Copyright
©The Author(s) 2015.
World J Gastroenterol. Nov 14, 2015; 21(42): 12171-12178
Published online Nov 14, 2015. doi: 10.3748/wjg.v21.i42.12171
Published online Nov 14, 2015. doi: 10.3748/wjg.v21.i42.12171
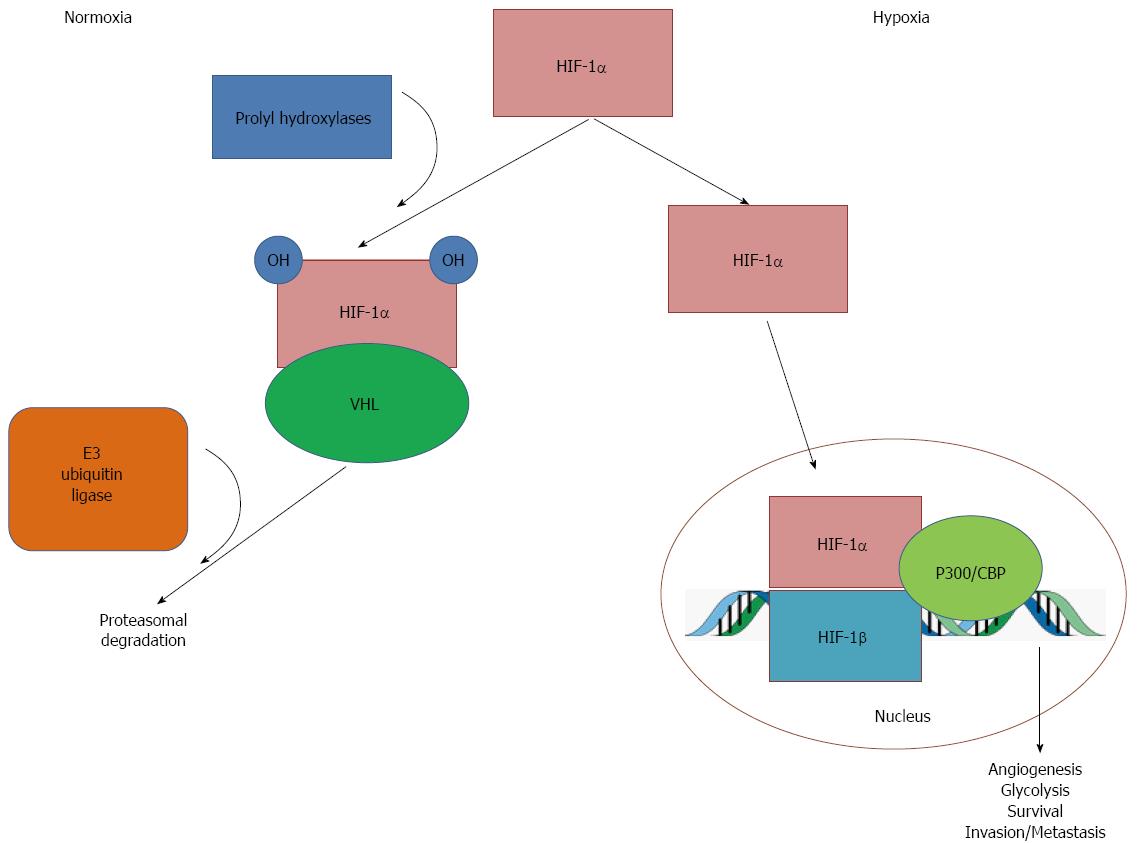
Figure 2 Oxygen-dependent mechanism of hypoxia-inducible factor-1α degradation.
In the presence of oxygen (normoxia), hypoxia-inducible factor (HIF)-1α undergoes hydroxylation via prolyl hydroxylases. This causes HIF-1α to interact with von Hippel Lindau (VHL) tumor suppressor protein, which is in turn recognized by E3 ubiquitin ligase, which targets HIF-1α for ubiquitination and degradation. Under hypoxic conditions, reduced oxygen leads to inactivation of prolyl hydoxylases, which diminishes hydroxylation and, therefore, reduces degradation of HIF-1α. Stabilized HIF-1α accumulates and translocates into the nucleus, where it dimerizes with HIF-1β and interacts with cofactors, such as p300 and CREB binding protein, to bind to DNA on hypoxia response elements (HREs). This activates transcription of HIF-1α target genes, leading to angiogenesis, glycolysis, survival, and invasion and metastasis of cancer cells.
- Citation: Lin D, Wu J. Hypoxia inducible factor in hepatocellular carcinoma: A therapeutic target. World J Gastroenterol 2015; 21(42): 12171-12178
- URL: https://www.wjgnet.com/1007-9327/full/v21/i42/12171.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i42.12171