Published online Jun 1, 2004. doi: 10.3748/wjg.v10.i11.1578
Revised: September 14, 2003
Accepted: September 25, 2003
Published online: June 1, 2004
AIM: To determine whether dendritic cells (DCs) from chronic hepatitis B patients could induce HBV antigen-specific T cell responses or not.
METHODS: DCs were generated from peripheral blood mononuclear cells of patients with chronic hepatitis B (CHB) infection and healthy donors. We compared the phenotypes of these DCs and their ability to secrete cytokines and to participate in mixed lymphocyte reactions. In addition, autologous lymphocytes were cultured with DCs loaded with HBV core region peptide HBcAg8-27, an epitope recognized by cytotoxic T lymphocytes (CTL), and bearing human leucocyte antigen (HLA) -A2 for 10 d. Cytokine secretion and lytic activity against peptide-pulsed target cells were assessed.
RESULTS: DCs with typical morphology were generated successfully by culturing peripheral blood mononuclear cells (PBMCs) from CHB patients with AIM-V containing GM-CSF and IL-4. Compared with DCs from normal donors, the level of CD80 expressed in DCs from CHB patients was lower, and DCs from patients had lower capacity of stimulate T cell proliferation. When PBMCs isolated from patients with chronic or acute hepatitis B infection and from normal donors were cocultured with HBcAg18-27 peptide, the antigen-specific memory response of PBMCs from acute hepatitis B patients was stronger than that of PBMCs from chronic hepatitis B patients or normal donors. PBMCs cocultured with DCs treated with HBcAg18-27 CTL epitope peptide induced an antigen-specific T cell reaction, in which the level of secreted cytokines and lytic activity were higher than those produced by memory T cells.
CONCLUSION: DCs from patients with CHB can induce HBV antigen-specific T cell reactions, including secretion of cytokines essential for HBV clearance and for killing cells infected with HBV.
- Citation: Li RB, Chen HS, Xie Y, Fei R, Cong X, Jiang D, Wang SX, Wei L, Wang Y. Dendritic cells from chronic hepatitis B patients can induce HBV antigen-specific T cell responses. World J Gastroenterol 2004; 10(11): 1578-1582
- URL: https://www.wjgnet.com/1007-9327/full/v10/i11/1578.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i11.1578
In patients infected by hepatitis B virus (HBV), the virus may easily escape from immune surveillance, thus inducing tolerance and becoming a persistent infection. Persistently infected patients produce a weak or undetectable HBV-specific CTL response[1]. Therefore, when treating chronically infected patients with HBV, the population of T cells recognizing HBV should be increased, thus producing an effective immune response.
Dendritic cells (DCs) are the most potent antigen presenting cells throughout the body. These cells express high levels of MHC class I and II antigens, as well as costimulatory molecules, and play essential roles in triggering primary immune responses[2,3]. The use of DCs, as a type of natural adjuvant, has been applied to the immunotherapy of melanomas and prostate gland carcinomas[4]. In addition, administration of DCs activated by cytokines to transgenic mice has been shown to break CTL immune tolerance and induce specific CTL reactions against HBV[5]. The aim of this study was to compare the anti-HBV activity of DCs derived from patients infected with HBV with those from healthy donors. DCs derived from different individuals were cultured, reacted with a peptide specific to the HBV antigen, HBcAg18-27, and cocultured with autogeneic PBMCs to determine if this procedure could induce antigen-specific CTL, which could then be utilized to kill target cells loaded with HBV antigens or to secrete specific cytokines.
Of the HBV patients, four were studied during an episode of acute hepatitis B, and 12 (8 males, 4 females; average age, 38.3 years) were chronically infected with HBV. In addition, 20 patients were HLA-A2 positive, and 4 patients were HLA-A2 negative. We also included eight uninfected healthy volunteers (4 males, 4 females; average age, 35.2 years) as normal controls, of them six were HLA-A2 positive and two were HLA-A2 negative.
The diagnosis of acute and chronic hepatitis B was based on standard diagnostic criteria, as formulated by the 5th China Infection Disease and Parasitology Conference in 2000. HBV-infected patients had no complications of other organs. Normal controls had no clinical history of HBV infection and were serologically negative for HBV markers. All patients and normal controls were serologically negative for antibodies to HIV and HCV.
Recombinant human GM-CSF was purchased from Leucomax Corp (Germany), and recombinant human IL-4 and TNF-α were purchased from Peprotech Corp (England). Ficoll-Hypaque fluid was from Dingguo Corporation, Beijing. Mouse anti-human HLA-A*02 monoclonal antibody (BB7.2 cell line) was a gift from Professor. Wei-Feng Chen, and T2 cell line was a gift from Professor. Yan-Fang Sui. FITC-conjugated mouse monoclonal antibodies to human CD86, CD14, and CD3, and PE-labeled mouse monoclonal antibodies to human CD80, HLA-DR, CD54, CD11c, and CD19, were obtained from B-D Corp (USA). Mouse monoclonal antibodies to human CD83 and CD1a, FITC- and PE-labeled goat anti-mouse Ig (H + L) antibodies, PE-labeled mouse anti-human IFN-γ, TNF-α, and IL-2 were all purchased from Pharmingen Corp (USA). CytoTox 96 non-radioactive cytotoxicity assay kits were from Promega Corp (USA). AIMV and RPMI 1640 were from GIBCO Corp (USA), and IL-12 ELISA kit was from Endogen Corp (USA).
HLA-A2 restricted HBcAg18-27 CTL epitope peptide, FLPSDFFPSV, was synthesized by Saibaisheng Biological Corporation (Beijing) and purified to > 90% homogeneity by HPLC. The lyophilized peptide was dissolved in DMSO at a concentration of 2 mg/mL, and stored at -20 °C.
Following informed consent obtained from each subject, 100 mL anticoagulated venous blood was obtained from each, and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation (density, 1.077). Cells at the interface were transferred to RPMI 1640 incomplete medium containing 100 U/mL penicillin and 100 U/mL streptomycin, and washed twice.
DCs were cultured as described previously[6,7] with slight modifications. PBMCs were suspended in DC culture medium (AIMV), plated at a density of 2 × 107 cells/well in 6-well polystyrene culture plates, and cultured overnight at 37 °C in 950 ml/L air/50 ml/L CO2. Nonadherent cells were removed by gently swirling the plate, and the adherent cells were cultured in AIMV containing 1000 U/mL recombinant human GM-CSF and 500 U/mL recombinant human IL-4 at 37°C in 950 ml/L air/50 ml/L CO2 and refed daily with half volumes of fresh medium. Cells and culture supernatants were harvested on d 3,5,7,9,11, 13, and 15. Where indicated, TNF-α (20 ng/mL) was added on d 7, and the cells and supernatant were harvested on d 9.
The phenotype of harvested DCs was analyzed by flow cytometry (Becton Dickinson, FACScalibur). Cells (5 × 105) were suspended in PBS, centrifuged at 313 r/min for 5 min at 4°C, incubated for 30 min at 4°C with fluorescent monoclonal antibody, and fixed in 4 g/L paraformaldehyde.
IL-12 concentration in cell supernatants was assayed by ELISA, using standard curves according to the manufacturer’s instructions.
To test T cell stimulatory function of DCs, DCs were irradiated with 60Co (3000 rad), suspended in AIMV and placed in 96-well flat bottom culture plates at densities of 0.5, 1.0, and 2.5 × 104 cells/well. Responder T cells were added to each well, at a density of 5 × 105 cells/well, and cultured for 120 h. The ratio of DC:T was 1:20, 1:50, and 1:100, and each assay was performed in triplicate. T cells plated without DCs were utilized as negative controls. During the last 16 h of incubation, 1Ci/well of [3H] thymidine was added. On d 5, the cells were harvested, and the amounts of [3H] thymidine incorporated into responder cells were counted with a beta counter.
Purified populations of T cells were isolated from PBMCs using magnetic beads. Briefly, PBMCs were incubated with anti-CD8 MACS (Miltenyi Biotech, Germany) according to the manufacturer’s protocol. Cells retained by MACS beads were regarded as CD8 positive cells, with purity greater than 90%.
PBMCs from patients and normal donors were plated in 24-well plates at densities of 2 × 106 cells/well, in 2 mL RPMI1640 containing IL-2 (50 mg/L). Synthetic peptides were added to the cell cultures at a final concentration of 20 mg/L. As a negative control, DMSO alone was added to the wells. After 1 h, 0.7 μL Golgistop was added per mL culture medium, cells were collected 4 h later, and intracellular cytokine stain (ICCS) was applied.
Harvested cells were washed twice with PBS, 10 μL FITC-labelled mouse anti-human CD8 Ab was added to each tube, and the tubes were incubated in the dark on ice for 30 min. To each tube 250 μL FACS permeabilization solution was added, and the tubes were incubated for 20 min on ice in the dark. Cells were washed twice with 1 mL/tube FACS perm-wash and incubated with PE-conjugated mouse monoclonal antibody to human IFN-γ, IL-2, or TNF-α for 30 min on ice in the dark. The cells were washed with FACS perm-wash solution, PBS + 1 g/L BSA was added, and the cells were loaded onto a FACS calibur flow cytometer within 24 h, with data analysis performed using Cell Quest software (Becton Dickinson). A total of 400000 events were measured in each analysis. PE-conjugated mouse Ab was used as isotype controls. A lymphocyte gate was set to exclude monocytes, debris and dead cells.
CD8+ responders for peptide-specific CTL were generated from nonadherent PBMCs. CD8+ T cells were stimulated with DCs loaded with peptide at a stimulator-to-responder (S:R) ratio of 1:30. These cells were cultured in RPMI containing 100 g/L fetal calf serum (FCS) and IL-2 (50 mg/L). Responders were restimulated every 5 d with DCs pulsed with peptide. As controls, CD8+ T cells were stimulated with unpulsed DC. Six h before harvesting, 0.7 μL Golgistop was added to per mL culture medium. On d 10, CD8+T cells were harvested, ICCS was applied, and CTL cytotoxicity assays were performed.
HLA-A2 restricted antigen recognition by CTL was assessed by a standard cytotox 96 non-radioactive cytotoxicity assay. Recognition of HBcAg18-27 peptide by CTL was assessed using T2 cells preincubated for 4 h with 20 μg/mL peptide. Target cells were resuspended at a density of 1 × 105 cells/mL, thus making the ratios of effectors to target cells 30:1, 10:1, and 3:1, with a triplicate set of wells prepared for each ratio and for the controls. The percent of lyses was calculated as:
Math 1
Result were expressed as mean ± SD and analyzed using the Student’s t test. P < 0.05 was regarded as statistically significant.
PBMCs were isolated from patients and healthy donors and cultured overnight. After removal of the nonadherent cells, the remaining adherent monocytes were cultured in AIMV containing GM-CSF and IL-4. Cell cultures were observed after 24 h, becoming larger after 48 h and exhibiting DC morphology with veiled processes and dendrites (Figure 1). The yields of DCs from hepatitis B patients and normal donors did not differ significantly.
DCs were identified by their typical morphology and by CD86/HLA-DR double positive expression. These cells were harvested and counted, and surface markers were measured over time. The number of DCs peaked on d 9, decreasing gradually thereafter. The expression of DC- related cell markers was first detected on d 3. After cultured for 7 d with GM-CSF and IL-4, these DCs expressed costimulatory and MHC molecules, showing that cells derived from both patients and controls were morphologically compatible with DCs. Among the MHC and costimulatory molecules examined, we found that CD80 expression in patients with hepatitis B was weaker than that in normal donors (Table 1).
| DC Antigen | Positive DCs of HB patients | Positive DCs of normal donors | P |
| CD86 | 89.89 ± 1.14 | 91.17 ± 3.41 | 0.633 |
| CD80 | 36.86 ± 2.30 | 59.87 ± 6.31 | 0.021 |
| CD1a | 23.25 ± 7.04 | 32.92 ± 4.58 | 0.405 |
| HLA-DR | 97.29 ± 1.09 | 97.11 ± 1.14 | 0.852 |
| CD83 | 23.25 ± 7.34 | 33.18 ± 11.12 | 0.265 |
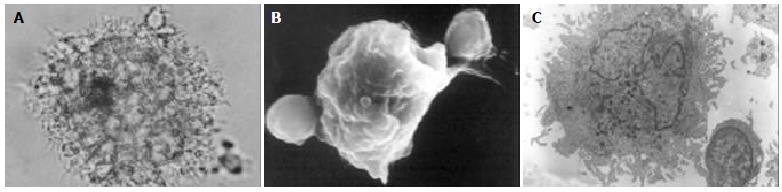
Following addition of 20 μg/mL TNF-α to the culture medium on the 5th d of culture, the cells and culture supernatants were harvested 48 h later. When cell phenotypes were assayed by flow cytometry, we found that the expression of CD83 was weaker in cells cultured in the absence of TNF-α than in its presence. However, culture in the presence of TNF-α did not alter the expression of CD86, CD80 or HLA-DR. While production of IL-12 by cultured DCs from hepatitis B patients and healthy donors did not differ, the secretion of IL-12 was significantly increased in cells cultured with TNF-α (Figure 2).
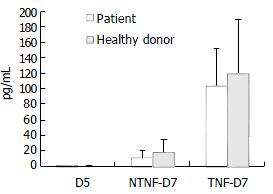
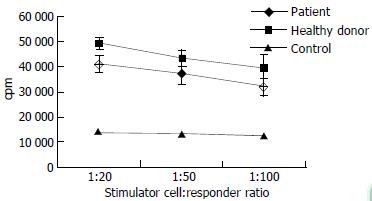
When we tested T cell-stimulatory activity of DCs in allogeneic MLR, we found that patients with chronic hepatitis B tended to have decreased T cell-stimulatory activity compared with normal donors, but the difference did not attain statistical significance (Figure 3).
Each MLR was set up in triplicate in flat-bottom 96-well plates. The responder T cells were obtained from a healthy donor, while irradiated DCs were used as stimulators at different ratios to T cells. Each point represents the mean ± SD. of measurements from 6 different individuals.
PBMCs isolated from patients with chronic hepatitis B (CHB) and acute hepatitis B (AHB) and from normal donors were incubated with HBcAg 18-27 peptide for 6 h, and ICCS was used to determine the frequency of HBcAg 18-27 peptide reactive T cells secreting cytokines. We found that stimulated PBMCs from the AHB group produced significantly stronger T cell memory reactions than PBMCs from either of the other groups (P < 0.05) (Table 2).
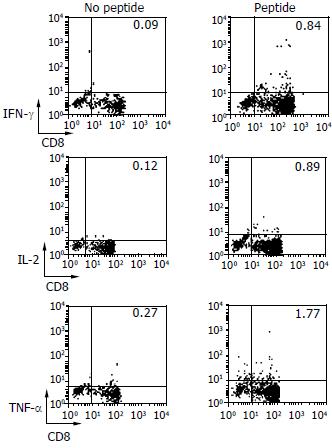
DCs primed with HBcAg18-27 peptide were cultured with lymphocytes for 10 d to determine whether peptide-reactive T cells were able to secrete various cytokines, including IFN-γ, IL-2 and TNF-α. Using the ICCS assay, we found that DCs from CHB patients induced peptide-reactive T cells to secrete cytokines (Figure 4), to a significantly greater degree than that secreted by peptide-stimulated PBMCs (P < 0.05). We also observed significant differences between DCs and PBMCs of CHB patients (Table 3).
| Group | n | Percent of CD8+ T cells secreting cytokines (%) | ||
| IFN-γ/CD8 | IL-2/CD8 | TNF-α/CD8 | ||
| CHB1 | 7 | 0.28 ± 0.15 | 0.46 ± 0.13 | 0.68 ± 0.15 |
| CHB2 | 4 | 0.59 ± 0.39 | 0.74 ± 0.16 | 1.52 ± 0.38 |
| P valuea | 0.043 | 0.049 | 0.028 | |
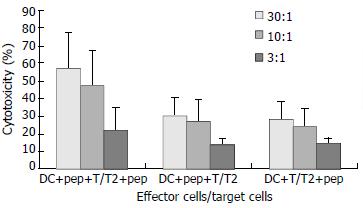
Target cells were divided into 2 groups, with one group incubated with peptide and the other group without. CTL cytotoxicity of the DCs from CHB patients against T cells incubated with peptide was (57.28 ± 20.00) %, (47.54 ± 20.70) %, and (21.38 ± 12.86) % at effector to target cell ratios of 30:1, 10:1, and 3:1, respectively, while CTL cytotoxicity of these same DCs against T cells incubated without peptide was (30.38 ± 10.00) %, (26.99 ± 12.72) %, and (13.74 ± 3.22) %, respectively (Figure 5).
DCs are the most potent antigen presenting cells crucial for activating CD8+ killer T cells and CD4+ helper T cells. DCs release bioactive cytokine IL-12, which can stimulate NK cells, induce Th1 type cytokine production and foster the development of cytotoxic T lymphocytes. DCs may thus be a powerful natural adjuvant for the treatment of tumors and viral infections. It is now known that the function of DCs is not fixed, depending on alterations in the cellular environment. Alterations in DC function may result from the effects of anti-inflammatory cytokines, including IL-10 and TGF-b, and on the characteristics of pathogens.
It was observed that, the functional activity of DCs was decreased, as measured by the stimulation of allogeneic T cell proliferation. In patients with chronic hepatitis B, however, it was unclear if DCs could act as natural adjuvants. In HBV transgenic (Tg) mice, DC function was normal, and there were CTLs specific for HBsAg, which were functionally quiescent. When cytokine-activated DCs were administered to these Tg mice, tolerance was broken, and antiviral reactions were induced[2]. In contrast, the expression of MHC II (I a) and CD86 and the ability of DCs to induce cell proliferation were lower in Tg than in normal mice[8].
In this study, we isolated PBMCs from the peripheral blood of patients with chronic hepatitis B infection and from healthy donors and compared the functional activity of their DCs. We found that, while CD80 expression was lower on DCs from patients than on those from healthy donors, other surface markers including HLA and costimulatory molecules were expressed to an equal extent. Functionally, DCs from CHB patients were able to stimulate the proliferation of allogeneic T cells, but to a lower extent than observed with DCs from non-CHB donors.
T cell activation requires costimulatory molecules (e.g., CD28-B7), which provide a second signal. The B7 family is comprised primarily of B7-1 (CD80) and B7-2 (CD86). Although their function is not fully known, several studies have shown that CD80 signals preferentially promote development of Th1 cells, whereas CD86 signals promote Th2 development. While CD80 gene knockout mice have a normal immunoglobulin superfamily repertoire, a complete deficiency of CD86 would destroy humoral immunity. B7 receptors have two ligands. CD28 provides a costimulatory signal essential to T cell activation, whereas CTLA-4 blocks T cell proliferation. CTLA-4 bound with greater affinity to CD80 than to CD86, suggesting that the binding of CD80 tends to inhibit T cell proliferation[9-11]. Our finding, that CD80 expression by DCs from patients with chronic hepatitis B was weaker than that from healthy donors, might be related to the inhibition of T cell activation seen in these patients and to the decreased function of DCs from these patients observed in our MLR assay. In addition, our results confirmed Akbar’s study[7] , in that the decreased functional activity of patient DCs results in the inability of these cells to present antigens to T cells, thus leading to reduced induction of a specific cytotoxic reaction. This, in turn, resulted in a lower rate of killing of infected cells and a reduction in the suppression of viral replication, finally leading to a state of persistent infection.
When pathogens invade a host, a potent immune response is produced to eliminate the pathogens. At the end stage of this immune response, the majority of effector T and B cells gradually undergo apoptosis, while a smaller number differentiate into long-lived memory T or B cells. Memory T cells produce vigorous secondary responses to antigen and rapidly eliminate pathogens. For example, the infected cells were cytolysed by CTLs. On the other hand, the primary difference between memory and naïve T cells is that, when they are activated, the former had higher reactivity to antigens and secreted more cytokines[12-14]. Thus, when an individual is infected with HBV, certain soluble products of the immune response, especially IFN-γ, IL-2, and TNF-α, can suppress HBV gene expression, as well as replication in vivo.
Hepatocellular HBV gene expression has been shown to be profoundly suppressed by non-cytolytic regulatory signals delivered by HBsAg-specific CTL through their secretion of IFN-γ and induction of TNF-α following antigen recognition. These effects were mediated by a posttranscriptional mechanism that selectively accelerates the degradation of cytoplasmic HBV mRNA[15]. We therefore assayed the cytokines secreted by CD8+T cells to illuminate the immune response of individuals to viral infection. We found that PBMCs from patients with CHB and AHB and from normal donors could be stimulated by HBcAg18-27 peptide. However, stimulated PBMCs from patients with AHB produced stronger memory reactions than those from patients with CHB or healthy donors, suggesting that AHB PBMCs have potent memory immune reactions to this specific antigen, whereas CHB PBMCs are at least partially tolerant to this antigen. The mechanism of tolerance includes factors from the host and from the virus. New-born babies contaminated by HBV transmitted from their mothers were in a state of immune tolerance to HBV and about 90% carry HBV as a persistent infection.
T cells play an important role in the immune system. Not only do they have effector functions, but also they are the major regulators of immune function. T cell activation requires two signals, one from the T cell receptor that recognizes the complex of MHC and antigen on the surfaces of antigen presenting cells (APCs), and the other from costimulatory molecules such as CD28-B7. To determine whether the immune tolerance of DCs from CHB patients could be broken, we primed DCs with HBcAg18-27 and incubated them with lymphocytes, and we assayed CTL generation. Our finding, that an antigen-specific T cell reaction was induced when these peptide-primed DCs were co-cultured with CD8+T cells, and that the cytotoxic activity of CTLs against target cells cultured with peptide was higher than that against target cells cultured without peptide, suggested that DCs from patients with CHB could break immune tolerance and induce an HBV antigen-specific T cell reactions.
DCs have great potential in anti-viral therapy as antigen presenting cells. Our findings showed that, although the function of patient DCs was lower than that of control DCs, the former still expressed signals necessary for T cell activation, released of IL-12 and stimulation of allogeneic T cell proliferation. Moreover DCs from these patients could break immune tolerance to induce HBV antigen-specific T cell responses. IL-12 is a critical cytokine secreted by DCs, which can induce the production of specific T cells, suggesting that TNF-α is an important factor in immunotherapy. Thus, our findings suggest that DCs originating from cytokine activated PBMCs of CHB patients can be utilized in immunotherapy, which will be a potential treatment for hepatitis B.
Co-correspondents: Yu Wang
Edited by Wang XL and Xu CT Proofread by Xu FM
| 1. | Chisari FV. Cytotoxic T cells and viral hepatitis. J Clin Invest. 1997;99:1472-1477. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245-3287. [PubMed] [Cited in This Article: ] |
| 3. | Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271-296. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Rescigno M, Granucci F, Ricciardi-Castagnoli P. Dendritic cells at the end of the millennium. Immunol Cell Biol. 1999;77:404-410. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Shimizu Y, Guidotti LG, Fowler P, Chisari FV. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J Immunol. 1998;161:4520-4529. [PubMed] [Cited in This Article: ] |
| 6. | Romani N, Reider D, Heuer M, Ebner S, Kämpgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137-151. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Romani N, Gruner S, Brang D, Kämpgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83-93. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Akbar SM, Inaba K, Onji M. Upregulation of MHC class II antigen on dendritic cells from hepatitis B virus transgenic mice by interferon-gamma: abrogation of immune response defect to a T-cell-dependent antigen. Immunology. 1996;87:519-527. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Freeman GJ, Boussiotis VA, Anumanthan A, Bernstein GM, Ke XY, Rennert PD, Gray GS, Gribben JG, Nadler LM. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523-532. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707-718. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Coyle AJ, Gutierrez-Ramos JC. The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nat Immunol. 2001;2:203-209. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54-60. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Sprent J, Surh CD. Generation and maintenance of memory T cells. Curr Opin Immunol. 2001;13:248-254. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Champagne P, Dumont AR, Sékaly RP. Learning to remember: generation and maintenance of T-cell memory. DNA Cell Biol. 2001;20:745-760. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Chisari FV. Rous-Whipple Award Lecture. Viruses, immunity, and cancer: lessons from hepatitis B. Am J Pathol. 2000;156:1117-1132. [PubMed] [DOI] [Cited in This Article: ] |