Copyright
©The Author(s) 2015.
World J Gastroenterol. Nov 14, 2015; 21(42): 12171-12178
Published online Nov 14, 2015. doi: 10.3748/wjg.v21.i42.12171
Published online Nov 14, 2015. doi: 10.3748/wjg.v21.i42.12171
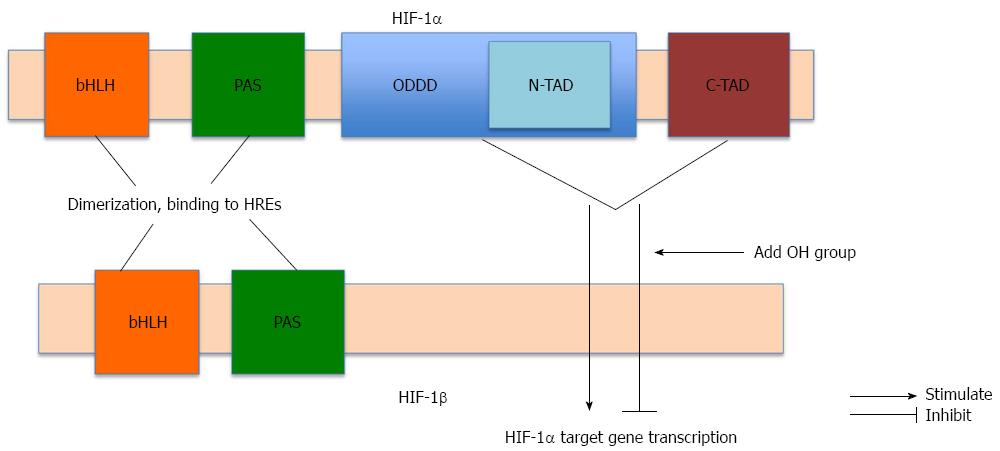
Figure 1 Structure of hypoxia-inducible factor-1.
Hypoxia-inducible factor (HIF)-1 is a heterodimer comprising HIF-1α and HIF-1β. Both subunits contain basic helix-loop helix (bHLH) motifs and PER-ARNT-SIM (PAS) domains needed for dimerization and binding to hypoxia response elements (HREs) in the promoter region of target regions. HIF-1α contains two transactivation domains: the NH2-terminal transactivation domain (N-TAD), located on the oxygen-dependent degradation domain (ODDD), and the carboxy-terminal transactivation domain (C-TAD). Both transactivation domains are essential for promoting HIF-1α target gene transcription. Hydroxylation of N-TAD and C-TAD of HIF-1α leads to inhibition of HIF-1α target gene transcription.
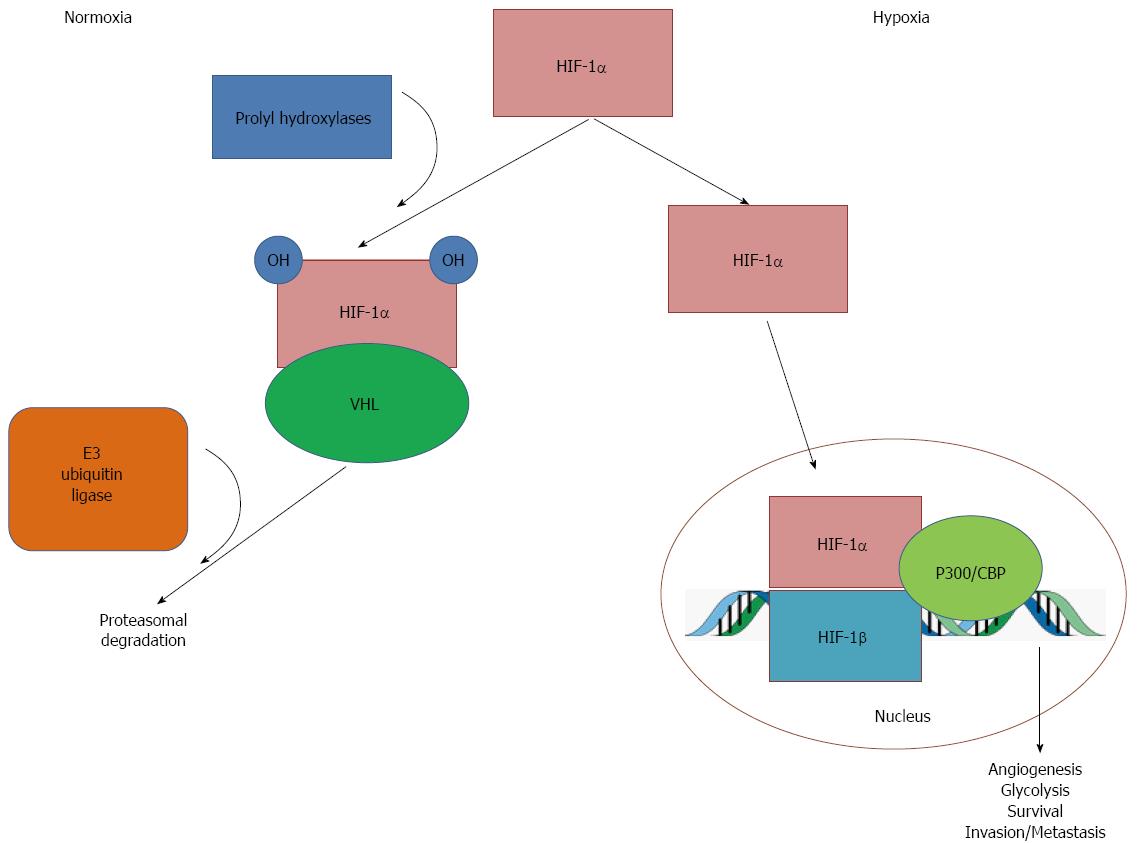
Figure 2 Oxygen-dependent mechanism of hypoxia-inducible factor-1α degradation.
In the presence of oxygen (normoxia), hypoxia-inducible factor (HIF)-1α undergoes hydroxylation via prolyl hydroxylases. This causes HIF-1α to interact with von Hippel Lindau (VHL) tumor suppressor protein, which is in turn recognized by E3 ubiquitin ligase, which targets HIF-1α for ubiquitination and degradation. Under hypoxic conditions, reduced oxygen leads to inactivation of prolyl hydoxylases, which diminishes hydroxylation and, therefore, reduces degradation of HIF-1α. Stabilized HIF-1α accumulates and translocates into the nucleus, where it dimerizes with HIF-1β and interacts with cofactors, such as p300 and CREB binding protein, to bind to DNA on hypoxia response elements (HREs). This activates transcription of HIF-1α target genes, leading to angiogenesis, glycolysis, survival, and invasion and metastasis of cancer cells.
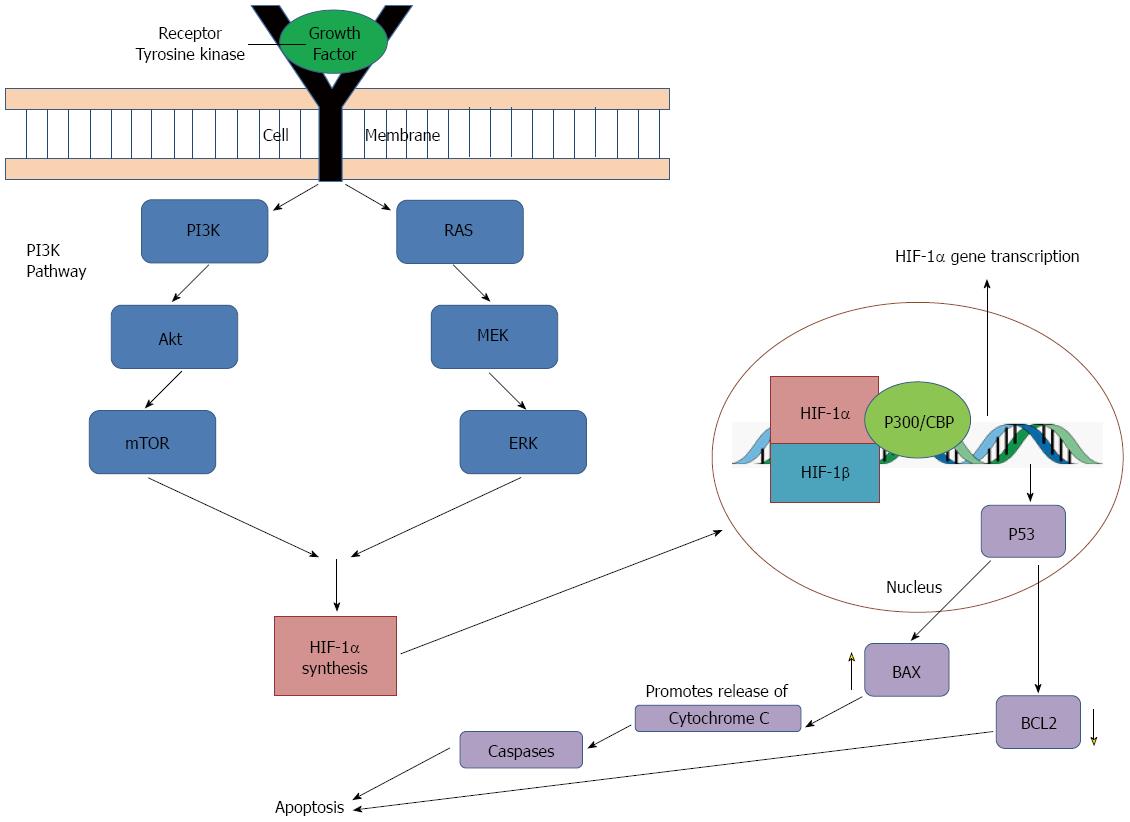
Figure 3 Hypoxia inducible factor 1α: protein synthesis and relation to apoptotis.
Hypoxia-inducible factor (HIF)-1α synthesis is upregulated by growth factor binding to tyrosine kinase receptors, causing activation of two pathways essential for cell proliferation and survival: the phosphatidylinositol 3-kinase (PI3K) pathway and the mitogen-activated protein kinase (MAPK) pathway. Extracellular signal-related kinase (ERK) and mitogen/extracellular signal-related kinase (MEK) represent members of the MAPK family which are activated as part of a signaling cascade. HIF-1α also interacts with p53, a tumor suppressor gene, which leads to transcription of pro-apoptotic genes. p53 activates transcription of BAX which acts on mitochondria to promote release of cytochrome C, activating a series of caspase signaling, which ultimately promotes apoptosis. In addition, p53 also downregulates BCL2, an anti-apoptotic protein. Together, these actions serve to increase apoptosis. mTOR: Mammalian target of rapamycin.
- Citation: Lin D, Wu J. Hypoxia inducible factor in hepatocellular carcinoma: A therapeutic target. World J Gastroenterol 2015; 21(42): 12171-12178
- URL: https://www.wjgnet.com/1007-9327/full/v21/i42/12171.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i42.12171