Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. Nov 14, 2012; 18(42): 6036-6059
Published online Nov 14, 2012. doi: 10.3748/wjg.v18.i42.6036
Published online Nov 14, 2012. doi: 10.3748/wjg.v18.i42.6036
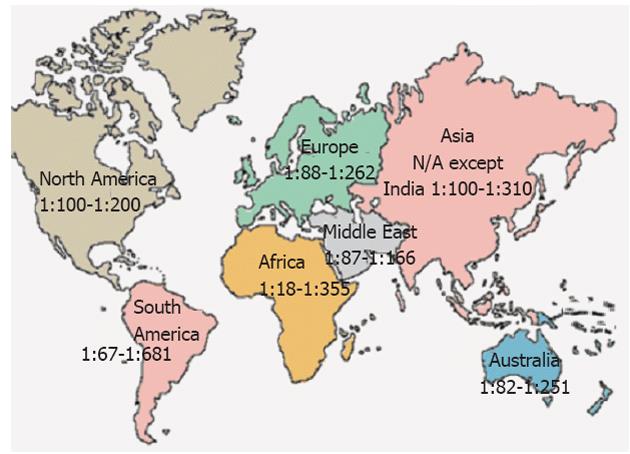
Figure 1 Prevalence of celiac disease worldwide.
N/A: Not available.
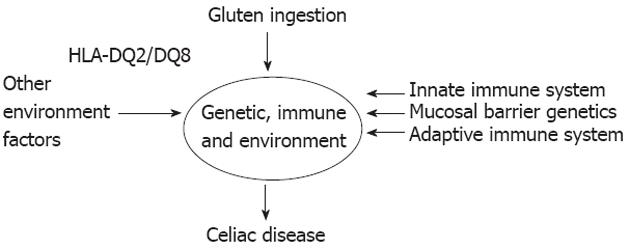
Figure 2 Factors necessary for celiac disease development[96] (adapted).
HLA: Human leukocyte antigen.
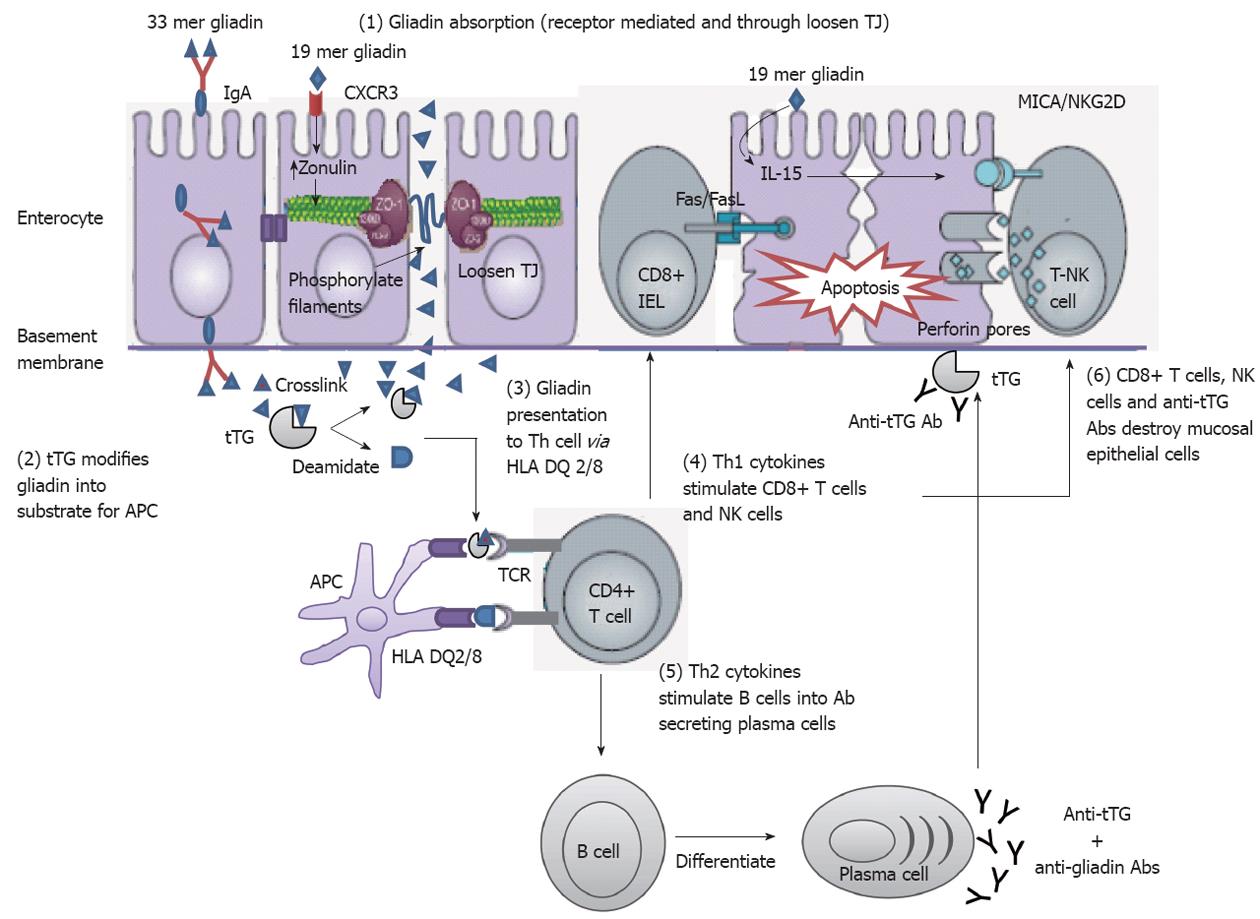
Figure 3 Mechanisms of mucosal damage in celiac disease[80] (adapted).
Gliadin peptides crosses the enterocyte by paracellular tight junctions (TJ) as a consequence of increased release of zonulin leading to impaired mucosal integrity upon 19 mer gliadin binding to chemokine (C-X-C motif) receptor 3 (CXCR3) receptor, or via transcytosis, or retrotranscytosis of secretory immunoglobulin A (IgA) through transferrin receptor CD71. Tissue transglutaminase (tTG) deamidates or crosslinks 33 mer gliadin which is then recognized by human leukocyte antigen (HLA)-DQ2 or -DQ8 molecules of antigen presenting cell (APC). APC presents the toxic peptide to CD4+ T cells. Activated gluten-reactive CD4+ T-cells produce high levels of pro-inflammatory cytokines. T helper 1 (Th1) cytokines promote increased cytotoxicity of intraepithelial lymphocytes (IELs) and natural killer (NK) T cells which cause apoptotic death of enterocytes by the Fas/Fas ligand (FasL) system, or interleukin 15 (IL-15)-induced perforin/granzyme and homodimeric natural killer-activating receptor-major histocompatibility-classIchain-related gene A comple (NKG2D–MICA) signaling pathways. The production of T-helper2 (Th2) cytokines activate and induce clonal expansion of B cells, which differentiate into (antigliadin and anti-tTG) antibody secreting plasma cells. Interaction between with the extracellular tTG and anti-tTG-autoantibody may induce epithelial damage. TCR: T cell receptor.
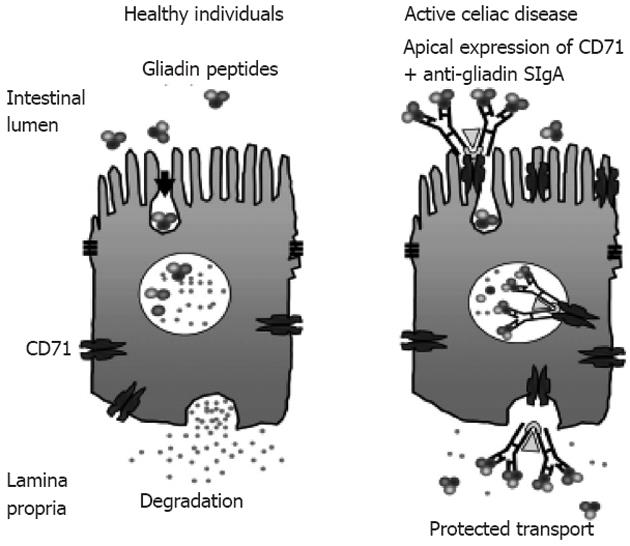
Figure 4 CD71 receptor-mediated transport of immunoglobulin A-gliadin complexes in celiac disease[136] (adapted).
Gliadin bound to apically expressed CD71 receptor in active celiac disease individual allows protected transport of gliadin into the lamina propria. SIgA: Secretory immunoglobulin A.
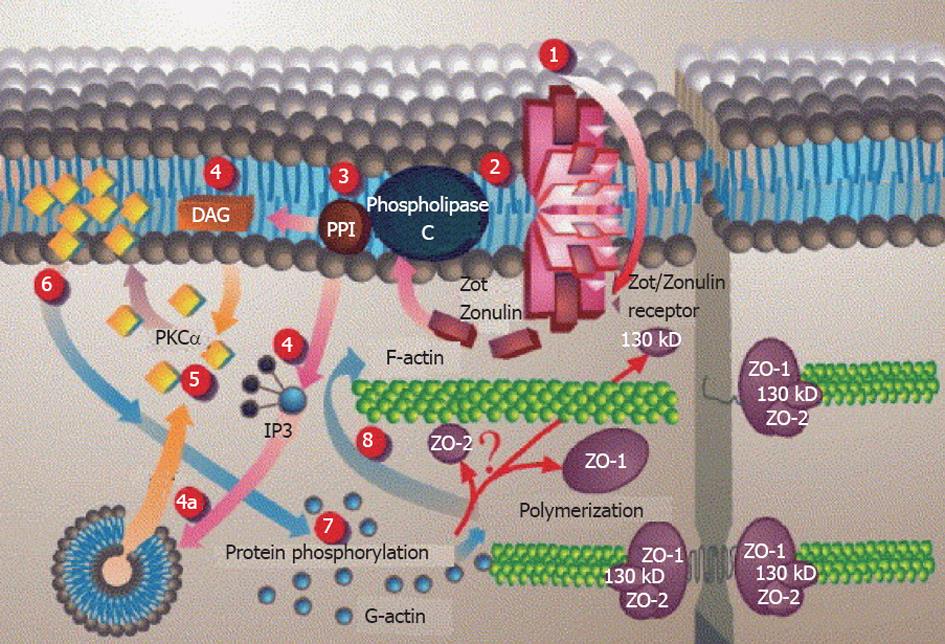
Figure 5 Proposed Zot intracellular signal mediated opening of intestinal tight junctions[146] (printed with permission).
1: Zot interacts with a specific Zot/Zonulin intestinal surface receptor; 2: Leading to protein internalization; 3: Activation of phospholipase C; 4: Hydrolyzes phosphatidyl inositol to release inositol 1,4,5-tris phosphate (PPI-3) and diacylglycerol (DAG), either via DAG or (4a) through the release of intracellular Ca2+via PPI-3; 5: Protein kinase C alpha (PKCα) is then activated; 6: Membrane-associated, activated PKCα catalyzes the phosphorylation of target protein(s); 7: With subsequent polymerization of soluble G-actin in F-actin; 8: This polymerization causes the rearrangement of the tight junctions (TJ) filaments and displacement of proteins [including zonula occludens-1 (ZO-1)]. As a result, intestinal TJ becomes loosened. IP3: Inositol trisphosphate.
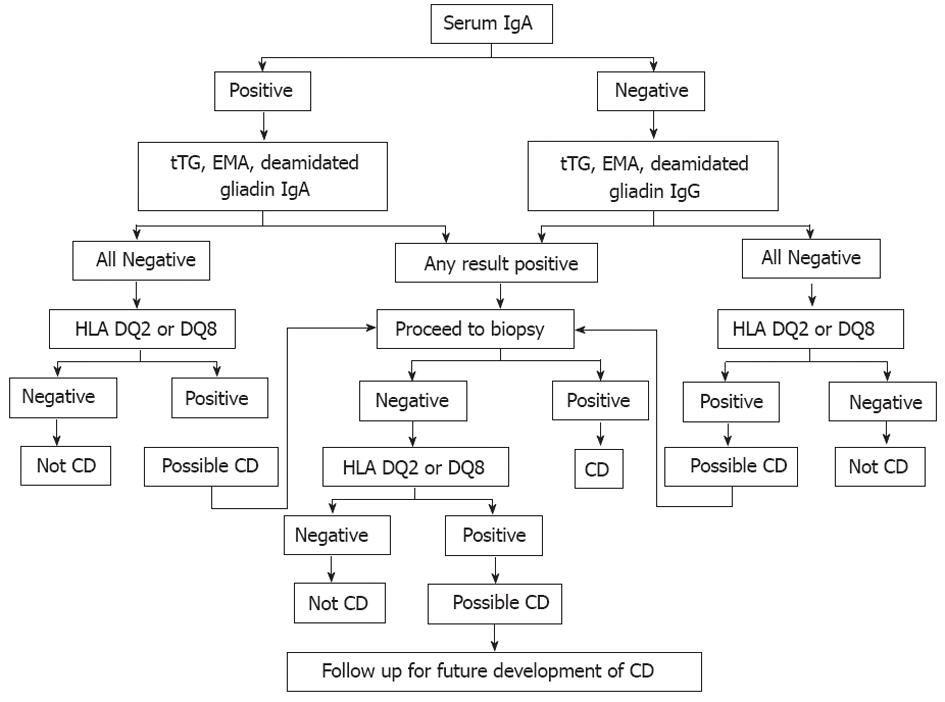
Figure 6 Celiac disease diagnostic testing algorithm (adapted from Mayo Medical Laboratories, Mayo Foundation for Medical Education and Research).
IgA: Immunoglobulin A; IgG: Immunoglobulin G; tTG: Tissue transglutaminase; EMA: Endomysial; HLA: Human leukocyte antigen; CD: Celiac disease.
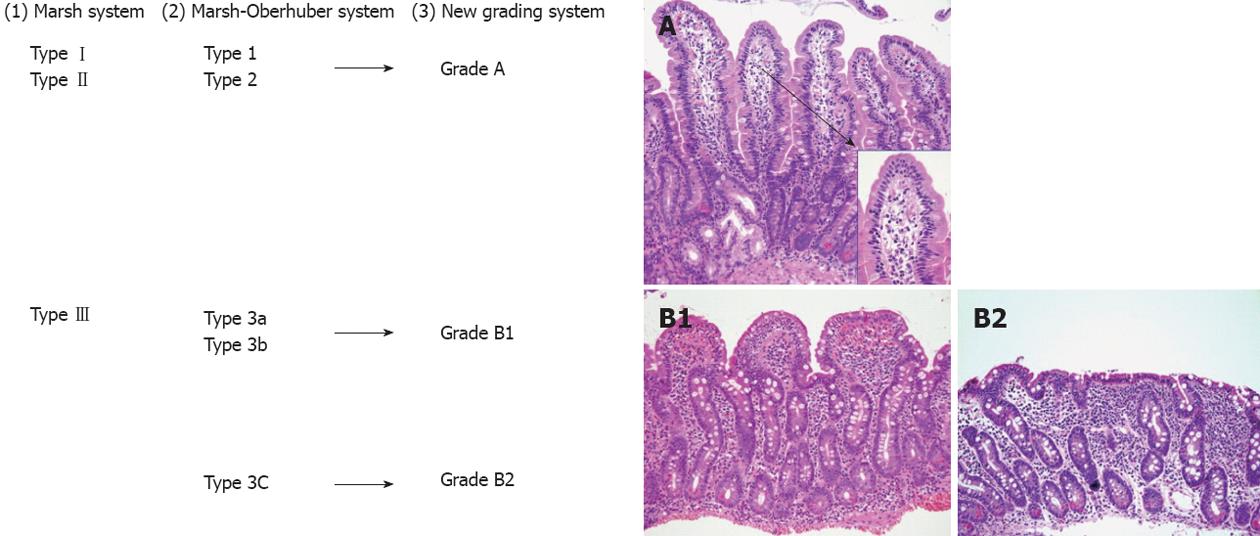
Figure 7 A comparison between the Marsh classification for celiac disease.
1: Marsh-Oberhuber; 2: Grading system for celiac disease, and the new grading system; 3: Representative pictures of the grades A (original magnification, 20×; insert, 60×), B1 (20×), and B2 (20×), proposed in the new grading system. An alternative classification may simply describe “mild”, “moderate” or “severe (flat)” architectural changes[186] (printed with permission).
- Citation: Gujral N, Freeman HJ, Thomson AB. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol 2012; 18(42): 6036-6059
- URL: https://www.wjgnet.com/1007-9327/full/v18/i42/6036.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i42.6036