E-cadherin and β-catenin expressions were detected immunohistochemically and their relationship with phenotypic classification, tumor progression and prognosis was analyzed.
A consecutive series of 66 patients with gastric SRC carcinoma were studied. All patients were treated by D2 resection in Beijing Cancer Hospital between May 1994 and July 2004. The criteria of the WHO classification for histological typing of gastric carcinomas was followed: a diagnosis of SRC carcinoma of the stomach was made if an adenocarcinoma contained a predominant component (> 50%) of isolated carcinoma cells with intracellular mucin[19]. There were 32 males and 34 females with a mean age of 53 (range, 29-91) years. Stage of gastric carcinoma was described according to the 1997 tumor-node-metastasis (TNM) classification of malignant tumors by the International Union against Carcinoma. Among the 66 cases, 20 cases showed intestinal metaplasia in the surrounding mucosa in H.E. staining, 38 cases had a minimum 3 years of follow-ups.
Tumor tissues were fixed in 10% formalin and embedded in paraffin. One paraffin-embedded block of tumorous tissue was selected from each case and was cut into 4 μm sections. The sections were put in the oven at 60°C for 4 h, deparaffinized in xylene, rehydrated in a graded ethanol series, and treated with 3% hydrogen peroxide solution for 10 min. Antigen retrieval was done by microwaving tissues in EDTA buffer (pH 8.0) at over 90°C for 10 min, then cooling at room temperature for 30 min. The sections were then incubated with primary antibodies in a appropriate dilutions (Table 1) at 4°C overnight. The primary antibodies were detected using the Powervision two-step histostaining reagent (PV-6001, Dako, Glostrop, Denmark) as the secondary antibody. Finally, the slides were visualized with 3,3’-diaminobenzedine and counterstained with haematoxylin. Positive controls for gastric phenotype markers were normal gastric mucosa; and for intestinal phenotype markers were normal small intestinal mucosa. Negative controls were performed by replacing the primary antibodies with PBS.
| Antibody | Clone | Diluent’sproportion | Company |
| Gastric phenotypic markers | |||
| MUC5AC | CLH2 | 1:100 | Novocastra, Newcastle, UK |
| MUC6 | CLH5 | 1:50 | Novocastra, Newcastle, UK |
| HGM | 45M1 | 1:50 | Novocastra, Newcastle, UK |
| Intestinal phenotypic markers | |||
| MUC2 | Ccp58 | 1:100 | Novocastra, Newcastle, UK |
| CDX2 | CDX2-88 | 1:50 | BioGenex, San Ramon CA |
| Li-cadherin | Clone 141713 | 1:200 | Neomarker, Fremont, CA, USA |
| VILLIN | CWWB1 | 1:200 | Novocastra, Newcastle, UK |
| Other antibodies | |||
| Ki -67 | K-2 | 1:50 | Zymed, San Francisco, CA, USA |
| CD44v6 | VFF-7 | 1:50 | Zymed , San Francisco, CA, USA |
| E-cadherin | 4A2C7 | 1:50 | Zymed , San Francisco, CA, USA |
| β-catenin | CAT-5H10 | 1:30 | Zymed , San Francisco, CA, USA |
Two experienced pathologists independently examined staining, being blind to the clinicopathologic data. At least 10 high-power field at 400 × magnification were chosen randomly and > 1000 carcinoma cells were counted for each section. The positive expressions for MUC2, VILLIN, MUC5AC, MUC6, HGM and Li-cadherin were located in the cytoplasm and cell membrane. CDX2 was located in nucleus. The cases were defined as positive when > 10% tumor cells were positively stained in each section[10,14].
According to the expression of phenotypic markers, tumors were classified into four differentiated phenotypes. Tumors that were positively stained by one or more gastric phenotypic markers, but no intestinal phenotypic marker, were classified as G type; those stained by one or more intestinal phenotypic markers, but no gastric marker ,were classified as I type; those positively stained by both gastric and intestinal phenotypic markers were GI (mixed) type; and those stained by none of the phenotypic markers were regarded as carcinomas of the UC (unclassified) type.
Ki67 labeling index was defined as positive nuclear stained cells ratio after counting 1000 cancer cells in 5-10 high power field. E-cadherin and β-catenin showed staining in cell membranes or ectopic staining in cell plasma/nucleus, more than 10% cancer cells stained in one slide was defined as weak positive, and more than 50% cancer cells stained as positive. Positive CD44v6 stain was defined if more than 25% cancer cells were stained in cell plasma and membrane[20].
All data were analysed using SPSS10.0 software. The association of antibody expression with various clinicopathologic parameters was analyzed using the t test, χ2 test, 2-sided Fisher’s test and Spearman’s rank correlation analysis. Cumulative survival was estimated by the Kaplan-Meier method and differences between survival curves were analyzed by the log-rank test. The influence of each variable in survival was analyzed by the multivariate analysis of Cox proportional hazard model (backward, stepwise). P≤ 0.05 was considered statistically significant.
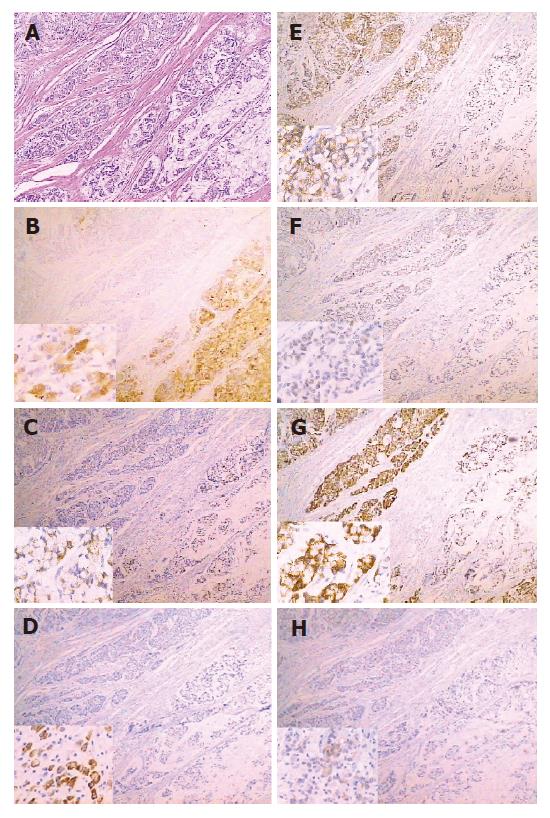
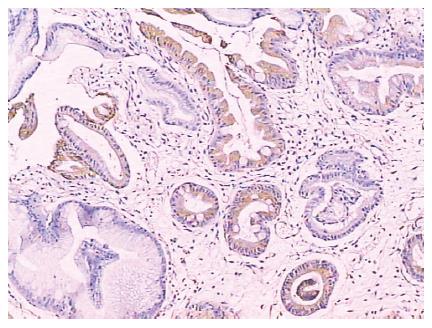
Both gastric and intestinal markers showed a heterogeneous staining pattern (Figure 1). The positive rates of gastric phenotypic markers MUC5AC, MUC6 and HGM expression in 66 cases were 56.1% (37/66), 10.6% (7/66) and 66.7% (44/66) respectively, while the positive rates of intestinal phenotypic markers MUC2, VILLIN, CDX2 and Li-cadherin were 48.5% (32/66), 7.6% (5/66), 21.2% (14/66) and 36.4% (24/66), respectively. The expression pattern of each case is shown in Table 2.
| Phenotype | Age/Gender | MUC2 | Li-cadherin | CDX2 | VILLIN | HGM | MUC5AC | MUC6 |
| G type (n = 17) | ||||||||
| 1 | 65/F | - | - | - | - | + | + | + |
| 2 | 60/M | - | - | - | - | + | + | - |
| 3 | 67/F | - | - | - | - | + | + | - |
| 4 | 29/F | - | - | - | - | + | + | + |
| 5 | 66/F | - | - | - | - | + | + | + |
| 6 | 49/M | - | - | - | - | - | + | - |
| 7 | 49/M | - | - | - | - | + | + | - |
| 8 | 69/F | - | - | - | - | + | + | - |
| 9 | 33/F | - | - | - | - | + | + | - |
| 10 | 38/F | - | - | - | - | - | + | - |
| 11 | 35/F | - | - | - | - | + | + | - |
| 12 | 56/M | - | - | - | - | + | + | - |
| 13 | 39/M | - | - | - | - | - | + | - |
| 14 | 70/F | - | - | - | - | + | + | - |
| 15 | 42/M | - | - | - | - | + | + | - |
| 16 | 42.M | - | - | - | - | - | + | - |
| 17 | 66/M | - | - | - | - | + | - | - |
| I type (n = 10) | ||||||||
| 1 | 63/M | + | + | + | - | - | - | - |
| 2 | 75/M | + | + | + | - | - | - | - |
| 3 | 59/F | + | + | + | - | - | - | - |
| 4 | 60/M | + | - | - | - | - | - | - |
| 5 | 31/F | + | - | - | - | - | - | - |
| 6 | 76/F | + | - | - | - | - | - | - |
| 7 | 73/F | + | - | - | + | - | - | - |
| 8 | 46/F | + | + | - | - | - | - | - |
| 9 | 62/M | - | + | - | + | - | - | - |
| 10 | 41/M | - | + | - | - | - | - | - |
| GI type (n = 31) | ||||||||
| 1 | 31/M | + | - | - | - | + | + | - |
| 2 | 69/F | + | - | - | - | + | + | - |
| 3 | 61/M | + | - | + | - | + | + | + |
| 4 | 67/M | + | - | - | - | + | + | + |
| 5 | 56/M | + | + | + | - | + | + | - |
| 6 | 47/F | + | + | - | - | + | + | - |
| 7 | 65/M | + | + | - | - | + | + | - |
| 8 | 64/M | + | - | - | - | + | + | - |
| 9 | 63/M | + | + | + | - | + | + | - |
| 10 | 48/F | + | + | - | - | + | + | + |
| 11 | 52/F | + | - | - | - | + | + | - |
| 12 | 65/M | + | + | + | - | + | + | - |
| 13 | 72/M | + | + | - | - | + | + | - |
| 14 | 39/F | + | + | + | - | + | + | - |
| 15 | 36/M | + | + | + | + | + | + | - |
| 16 | 51/M | + | + | - | - | + | + | - |
| 17 | 60/F | + | + | - | - | + | - | - |
| 18 | 46/F | + | - | - | - | + | - | - |
| 19 | 45/F | + | - | - | - | + | - | - |
| 20 | 45/F | + | - | + | - | + | - | - |
| 21 | 55/M | + | + | + | - | + | - | - |
| 22 | 65/F | + | - | - | - | + | - | - |
| 23 | 47/F | + | - | - | - | + | - | - |
| 24 | 60/F | + | + | - | - | + | - | - |
| 25 | 46/F | - | - | + | - | + | + | + |
| 26 | 50/F | - | + | - | - | + | + | - |
| 27 | 54/F | - | + | + | - | + | + | - |
| 28 | 32/M | - | - | - | + | + | + | - |
| 29 | 55/M | - | + | + | + | + | + | - |
| 30 | 38/F | - | + | - | - | + | - | - |
| 31 | 59/M | - | + | - | - | + | - | - |
| U type (n = 8) | ||||||||
| 1 | 36/M | - | - | - | - | - | - | - |
| 2 | 49/F | - | - | - | - | - | - | - |
| 3 | 39/M | - | - | - | - | - | - | - |
| 4 | 61/F | - | - | - | - | - | - | - |
| 5 | 70/F | - | - | - | - | - | - | - |
| 6 | 67/M | - | - | - | - | - | - | - |
| 7 | 64/M | - | - | - | - | - | - | - |
| 8 | 34/F | - | - | - | - | - | - | - |
According to the expression of phenotype markers, we classified the 66 cases of SRC carcinomas into four phenotypes: 17 cases (25.8%) were G type, 10 cases (15.2%) were I type, 31 cases (47.0%) were GI type and 8 cases (12.1%) were UC type.
Based on the number of positive markers, G type cases can be divided into three subgroups G1-G3 (Table 3). Five cases (29.4%) showed only one positive marker among MUC5AC, MUC6 and HGM, 9 cases (52.9%) showed two positive markers and 3 cases (17.6%) expressed all three gastric markers.
| β-catenin | Plasma/Nucleusβ-cateninexpression | |||||
| Phenotype of gastricSRC carcinomas | Number ofpositive GPM | Number ofpositive IPM | Cases | Ki67 index> 25% | membraneexpression | |
| G type | 17 | 2 (11.8%) | 11 (64.7%) | 2 (11.8%) | ||
| G1 | 1 | 0 | 5 | 0 | 3 (60.0%) | 0 |
| G2 | 2 | 0 | 9 | 2 (22.2%) | 6 (66.7%) | 1 (11.1%) |
| G3 | 3 | 0 | 3 | 0 | 2 (66.7%) | 1 (33.3%) |
| I type | 10 | 5 (50.0%) | 1 (10.0%)b | 4 (40.0%) | ||
| I1 | 0 | 1 | 4 | 1 (25.0%) | 0 | 1 (25.0%) |
| I2 | 0 | 2 | 3 | 3 (100%) | 0 | 2 (66.7%) |
| I3 | 0 | 3 | 3 | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) |
| GI type | 31 | 15 (48.4%)a | 12 (36.4%) | 14 (45.2%)a | ||
| GI1 | 1 | 1 | 6 | 3 (50.0%) | 2 (33.3%) | 3 (50.0%) |
| GI2 | 1 | 2 | 3 | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) |
| GI3 | 1 | 3 | 1 | 0 | 0 | 1 (100%) |
| GI4 | 2 | 1 | 6 | 3 (50.0%) | 3 (50.0%) | 3 (50.0%) |
| GI5 | 2 | 2 | 5 | 4 (80.0%) | 4 (80.0%) | 2 (40.0%) |
| GI6 | 2 | 3 | 5 | 2 (40.0%) | 1 (20.0%) | 1 (20.0%) |
| GI7 | 2 | 4 | 1 | 0 | 0 | 1 (100%) |
| GI8 | 3 | 1 | 2 | 1 (50.0%) | 1 (50.0%) | 1 (50.0%) |
| GI9 | 3 | 2 | 2 | 1 (50.0%) | 0 | 1 (50.0%) |
| U type | 0 | 0 | 8 | 3 (37.5%) | 4 (50.0%) | 3 (37.5%) |
I type cases also can be divided into three subgroups I1-I3 based on the number of positive markers. Four cases (40%) expressed only one of the four intestinal phenotypic markers MUC2, VILLIN, CDX2 and Li-cadherin, 3 cases (30%) showed two positive markers and 3 (30%) expressed three markers, and no case expressed all intestinal markers in I type.
There were nine expression patterns GI1-GI9 in GI type cases according to the expression of seven phenotypic markers. Among these subgroups, the cases expressing one gastric marker with one intestinal marker and the cases expressing two gastric markers together with one intestinal marker were most frequently seen, both were 19.4% (6/31). The second common situations were two positive gastric markers coexisting with two or three positive intestinal markers, both being 16.1% (5/31). The other five expression patterns were less common (9 cases or 29.0%). No case expressed three gastric markers together with more than three intestinal markers or one gastric marker together with four intestinal markers.
Among 66 cases, 22 (33.3%) showed Ki67 labeling index of less than 5%, 18 (27.3%) between 6%-25%, 15 (22.7%) between 26%-50% and 11 (16.7%) > 50%. The proportion of cases with Ki67 labeling index > 25% was 11.8% (2/17) in G type, 50.0% (5/10) in I type, 48.4% (15/31) in GI type and 37.5% (3/8) in UC type (Table 3). χ2 test showed that the proportion of cases with Ki67 labeling index > 25% was significantly lower in G type than in GI type, P = 0.011. Compared with G type, the proportion of cases with Ki67 labeling index > 25% was higher in I type, but the difference was not significant after 2-sided Fisher's test, P = 0.065. No difference was found between I type and GI type or UC type and other three types. Compared with G + UC type (cases expressed none of the intestinal markers), GI + I type (cases expressed one or more intestinal markers) had significantly higher proportion of cases with Ki67 labeling index > 25%, P = 0.012.
β-catenin stained extensively in positive cases of gastric SRC carcinomas. In 28 cases (42.4%), β-catenin mainly expressed on membrane, and 23 cases (34.8%) were mainly stained in plasma/nucleus (Figure 2). Both membrane and plasma/nucleus stained cases accounted for 13.6% (9/66) while negative stained cases comprised 36.4% (24/66) (Table 3). Significant difference of β-catenin plasma/nucleus staining was found between GI + I type and G + UC type (43.9% vs 20.0% P = 0.048). The proportion of cases with Ki67 labeling index > 25% was significantly higher in β-catenin plasma/nucleus positive cases than in plasma/nucleus negative cases (56.5% vs 30.2%, P = 0.037).
The positive rate of CD44v6 in 66 gastric SRC carcinomas was 7.6% (5/66), two CD44v6 positive cases were G type, three were GI type and one belonged to I type. Only 2 cases (3.0%) in this study showed weakly stained E-cadherin, including one G type case and one GI type case. No difference was found between CD44v6, E-cadherin expression and phenotypes.
MUC2 (+) cases had a significantly higher lymph node metastasis rate (75.0% vs 38.2%) and vascular invasion rate (59.4% vs 20.6%) than MUC2 (-) cases (P = 0.003 and 0.001 respectively), (Table 4). The expression of MUC2 was also significantly higher in cases with larger tumor diameter (≥ 5 cm), deeper wall invasion (T2 + T3 + T4) and higher TNM stage (III + IV) (P = 0.001, 0.008 and 0.007, respectively). No significant correlation was found between MUC2 expression and gender, age and distant metastasis. There was significantly higher CDX2 expression in cases with larger tumor diameter (≥ 5 cm) (P < 0.01), and higher Li-cadherin expression in vascular invaded cases (P < 0.01). The expression of CDX2 and Li-cadherin was not found to correlate with other clinicopathologic parameters. No significant association was observed between VILLIN, MUC5, MUC6, HGM expression and any of the clinicopathologic parameters.
| MUC2 expression | CDX2 expression | Li-cadherin expression | Ki-67 index | ||||||||||
| Factors | Cases | MUC2-(n = 34) | MUC2+(n = 32) | P | CDX2-(n = 52) | CDX2+(n = 14) | P | Li-cad-(n = 42) | Li-cad+(n = 24) | P | ≤ 25%(n = 40) | > 25%(n = 26) | P |
| Gender | |||||||||||||
| Male | 32 | 17 (50.0) | 15 (46.9) | > 0.05 | 23 (44.2) | 9 (64.3) | > 0.05 | 18 (42.9) | 14 (58.3) | > 0.05 | 18 (45.0) | 14 (53.8) | > 0.05 |
| Female | 34 | 17 (50.0) | 17 (53.1) | 29 (55.8) | 5 (35.7) | 24 (57.1) | 10 (41.7) | 22 (55.0) | 12 (46.2) | ||||
| Age (yr) | |||||||||||||
| Mean ± SE | 66 | 50.9 ± 2.2 | 56.0 ± 2.2 | > 0.051 | 53.5 ± 1.9 | 52.9 ± 3.1 | > 0.051 | 51.9 ± 3.0 | 54.6 ± 2.6 | > 0.051 | 53.9 ± 2.2 | 51.0 ± 2.3 | > 0.051 |
| Tumor diameter | |||||||||||||
| < 5.0 cm | 32 | 23 (67.6) | 9 (28.1) | 0.001 | 30 (57.7) | 2 (14.3) | 0.004 | 24 (57.1) | 8 (33.3) | > 0.05 | 24 (60.0) | 8 (30.8) | 0.02 |
| ≥ 5.0 cm | 34 | 11 (32.4) | 23 (71.9) | 22 (42.3) | 12 (85.7) | 18 (42.9) | 16 (66.7) | 16 (40.0) | 18 (69.2) | ||||
| Depth of wall invasion | |||||||||||||
| T1 | 13 | 11 (32.4) | 2 (6.3) | 0.0082 | 13 (25.0) | 0 | > 0.05 | 10 (23.8) | 3 (12.5) | > 0.05 | 11 (27.5) | 2 (7.7) | 0.0482 |
| T2 | 22 | 10 (29.4) | 12 (37.5) | 16 (30.8) | 6 (42.9) | 15 (35.7) | 7 (29.2) | 14 (35.0) | 8 (30.8) | ||||
| T3 | 22 | 10 (29.4) | 12 (37.5) | 15 (28.8) | 7 (50.0) | 11 (26.2) | 11 (45.8) | 10 (25.0) | 12 (46.2) | ||||
| T4 | 9 | 3 (8.8) | 6 (18.8) | 8 (15.4) | 1 (7.1) | 6 (14.3) | 3 (12.5) | 5 (12.5) | 4 (15.4) | ||||
| Lymph node metastasis | |||||||||||||
| LN (–) | 29 | 21 (61.8) | 8 (25.0) | 0.003 | 25 (48.1) | 4 (28.6) | > 0.05 | 20 (47.6) | 9 (37.5) | > 0.05 | 22 (55.0) | 7 (26.9) | 0.025 |
| LN (+) | 37 | 13 (38.2) | 24 (75.0) | 27 (51.9) | 10 (71.4) | 22 (52.4) | 15 (62.5) | 18 (45.0) | 19 (70.1) | ||||
| Distant metastasis | |||||||||||||
| M0 | 60 | 32 (94.1) | 28 (87.5) | > 0.05 | 48 (92.3) | 12 (85.7) | > 0.05 | 39 (92.9) | 21 (87.5) | > 0.05 | 36 (90.0) | 24 (92.3) | > 0.05 |
| M1 | 6 | 2 (5.9) | 4 (12.5) | 4 (7.7) | 2 (14.3) | 3 (7.1) | 3 (12.5) | 4 (10.0) | 2 (7.7) | ||||
| Vascular invasion | |||||||||||||
| V (–) | 40 | 27 (79.4) | 13 (40.6) | 0.001 | 34 (65.4) | 6 (42.9) | > 0.05 | 31 (73.8) | 9 (37.5) | 0.004 | 28 (70.0) | 12 (46.2) | > 0.05 |
| V (+) | 26 | 7 (20.6) | 19 (59.4) | 18 (34.6) | 8 (57.1) | 11 (26.2) | 15 (62.5) | 12 (30.0) | 14 (53.8) | ||||
| TNM stage | |||||||||||||
| I+ II | 32 | 22 (64.7) | 10 (31.2) | 0.007 | 27 (51.9) | 5 (35.7) | > 0.05 | 21 (50.0) | 11 (45.8) | > 0.05 | 24 (60.0) | 8 (30.8) | 0.02 |
| III + IV | 34 | 12 (35.3) | 22 (68.8) | 25 (48.1) | 9 (64.3) | 21 (50.0) | 13 (54.2) | 16 (40.0) | 18 (69.2) | ||||
The relationship between phenotypic classification and clinicopathologic parameters is shown in Table 5. The proportions of positive lymph node metastatic cases in four phenotypes were 23.5% (4/17) in G type, 60.0% (6/10) in I type, 74.2% (23/31) in GI type and 50.0% (4/8) in UC type, respectively. The frequency of vascular invasion was 11.8% (2/17) in G type, 30.3% (3/10) in I type, 61.3% (19/31) in GI type and 25.0% (2/8) in UC type. G phenotype cases had significantly lower rates of lymph node metastasis and vascular invasion than other phenotypes (both P < 0.01). More cases of GI type SRC carcinomas had tumor diameters ≥ 5 cm (67.7% vs 11.8%, P < 0.001), wall invasion deeper than submucosa layer (96.8% vs 58.8%, P = 0.002) and higher (III + IV) TNM stage (63.1% vs 29.1%, P = 0.035) than G type cases. I type also had more cases with tumor diameters ≥ 5cm than G type (70.0% vs 11.8%, P = 0.002). No difference was found between UC type and other three types. Phenotypic classification had no correlation with patient’s gender, age and distant metastasis.
| Four phenotypes of gastric SRC carcinoma | |||||
| Factors | n | G type (n = 17) | I type (n = 10) | GI type (n = 31) | UC type (n = 8) |
| Gender | |||||
| Male | 32 | 8 (47.1) | 5 (50.0) | 15 (48.4) | 4 (50.0) |
| Female | 34 | 9 (52.9) | 5 (50.0) | 16 (51.6) | 4 (50.0) |
| Age (yr) | |||||
| Mean ± SE | 66 | 53.7 ± 2.9 | 49.1 ± 4.0 | 54.7 ± 2.5 | 52.8 ± 4.3 |
| Tumor diameter | |||||
| < 5.0 cm | 32 | 15 (88.2)a | 3 (30.0)b | 10 (32.3) | 4 (50.0) |
| ≥ 5.0 cm | 34 | 2 (11.8)a | 7 (70.0)b | 21 (67.7) | 4 (50.0) |
| Depth of wall invasion | |||||
| T1 | 13 | 7 (41.2)c | 3 (30.0) | 1 (3.2) | 2 (25.0) |
| T2 | 22 | 5 (29.1)c | 2 (20.0) | 12 (38.7) | 3 (37.5) |
| T3 | 22 | 4 (23.5)c | 2 (20.0) | 13 (41.9) | 3 (37.5) |
| T4 | 9 | 1 (5.9)c | 3 (30.0) | 5 (16.1) | 0 |
| Lymph node metastasis | |||||
| LN (–) | 29 | 13 (76.5)c | 4 (40.0) | 8 (25.8) | 4 (50.0) |
| LN (+) | 37 | 4 (23.5)c | 6 (60.0) | 23 (74.2) | 4 (50.0) |
| Distant metastasis | |||||
| M0 | 60 | 16 (94.1) | 8 (80.0) | 28 (90.3) | 8 (100) |
| M1 | 6 | 1 (5.9) | 2 (20.0) | 3 (9.7) | 0 |
| Vascular invasion | |||||
| V (–) | 40 | 15 (88.2)c | 7 (70.0) | 12 (38.7) | 6 (75.0) |
| V (+) | 26 | 2 (11.8)c | 3 (30.0) | 19 (61.3) | 2 (25.0) |
| TNM stage | |||||
| I+ II | 32 | 12 (70.6)d | 4 (40.0) | 12 (38.7) | 4 (50.0) |
| III + IV | 34 | 5 (29.1)d | 6 (60.0) | 19 (61.3) | 4 (50.0) |
| IM | |||||
| IM (–) | 46 | 11 (64.7) | 6 (60.0) | 23 (74.2) | 6 (75.0) |
| IM (+) | 20 | 6 (35.3) | 4 (40.0) | 8 (25.8) | 2 (25.0) |
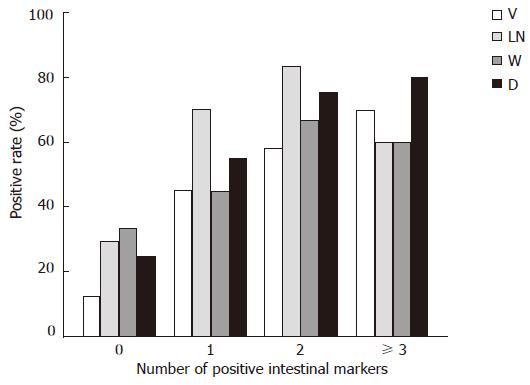
Differences in biological behaviors were observed between G + UC type (cases expressed none of the intestinal markers) and GI + I type (cases expressed one or more intestinal markers), the latter had higher proportion of vascular invasion (53.7% vs 16.0%, P = 0.002) and lymph node metastasis (70.0% vs 32.0%, P = 0.002), larger tumor diameters (68.3% vs 24.0%, P < 0.001), deeper wall invasion (90.2% vs 64.0%, P = 0.023) and higher TNM stage (61.0% vs 36.0%, P = 0.048). Spearman’s rank correlation analysis showed that the number of positive intestinal phenotype markers in one case was positively correlated with higher rates of lymph node metastasis (P < 0.01) and vascular invasion (P < 0.01), larger tumor diameter (P < 0.01) and deeper wall invasion (P < 0.05), (Figure 3).
χ2 test showed that, comparing with the cases with Ki67 labeling index ≤ 25%, the cases with Ki67 labeling index > 25% had a higher rate of lymph node metastasis (70.1% vs 45.0%, P = 0.025), larger tumor diameters (69.2% vs 40.0%, P = 0.020), deeper wall invasion (92.3% vs 72.5%, P = 0.048) and higher TNM stage (69.2% vs 40.0%, P = 0.020), (Table 4). No correlation was found between expression of β-catenin, CD44v6, E-cadherin and clinicopathologic parameters.
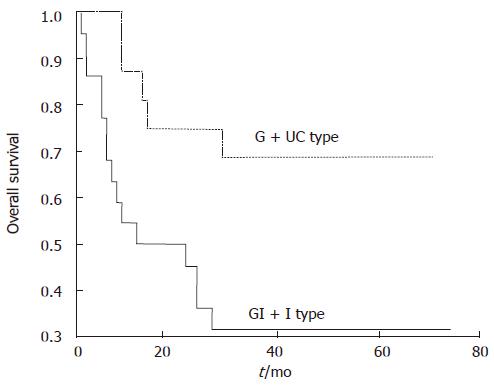
In the 38 cases with 3-year follow-up, vascular invasion, lymph node metastasis, TNM stage and Ki67 labeling index > 25% were found correlated with poor prognosis (P = 0.0001, 0.0010, 0.0012 and 0.0141, respectively). GI + I type had significantly a lower overall survival rate than G + UC type (31.82% vs 68.75%, P = 0.0146 (Figure 4). Cox proportional hazard model (backward, stepwise) showed that vascular invasion and TNM stage were independent prognosis factors (P = 0.017 and 0.037, respectively), while GI + I type, lymph node metastasis and Ki67 labeling index were not independent prognosis factors.
Among 66 SRC carcinomas, 20 cases (30.3%) possessed intestinal metaplasia in the surrounding mucosa in H.E. staining slides. The proportions of metaplastic cases in four phenotypes were between 25%-40%, no significant difference was found (Table 5). Expression of seven intestinal and gastric phenotypic markers was not correlated with metaplasia in the surrounding mucosa (data not shown).
The phenotypic marker expression of tumors is conventionally thought to imitate that of the tissue of origin. In this study, immunohistochemistry showed that three gastric phenotypic markers were positively stained in normal gastric mucosa, but negatively in intestinal mucosa, while four intestinal phenotypic markers were positive in intestinal mucosa and metaplasia of stomach, but negative in normal gastric mucosa. These results indicated that the phenotypic markers in our study possessed tissue specificity.
The expression of MUC2, CDX2 and Li-cadherin was positively associated with tumor growth or invasion, and cases expressing more positive intestinal markers showed higher invasive potential, and cases expressing one or more intestinal markers had a lower survival rate than cases with none of intestinal markers positive. Combined analysis of gastric and intestinal phenotypic markers displayed that cases only expressing gastric markers had better biologic behaviors than the cases expressing both gastric and intestinal phenotype. These results suggest that different expression patterns of phenotype are associated with clinicopathologic parameters and prognosis.
In our study, Ki67 was positively associated with vascular invasion, lymph node metastasis, tumor size and TNM stage. Ki67 is well known to express in all cell cycle phases except in the resting cell (G0 phase). Ki67 immunohistochemistry has been substituted extensively for mitotic counting in assessing tumor cell proliferation[21]. A great deal of studies have demonstrated that Ki67 is correlated with poor differentiation and worse biological behaviors in many malignancies[22-24]. In gastric SRC carcinomas of this study, the intestinal phenotype positive cases (GI + I type) had higher Ki67 labeling index, indicating that these cases had a higher proliferative potential which was concordant with the worse biological behaviors shown by this kind of gastric SRC carcinomas.
β-catenin is a multifunctional protein, which takes part in intercellular adhesion on cell membrane and also plays an important role in Wnt signal pathway. Most β-catenins are located on cell membrane in normal mature cells and mediate cell adhesion by connecting with E-cadherin. If β-catenin accumulates in cytoplasm and translocates to the nucleus, it binds to the Tcf/Lef family of transcription factors, resulting in increased transcription of numerous genes, including c-myc and cyclin D1, and abnormal proliferation[25]. Our finding is consistent with previous reports that plasma/nucleus staining of β-catenin is positively correlated with higher Ki67 labeling index. The intestinal phenotype positive cases with high plasma/nucleus β-catenin staining rates indicated that the high proliferative activity of these cases is associated with activation of Wnt signal pathway. GI type SRC carcinomas had lower β-catenin membrane staining rates than G type, indicating that the higher metastasis ability of GI type may be associated with low cellular adhesion due to reduction of membrane β-catenin.
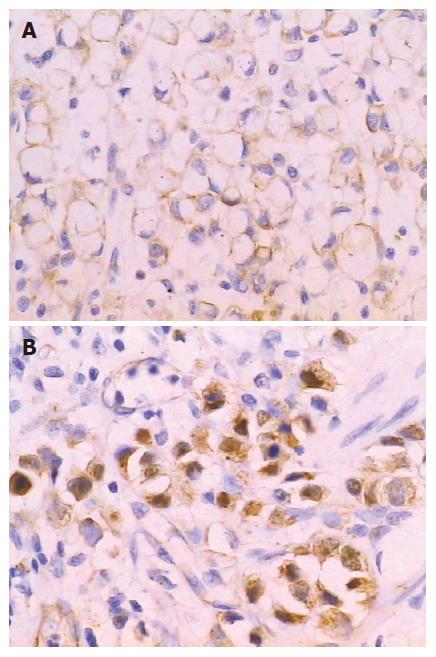
Li-cadherin is a novel member of the cadherin family specifically expressed in polarized epithelia of the liver and intestine of rats which was discovered in 1994[26]. Human Li-cadherin is an intestine specific cell adhesive molecule that also ectopically expressed in the metaplastic mucosa of the stomach as well as in gastric adenocarcinomas of the intestinal type[27]. Ko et al[28] reported that lymph node metastasis was significantly associated with the expression of Li-cadherin. Ito et al[29] reported Li-cadherin had higher expression in advanced gastric carcinoma than early gastric cancer, and associated with poor prognosis. Oue et al[30] discovered that Li-cadherin is positively correlated with tumor stages. Our result is concordant with these reports, which indicates that the intestinal phenotype positive cases possessed worse biological behavior possibly due to the expression of intestinal specific adhesive molecule Li-cadherin. However, the mechanism of Li-cadherin expression associated with worse biological behavior remains to be investigated[30]. In addition, we studied Li-cadherin immunohistochemically in 20 gastric biopsies with intestinal metaplasia and found that Li-cadherin was positively detected in all cases (Figure 5). Taken together, ours and previous studies suggest that Li-cadherin may be a novel specific marker for intestinal metaplasia.
In this study, we found an increased intestinal phenotype expression along with the progression of gastric SRC carcinoma. This phenomenon may be an appearance of abnormal differentiation and increased heterogeneity. Similar results can be seen in several Japanese reports: Yamachika et al[12] classified 203 gastric signet ring cell carcinomas into gastric phenotype and intestinal phenotype with PCS, GOS, S-GOS, anti-PgII, SH-9 and TKH-2 immunohistochemical staining. Their results showed that the proportion of gastric phenotype carcinoma cells decreases with the depth of invasion and the intestinal phenotype in four carcinomas involved the serosa. Bamba et al[10] applied immunohistochemistry with MUC2, M1 and PCS (III) to define the phenotype of 54 gastric signet ring cell carcinomas. They found that the larger size of the mucosal lesion was, the more frequently the intestinal phenotype was demonstrated. Aihara et al[13] examined MUC2, M1 and MUC6 staining of 69 early gastric SRC carcinomas; they classified these cases into G type and GI type and found the GI type was correlated with the depth of wall invasion. The findings of these studies showed that there is a phenotypic shift of cancer cell during gastric SRC carcinoma progression. This phenotypic shift may be a comitant phenomenon of tumor progression due to increasing heterogeneity.
A number of clinical studies revealed the difference of biological behaviors and prognosis among patients with gastric SRC carcinoma, indicating that morphologic classification is not enough for us to predict the progression and outcome of this kind of gastric carcinoma, and subtype classification needs further investigations. Our study showed that different phenotypic expression patterns were significantly associated with clinicopathologic parameters and prognosis of SRC carcinomas of stomach. Examination of phenotype expression may be a useful evidence for further classification and prognostic prediction in gastric SRC carcinomas.
S- Editor Zhu LH L- Editor Ma JY E- Editor Lu W
| 1. | Japanese Classification of Gastric Carcinoma - 2nd English Edition – Gastric. Cancer. 1998;1:10-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2009] [Cited by in F6Publishing: 1923] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 2. | Kim DY, Park YK, Joo JK, Ryu SY, Kim YJ, Kim SK, Lee JH. Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg. 2004;74:1060-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Antonioli DA, Goldman H. Changes in the location and type of gastric adenocarcinoma. Cancer. 1982;50:775-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 4. | Maehara Y, Sakaguchi Y, Moriguchi S, Orita H, Korenaga D, Kohnoe S, Sugimachi K. Signet ring cell carcinoma of the stomach. Cancer. 1992;69:1645-1650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 5. | Otsuji E, Yamaguchi T, Sawai K, Takahashi T. Characterization of signet ring cell carcinoma of the stomach. J Surg Oncol. 1998;67:216-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 6. | Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, Kikuchi S, Yamauchi H. Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku J Exp Med. 1998;186:121-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Theuer CP, Nastanski F, Brewster WR, Butler JA, Anton-Culver H. Signet ring cell histology is associated with unique clinical features but does not affect gastric cancer survival. Am Surg. 1999;65:915-921. [PubMed] [Cited in This Article: ] |
| 8. | Hyung WJ, Noh SH, Lee JH, Huh JJ, Lah KH, Choi SH, Min JS. Early gastric carcinoma with signet ring cell histology. Cancer. 2002;94:78-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Laurén P. Histogenesis of intestinal and diffuse types of gastric carcinoma. Scand J Gastroenterol Suppl. 1991;180:160-164. [PubMed] [Cited in This Article: ] |
| 10. | Bamba M, Sugihara H, Kushima R, Okada K, Tsukashita S, Horinouchi M, Hattori T. Time-dependent expression of intestinal phenotype in signet ring cell carcinomas of the human stomach. Virchows Arch. 2001;438:49-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol. 2004;121:884-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Yamachika T, Inada K, Fujimitsu Y, Nakamura S, Yamamura Y, Kitou T, Itzkowitz SH, Werther JL, Miki K, Tatematsu M. Intestinalization of gastric signet ring cell carcinomas with progression. Virchows Arch. 1997;431:103-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Aihara R, Mochiki E, Kamiyama Y, Kamimura H, Asao T, Kuwano H. Mucin phenotypic expression in early signet ring cell carcinoma of the stomach: its relationship with the clinicopathologic factors. Dig Dis Sci. 2004;49:417-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Mizoshita T, Inada K, Tsukamoto T, Nozaki K, Joh T, Itoh M, Yamamura Y, Ushijima T, Nakamura S, Tatematsu M. Expression of the intestine-specific transcription factors, Cdx1 and Cdx2, correlates shift to an intestinal phenotype in gastric cancer cells. J Cancer Res Clin Oncol. 2004;130:29-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Machado JC, Nogueira AM, Carneiro F, Reis CA, Sobrinho-Simões M. Gastric carcinoma exhibits distinct types of cell differentiation: an immunohistochemical study of trefoil peptides (TFF1 and TFF2) and mucins (MUC1, MUC2, MUC5AC, and MUC6). J Pathol. 2000;190:437-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 16. | Osborn M, Mazzoleni G, Santini D, Marrano D, Martinelli G, Weber K. Villin, intestinal brush border hydrolases and keratin polypeptides in intestinal metaplasia and gastric cancer; an immunohistologic study emphasizing the different degrees of intestinal and gastric differentiation in signet ring cell carcinomas. Virchows Arch A Pathol Anat Histopathol. 1988;413:303-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Reis CA, David L, Nielsen PA, Clausen H, Mirgorodskaya K, Roepstorff P, Sobrinho-Simões M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer. 1997;74:112-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 18. | Tajima Y, Yamazaki K, Nishino N, Morohara K, Yamazaki T, Kaetsu T, Suzuki S, Kawamura M, Kumagai K, Kusano M. Gastric and intestinal phenotypic marker expression in gastric carcinomas and recurrence pattern after surgery-immunohistochemical analysis of 213 lesions. Br J Cancer. 2004;91:1342-1348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Stanley RH, Lauri AA. Pathology and Genetics of tumours of the Digestive System. WHO. Classification of Tumours. Lyon: IARC Press 2000; 44-45. [Cited in This Article: ] |
| 20. | Yamaguchi A, Goi T, Yu J, Hirono Y, Ishida M, Iida A, Kimura T, Takeuchi K, Katayama K, Hirose K. Expression of CD44v6 in advanced gastric cancer and its relationship to hematogenous metastasis and long-term prognosis. J Surg Oncol. 2002;79:230-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Jalava P, Kuopio T, Juntti-Patinen L, Kotkansalo T, Kronqvist P, Collan Y. Ki67 immunohistochemistry: a valuable marker in prognostication but with a risk of misclassification: proliferation subgroups formed based on Ki67 immunoreactivity and standardized mitotic index. Histopathology. 2006;48:674-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Xu L, Zhang SM, Wang YP, Zhao FK, Wu DY, Yan X. Relationship between DNA ploidy,expression of ki-67 antigen and gastric cancer metastasis. World J Gastroenterol. 1999;5:10-11. [PubMed] [Cited in This Article: ] |
| 23. | Bubendorf L, Sauter G, Moch H, Schmid HP, Gasser TC, Jordan P, Mihatsch MJ. Ki67 labelling index: an independent predictor of progression in prostate cancer treated by radical prostatectomy. J Pathol. 1996;178:437-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 24. | Stavropoulos NE, Ioackim-Velogianni E, Hastazeris K, Kitsiou E, Stefanaki S, Agnantis N. Growth fractions in bladder cancer defined by Ki67: association with cancer grade, category and recurrence rate of superficial lesions. Br J Urol. 1993;72:736-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Lu Z, Hunter T. Wnt-independent beta-catenin transactivation in tumor development. Cell Cycle. 2004;3:571-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Berndorff D, Gessner R, Kreft B, Schnoy N, Lajous-Petter AM, Loch N, Reutter W, Hortsch M, Tauber R. Liver-intestine cadherin: molecular cloning and characterization of a novel Ca(2+)-dependent cell adhesion molecule expressed in liver and intestine. J Cell Biol. 1994;125:1353-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 142] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Grötzinger C, Kneifel J, Patschan D, Schnoy N, Anagnostopoulos I, Faiss S, Tauber R, Wiedenmann B, Gessner R. LI-cadherin: a marker of gastric metaplasia and neoplasia. Gut. 2001;49:73-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Ko S, Chu KM, Luk JM, Wong BW, Yuen ST, Leung SY, Wong J. Overexpression of LI-cadherin in gastric cancer is associated with lymph node metastasis. Biochem Biophys Res Commun. 2004;319:562-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Ito R, Oue N, Yoshida K, Kunimitsu K, Nakayama H, Nakachi K, Yasui W. Clinicopathological significant and prognostic influence of cadherin-17 expression in gastric cancer. Virchows Arch. 2005;447:717-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Oue N, Hamai Y, Mitani Y, Matsumura S, Oshimo Y, Aung PP, Kuraoka K, Nakayama H, Yasui W. Gene expression profile of gastric carcinoma: identification of genes and tags potentially involved in invasion, metastasis, and carcinogenesis by serial analysis of gene expression. Cancer Res. 2004;64:2397-2405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |