Published online Apr 1, 2004. doi: 10.3748/wjg.v10.i7.1043
Revised: November 11, 2003
Accepted: December 8, 2003
Published online: April 1, 2004
AIM: To investigate whether octreotide can inhibit the growth of human gallbladder cancer cells in vitro and to elucidate the antineoplastic mechanism of octreotide in gallbladder cancer.
METHODS: A human gallbladder cancer cell line, GBC-SD, was cultured in vitro. The antiproliferative effects of octreotide were examined by means of an MTT assay and a colony forming ability assay. Morphological variation was investigated under scanning electron microscopy and transmission electron microscopy. Cell cycle analysis and apoptosis rate was evaluated by flow cytometry (FCM) after staining by propidium iodide. DNA fragmentation was assayed by agarose gel electrophoresis. Immunohistochemical staining was performed to evaluate the expressions of mutant-type p53 and bcl-2.
RESULTS: The growth curve and colony forming ability assay showed significant inhibition of octreotide to the proliferation of GBC-SD cells in culture in a time- and dose-dependent manner. After exposure to octreotide, GBC-SD cells showed typically apoptotic characteristics, including morphological changes of chromatin condensation, vacuolar degeneration, nucleus fragmentation and apoptotic body formation. In FCM profile apoptotic cells showed increased sub-G1 peaks in the octreotide group, significantly higher than the control group (P = 0.013). There was also an augmentation in the cell proportion of G0/G1 phase (P = 0.015), while the proportion of S phase and G2/M phase remained unchanged (P = 0.057 and P = 0.280, respectively). DNA agarose gel electrophoresis displayed a ladder after exposure to 1 000 nmol/L octreotide. After being treated with octreotide, the expressions of both mutant-type p53 and bcl-2 decreased considering the percentage of positive cells (P < 0.05).
CONCLUSION: Octreotide has a negative action to the proliferation of GBC-SD cells, and the mechanism may be related to cytostatic and cytotoxic effects. The reduction of mutant-type p53 and bcl-2 expressions may be associated with the apoptosis induced by octreotide.
- Citation: Wang JH, Xing QT, Yuan MB. Antineoplastic effects of octreotide on human gallbladder cancer cells in vitro. World J Gastroenterol 2004; 10(7): 1043-1046
- URL: https://www.wjgnet.com/1007-9327/full/v10/i7/1043.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i7.1043
Gallbladder cancer is the commonest tumor of the biliary system[1]. Because of the absence of characteristic early symptoms, the majority of cases are diagnosed at a late stage when most patients already have occult or overt metastasis. As most gallbladder cancers are unresectable, the prognosis is dismal with the median survival time hardly exceeding 6-mo and 5-yr survival less than 5%. Due to the limited efficacy and considerable toxicity of conventional chemotherapy, novel cytotoxic agents and innovative noncytotoxic approaches are being developed. Amongst the various agents, our attention was being directed to somatostatin. Somatostatin and its analogs (SSTA) such as octreotide[2] inhibit tumor cell growth in vitro and in vivo[3,4]. Their effects are mediated by a family of G-protein-coupled receptors (SSTR1-5) that can couple to diverse signal transduction pathways such as inhibition of adenylate cyclase and guanylate cyclase, modulation of ionic conductance channels, and protein dephosphorylation. There are both ‘direct’ mechanisms that are sequellae of binding of SSTA to somatostatin receptors present on neoplastic cells and ‘indirect’ mechanisms related to effects of SSTA on the host[3] including antiangiogenetic effect[5] and inhibited secretion of tumor trophic factors or hormones such as insulin-like growth factor[6]. Various tumors are inhibited by octreotide, but little is known about whether octreotide has any antineoplastic effects on human gallbladder cancers[7].
Octreotide was a generous gift from Jiuyuan Gene Engineering Co. (Hangzhou, China). RPMI 1640 medium was obtained from Gibco. Newborn bovine serum was supplied by Sijiqing Biotechnology Co. (Hangzhou, China). MTT and propidium iodide (PI) were from Sigma. DNA Extract kit was from Dingguo Biotechnology Co. (Beijing, China). Mouse anti-human P53 and Bcl-2 monoclonal antibodies were purchased from Santa Cruz. ABC kit was provided by Vector.
Cell culture Human gallbladder cancer line GBC-SD (obtained from Doctor Bo Liu in Department of Hepato-biliary Surgery, General Hospital of PLA) was cultured in RPMI 1640, supplemented with 100 mL/L heat-inactivated newborn bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified atmosphere of 50 mL/L CO2 at 37 °C.
MTT assay Equal numbers of cells were seeded into 96-well tissue culture plates. One day later, the cells were treated at different concentrations of octreotide. Cell viability was determined in 5 wells for each drug concentration using MMT assay 12, 24, 36, 48, 60 h later. Optical density was measured at 570 and 630 nm using a microplate reader. Growth curve was made according to the optical density.
Colony-forming ability after drug exposure Aliquots of about 1 000 dispersed cells were seeded into Petri dishes in triplicates and treated with octreotide of different concentrations 24 h later. Colonies consisting of more than 50 cells were scored and the colony-forming rate was compared with that of untreated controls six days later.
Scanning electron microscopy (SEM) Cells grown on a small coverslip were prefixed with 25 mL/L glutaraldehyde, treated with OTO method, dehydrated in graded ethanol, critical point dried with CO2 and gold coated. The specimens were examined with a JSM-T300 scanning electron microscope.
Transmission electron microscopy (TEM) Cell cultures to be analyzed by transmission electron microscopy were fixed in 25 mL/L glutaraldehyde in phosphate buffer and post fixed in 10 g/L osmium tetraoxide, dehydrated in graded ethanol, embedded in an Epon 812 mixture, and sectioned on an ultramicrotome. After being stained with uranyl acetate and lead citrate, they were observed in a HITACHI H-800 electron microscope.
Agarose gel electrophoresis of DNA Cells treated with octreotide at 1 000 nmol/L for 72 h were collected, and DNA was extracted and analyzed in 10 g/L agarose gel for 1.5 h. After being stained with ethidium bromide solution for 10 to 15 min, the gel was viewed on a long wave UV transilluminator.
Flow cytometry assay (FCM) The cells subjected to treatment with octreotide were harvested, rinsed with PBS, resuspended and fixed in 700 mL/L ethanol at 4 °C overnight. The fixed cells were centrifuged, resuspended in 100 mg/L RNase A at 37 °C for 30 min and stained with PI at 4 °C for 30 min. FACS flow cytometer fluorescence intensity was measured by FACScan FCM and the DNA content in cells was analyzed by Modfit software. For each sample, 20 000 cells were measured.
Immunohistochemical analysis GBC-SD cells treated with octreotide for 24 h on coverslips were fixed with cold acetone for 5 min, rinsed in PBS for 5 min. Immunohistochemical staining proceeded according to kit instructions. Blank control, negative control (PBS substituting for primary antibodies) and positive control (parafin section of human gastric cancer) were set up at the same time. Cells from at least 10 randomly selected fields ( × 400) were counted. The percentage of positive cells was calculated as (Number of positive cells/Total number in the same visual field) × 100%.
Data were analyzed employing the paired two-tailed Student t test and nonparameter analysis, and significance was assumed at P < 0.05.
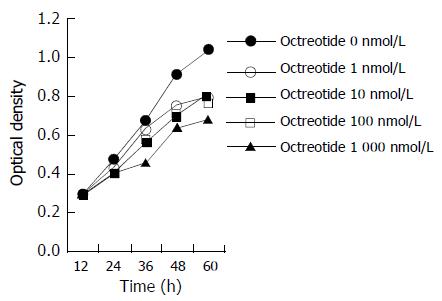
The growth curve (Figure 1) and colony forming ability assay (Table 1) showed significant inhibition of octreotide to the proliferation of GBC-SD cells in culture, inducing time- and dose-dependent effects.
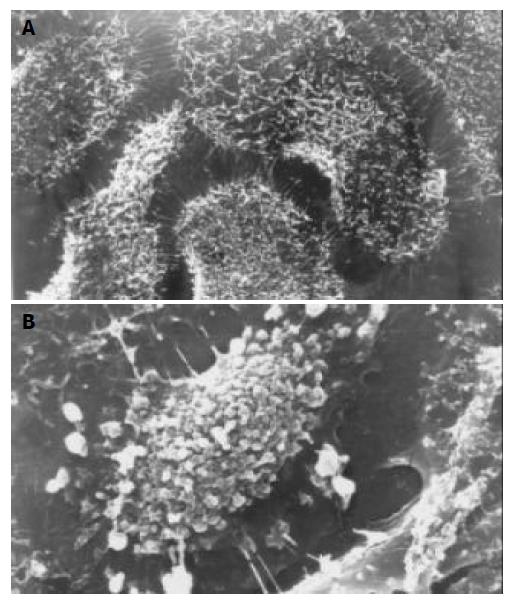
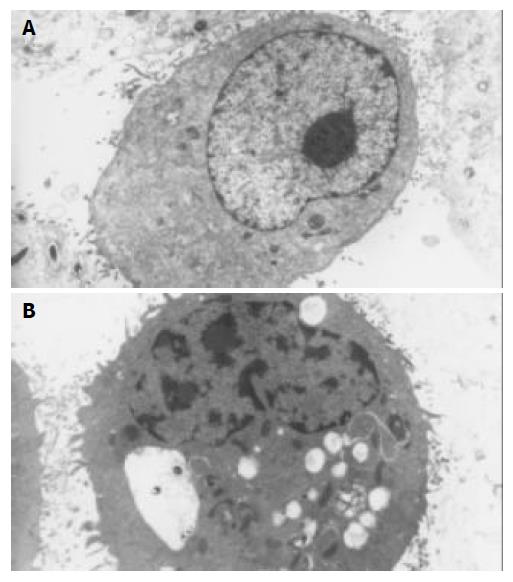
After exposure to octreotide, some GBC-SD cells showed typically apoptotic morphology, including chromatin condensation, vacuolar degeneration, nucleus fragmentation and formation of apoptotic body, which could be seen under SEM and TEM (Figure 2 and Figure 3).
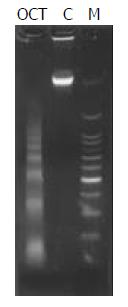
DNA of cells undergoing apoptosis usually displays a ladder in agarose gel electrophoresis. In the present study, a DNA ladder was characteristically identified in cells treated with 1 000 nmol/L of octreotide for 72 h as shown in Figure 4.
Usually, a reduced content in apoptotic cells under PI staining displays a ‘sub-G1’ peak in FCM profile and apoptotic cells can be quantified in this way. As demonstrated in Table 2, GBC-SD cells exposed to octreotide showed increased sub-G1 peaks, significantly higher than those of the control group (P = 0.013). Compared with the control group, there was also an augmentation in the cell proportion of G0/G1 phase (P = 0.015), while the proportion of S phase and G2/M phase remained unchanged (P = 0.057 and P = 0.280, respectively). This indicated that octreotide could arrest the GBC-SD cells at G0/G1 phase.
After being treated with octreotide, the expressions of both mutant-type p53 and bcl-2 decreased considering the percentage of positive cells (P < 0.05), as demonstrated in Table 3.
Somatostatin and SSTA show antineoplastic activity in a variety of experimental models in vivo and in vitro[3,4]. There is considerable evidence for antineoplastic activity of SSTA for neoplasms of breast, prostate, pancreas, colon, stomach, liver, and other common solid tumors for which current treatments are inadequate[8-18]. Some clinical trials employed octreotide in the treatment of human advanced tumors, showing increased survival, favorable toxicity profiles and improved life quality[19,20]. Recent studies described some aspects of the molecular mechanisms underlying this antineoplastic activity[21]. These researches suggest somatostatin and SSTA as drug candidates in oncology.
In this research we observed the effect of octreotide on the growth of GBC-SD cells in culture through two methods. Growth curve reflected group proliferative ability, while colony-forming assay reflected individual proliferative ability. Both methods showed significant inhibition of octreotide to the proliferation of GBC-SD cells, inducing time- and dose-dependent effects.
Over the past decade, a variety of studies revealed that somatostatin and SSTA mediated their action through both indirect and direct effects[3]. In our in vitro experiments, only direct effect was concerned, including cytostatic and cytotoxic effects.
After exposure to octreotide, FCM demonstrated an increased number of GBC-SD cells at G0/G1 phase. In cholangiocarcinoma cells, the G0/G1 cycle arrest was also induced[22]. This effect was attributed to the inhibition of signal transduction of some tumor trophic factors or hormones such as insulin and epidermal growth factor, which initiated tyrosine kinase pathway, activated the kinase cascade, increased the expression of cyclin, and promoted the cell cycle from G1 to S phase[23]. Some researches found octreotide activated SSTR on the membrane, down-regulated cyclin and up-regulated cyclin-dependent kinase inhibitor, thus leading to the inhibition of the mitogenic signal initiated by tyrosine kinase receptor family. Researchers reported specific phosphotyrosine phosphotases were required for maintaining high inhibitory levels of cyclin-dependent kinase inhibitor p27Kip1 and inactivating complex of cyclin E and cyclin dependent kinase 2[23-26]. Another research reported octreotide-induced growth arrest was mediated by inhibition of phosphatidylinositol 3-kinase pathway and by enhanced expressions of p21Cip and p27Kip1 [27].
A variety of findings indicated that apoptosis could be induced in cancer cells by octreotide, which was implicated in its antineoplastic mechanism[8,28]. In GBC-SD, spontaneous apoptosis was observed, showing an apoptosis rate varying from 0.21% to 0.69% in the control group and apoptosis could be induced by octreotide, displaying typical morphological changes, DNA ladder, and elevated sub-G1 peaks in FCM profiles. Octreotide-inducing apoptosis was reported to be associated with tyrosine proteinphosphotase pathway, intracellular acidification, activation of endonuclease and modulation of some genes such as wide-type p53, bax and bcl-2[28-31]. Sharma et al[29] reported only SSTR3 mediated apoptosis, depending on the expression of wide-type p53. Recent studies demonstrated SSTR2 mediated apoptosis in human pancreatic cancer cells and HL60 cells expressing mutated p53[32,33]. These results indicate that somatostatin can induce apoptosis by p53-dependent and p53-independent mechanisms[26].
Two main signaling pathways initiate the apoptotic program in mammalian cells. The cell-extrinsic pathway triggers apoptosis in response to activation by their respective ligand of the tumor necrosis factor (TNF) family of death receptors, while the cell-intrinsic pathway triggers apoptosis in response to DNA damage, loss of survival factors, or other types of cell distress. Guillermet and colleagues reported SSTR2 affected both pathways[32].
By means of immunohistochemical staining, we found high expressions of mutant-type p53 and bcl-2 in GBC-SD cells. In the process of octreotide-inducing apoptosis, expressions of both genes decreased. This suggests the down-regulation of both genes may be related to octreotide-inducing apoptosis.
In conclusion, we demonstrate that octreotide could inhibit proliferation in vitro in human gallbladder cancer cells, and speculate that cycle arrest and apoptotic induction might be involved in the mechanism.
Edited by Hu DK Proofread by Xu FM
| 1. | Fong YM, Kemeny N, Lawrence TS. Cancer of the liver and bil-iary tree In: Devita VT, Hellman S, Rosenberg S, eds. Cancer: principles and practice of oncology. 6th ed. Philadelphia:. Linppincott Williams and Wlikins. 2001;1162-1203. [Cited in This Article: ] |
| 2. | Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide. N Engl J Med. 1996;334:246-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 845] [Cited by in F6Publishing: 750] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 3. | Pollak MN, Schally AV. Mechanisms of antineoplastic action of somatostatin analogs. Proc Soc Exp Biol Med. 1998;217:143-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Scarpignato C, Pelosini I. Somatostatin analogs for cancer treatment and diagnosis: an overview. Chemotherapy. 2001;47 Suppl 2:1-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Woltering EA, Watson JC, Alperin-Lea RC, Sharma C, Keenan E, Kurozawa D, Barrie R. Somatostatin analogs: angiogenesis inhibitors with novel mechanisms of action. Invest New Drugs. 1997;15:77-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21:215-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 456] [Cited by in F6Publishing: 476] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 7. | Fiebiger WC, Scheithauer W, Traub T, Kurtaran A, Gedlicka C, Kornek GV, Virgolini I, Raderer M. Absence of therapeutic efficacy of the somatostatin analogue lanreotide in advanced primary hepatic cholangiocellular cancer and adenocarcinoma of the gallbladder despite in vivo somatostatin-receptor expression. Scand J Gastroenterol. 2002;37:222-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Diaconu CC, Szathmári M, Kéri G, Venetianer A. Apoptosis is induced in both drug-sensitive and multidrug-resistant hepatoma cells by somatostatin analogue TT-232. Br J Cancer. 1999;80:1197-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Raderer M, Hejna MH, Muller C, Kornek GV, Kurtaran A, Virgolini I, Fiebieger W, Hamilton G, Scheithauer W. Treatment of hepatocellular cancer with the long acting somatostatin analog lanreotide in vitro and in vivo. Int J Oncol. 2000;16:1197-1201. [PubMed] [Cited in This Article: ] |
| 10. | Gao S, Yu BP, Li Y, Dong WG, Luo HS. Antiproliferative effect of octreotide on gastric cancer cells mediated by inhibition of Akt/PKB and telomerase. World J Gastroenterol. 2003;9:2362-2365. [PubMed] [Cited in This Article: ] |
| 11. | Wang CH, Tang CW, Liu CL, Tang LP. Inhibitory effect of octreotide on gastric cancer growth via MAPK pathway. World J Gastroenterol. 2003;9:1904-1908. [PubMed] [Cited in This Article: ] |
| 12. | Wang XB, Wang X, Zhang NZ. Inhibition of somatostatin ana-log Octreotide on human gastric cancer cell MKN45 growth in vitro. Shijie Huaren Xiaohua Zazhi. 2002;10:40-42. [Cited in This Article: ] |
| 13. | Dolan JT, Miltenburg DM, Granchi TS, Miller CC, Brunicardi FC. Treatment of metastatic breast cancer with somatostatin analogues--a meta-analysis. Ann Surg Oncol. 2001;8:227-233. [PubMed] [Cited in This Article: ] |
| 14. | Tejeda M, Gaal D, Barna K, Csuka O, Kéri G. The antitumor activity of the somatostatin structural derivative (TT-232) on different human tumor xenografts. Anticancer Res. 2003;23:4061-4066. [PubMed] [Cited in This Article: ] |
| 15. | Lee JU, Hosotani R, Wada M, Doi R, Koshiba T, Fujimoto K, Miyamoto Y, Tsuji S, Nakajima S, Hirohashi M. Antiproliferative activity induced by the somatostatin analogue, TT-232, in human pancreatic cancer cells. Eur J Cancer. 2002;38:1526-1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Jia WD, Xu GL, Xu RN, Sun HC, Wang L, Yu JH, Wang J, Li JS, Zhai ZM, Xue Q. Octreotide acts as an antitumor angiogenesis compound and suppresses tumor growth in nude mice bearing human hepatocellular carcinoma xenografts. J Cancer Res Clin Oncol. 2003;129:327-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | González-Barcena D, Schally AV, Vadillo-Buenfil M, Cortez-Morales A, Hernández L V, Cardenas-Cornejo I, Comaru-Schally AM. Response of patients with advanced prostatic cancer to administration of somatostatin analog RC-160 (vapreotide) at the time of relapse. Prostate. 2003;56:183-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | He SW, Shen KQ, He YJ, Xie B, Zhao YM. Regulatory effect and mechanism of gastrin and its antagonists on colorectal carcinoma. World J Gastroenterol. 1999;5:408-416. [PubMed] [Cited in This Article: ] |
| 19. | Kouroumalis E, Skordilis P, Thermos K, Vasilaki A, Moschandrea J, Manousos ON. Treatment of hepatocellular carcinoma with octreotide: a randomised controlled study. Gut. 1998;42:442-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 196] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Cascinu S, Del Ferro E, Catalano G. A randomised trial of octreotide vs best supportive care only in advanced gastrointestinal cancer patients refractory to chemotherapy. Br J Cancer. 1995;71:97-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Bousquet C, Puente E, Buscail L, Vaysse N, Susini C. Antiproliferative effect of somatostatin and analogs. Chemotherapy. 2001;47 Suppl 2:30-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Zhao B, Zhao H, Zhao N, Zhu XG. Cholangiocarcinoma cells express somatostatin receptor subtype 2 and respond to octreotide treatment. J Hepatobiliary Pancreat Surg. 2002;9:497-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Pagès P, Benali N, Saint-Laurent N, Estève JP, Schally AV, Tkaczuk J, Vaysse N, Susini C, Buscail L. sst2 somatostatin receptor mediates cell cycle arrest and induction of p27(Kip1). Evidence for the role of SHP-1. J Biol Chem. 1999;274:15186-15193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Florio T, Arena S, Thellung S, Iuliano R, Corsaro A, Massa A, Pattarozzi A, Bajetto A, Trapasso F, Fusco A. The activation of the phosphotyrosine phosphatase eta (r-PTP eta) is responsible for the somatostatin inhibition of PC Cl3 thyroid cell proliferation. Mol Endocrinol. 2001;15:1838-1852. [PubMed] [Cited in This Article: ] |
| 25. | Lopez F, Estève JP, Buscail L, Delesque N, Saint-Laurent N, Théveniau M, Nahmias C, Vaysse N, Susini C. The tyrosine phosphatase SHP-1 associates with the sst2 somatostatin receptor and is an essential component of sst2-mediated inhibitory growth signaling. J Biol Chem. 1997;272:24448-24454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 125] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Bousquet C, Delesque N, Lopez F, Saint-Laurent N, Estève JP, Bedecs K, Buscail L, Vaysse N, Susini C. sst2 somatostatin receptor mediates negative regulation of insulin receptor signaling through the tyrosine phosphatase SHP-1. J Biol Chem. 1998;273:7099-7106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Charland S, Boucher MJ, Houde M, Rivard N. Somatostatin inhibits Akt phosphorylation and cell cycle entry, but not p42/p44 mitogen-activated protein (MAP) kinase activation in normal and tumoral pancreatic acinar cells. Endocrinology. 2001;142:121-128. [PubMed] [Cited in This Article: ] |
| 28. | Sharma K, Srikant CB. Induction of wild-type p53, Bax, and acidic endonuclease during somatostatin-signaled apoptosis in MCF-7 human breast cancer cells. Int J Cancer. 1998;76:259-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 29. | Sharma K, Patel YC, Srikant CB. Subtype-selective induction of wild-type p53 and apoptosis, but not cell cycle arrest, by human somatostatin receptor 3. Mol Endocrinol. 1996;10:1688-1696. [PubMed] [Cited in This Article: ] |
| 30. | Sharma K, Srikant CB. G protein coupled receptor signaled apoptosis is associated with activation of a cation insensitive acidic endonuclease and intracellular acidification. Biochem Biophys Res Commun. 1998;242:134-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Thangaraju M, Sharma K, Liu D, Shen SH, Srikant CB. Interdependent regulation of intracellular acidification and SHP-1 in apoptosis. Cancer Res. 1999;59:1649-1654. [PubMed] [Cited in This Article: ] |
| 32. | Guillermet J, Saint-Laurent N, Rochaix P, Cuvillier O, Levade T, Schally AV, Pradayrol L, Buscail L, Susini C, Bousquet C. Somatostatin receptor subtype 2 sensitizes human pancreatic cancer cells to death ligand-induced apoptosis. Proc Natl Acad Sci U S A. 2003;100:155-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Teijeiro R, Rios R, Costoya JA, Castro R, Bello JL, Devesa J, Arce VM. Activation of human somatostatin receptor 2 promotes apoptosis through a mechanism that is independent from induction of p53. Cell Physiol Biochem. 2002;12:31-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |