Published online May 31, 2019. doi: 10.13105/wjma.v7.i5.234
Peer-review started: March 26, 2019
First decision: April 30, 2019
Revised: May 20, 2019
Accepted: May 22, 2019
Article in press: May 22, 2019
Published online: May 31, 2019
Processing time: 66 Days and 21.5 Hours
Liver resection has become safer as it has become less invasive. However, the minimum residual liver volume (RLV) required to maintain homeostasis is unclear. Furthermore, the formulae used to calculate standard liver volume (SLV) are complex.
To review previously reported SLV formulae and the methods used to evaluate the minimum RLV, and explore the association between liver volume and mortality.
A systematic review of Medline, PubMed, and grey literature was performed. References in the retrieved articles were cross-checked manually to obtain further studies. The last search was conducted on January 20, 2019. We developed an SLV formula using data for 86 consecutive patients who underwent hepatectomy at our institution between July 2009 and August 2011.
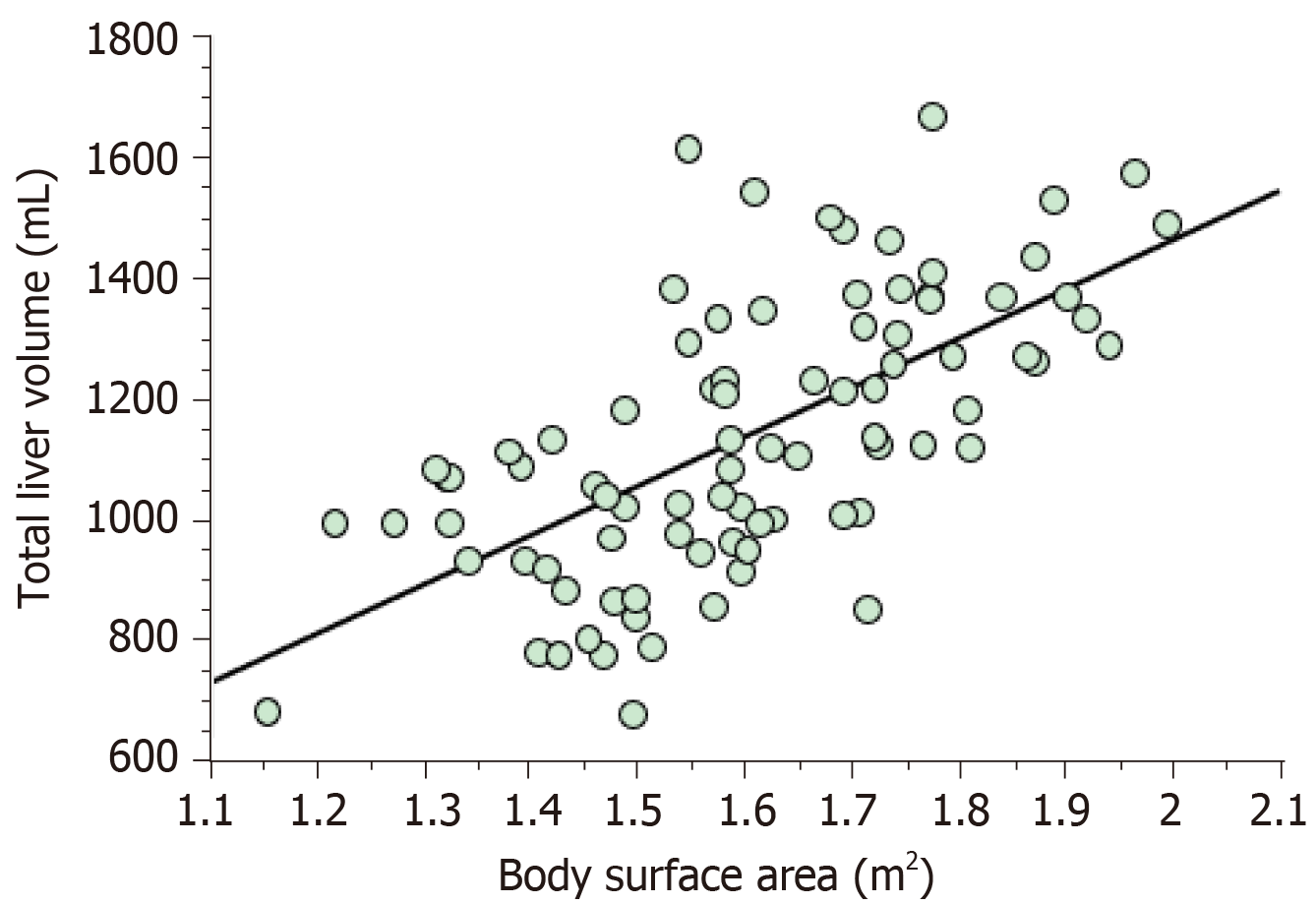
Linear regression analysis revealed the following formula: SLV (mL) = 822.7 × body surface area (BSA) − 183.2 (R2 = 0.419 and R = 0.644, P < 0.001). We retrieved 25 studies relating to SLV formulae and 12 studies about the RLV required for safe liver resection. Although the previously reported formulae included various coefficient and constant values, a simplified version of the SLV, the common SLV (cSLV), can be calculated as follows: cSLV (mL) = 710 or 770 × BSA. The minimum RLV for normal and damaged livers ranged from 20%-40% and 30%-50%, respectively. The Sapporo score indicated that the minimum RLV ranges from 35%-95% depending on liver function.
We reviewed SLV formulae and the minimum RLV required for safe liver resection. The Sapporo score is the only liver function-based method for determining the minimum RLV.
Core tip: We systematically reviewed standard liver volume (SLV) formulae, methods for assessing the minimum residual liver volume (RLV) required for safe liver resection, and the association between liver volume and mortality. Although the reported SLV formulae contained different coefficient/constant values, a simplified version of the SLV, the common SLV (cSLV), can be calculated as follows: cSLV (mL) = 710 or 770 × body surface area. The Sapporo score is the only liver function-based method for determining the minimum RLV.
- Citation: Harada K, Nagayama M, Ohashi Y, Chiba A, Numasawa K, Meguro M, Kimura Y, Yamaguchi H, Kobayashi M, Miyanishi K, Kato J, Mizuguchi T. Scoring criteria for determining the safety of liver resection for malignant liver tumors. World J Meta-Anal 2019; 7(5): 234-248
- URL: https://www.wjgnet.com/2308-3840/full/v7/i5/234.htm
- DOI: https://dx.doi.org/10.13105/wjma.v7.i5.234
Liver resection is a potentially curative treatment for malignant liver tumors, such as hepatocellular carcinoma (HCC) and metastatic liver cancer, in cases in which no metastasis is present in other organs[1,2]. Although the mortality rate associated with liver resection has decreased, surgical complications still occur[3-7]. To ensure that liver resection is performed safely, it is important to preoperatively evaluate patients’ liver function so that it is possible to estimate the maximum liver volume that can be safely removed[8,9]. The Child-Pugh classification and the liver damage classification established by the Liver Cancer Study Group of Japan are used to evaluate liver function[10-13]. The indications for liver resection are grade A or B liver function, according to either classification system. However, liver function varies greatly between grade A and B in patients with HCC. Therefore, in HCC patients it is difficult to accurately predict the maximum safe extent of liver resection.
Recent advances in radiological assessments of the liver have made it possible to precisely calculate liver volume prior to liver resection[8,9,14-16]. Multi-detector-row computed tomography (MDCT) can be used to evaluate not only liver volume, but also patients’ individual anatomies prior to liver resection[17-19]. The aim of this systematic review was to summarize the methods used to assess liver volume in order to aid the establishment of a standard formula for calculating standard liver volume (SLV). In addition, we attempted to summarize the relationship between liver volume and liver failure in order to facilitate safe liver resection.
This study was approved by the internal review board of Sapporo Medical University (approval ID: 302-195 and approval date: February 14, 2019). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines for conducting and reporting meta-analyses were followed[15]. To conduct this study, the study protocol was published on PROSPERO, which is the international prospective register of systematic reviews (reference: No. CRD42019123642).
Between July 2009 and August 2011, 86 consecutive patients who underwent liver resection for malignant tumors were enrolled in this study. Clinical laboratory tests, including of the serum levels of aspartate aminotransferase, alanine aminotransferase, albumin, hyaluronic acid, hepatocyte growth factor, and antithrombin III (ATIII); the prothrombin time (PT); the indocyanine green retention rate at 15 min (ICGR15); and the platelet count were evaluated prior to liver resection. The uptake ratio of the heart at 15 min to that seen at 3 min (HH15) and the uptake ratio of the liver to the liver plus heart at 15 min (LHL15) were obtained from time activity curves of 99 m Tc-galactosyl human serum albumin scintigraphy.
Liver volume was evaluated using 64-row MDCT (LightSpeed VCT VISION; GE Healthcare, Milwaukee, WI, United States). The images were obtained in four phases, the early arterial phase, portal vein phase, hepatic vein phase, and late phase. A ZIO STATION 2 (Ziosoft Inc., Tokyo, Japan) was used to calculate liver volume. The images of the hepatic vein phase were used for volumetry, and image analysis was restricted to the first and second branches of the portal and hepatic veins, as described previously[20].
Liver failure was defined as a serum bilirubin concentration of > 10 mg/dL for > 2 d. Liver dysfunction was defined as a total bilirubin level of ≥ 3 mg/dL and a PT value of < 50% within 7 d after liver resection[21].
A systematic review of Medline, PubMed, and grey literature was performed. References from the retrieved articles were also cross-checked manually to obtain further studies. When more than one study from the same institution was found, only the publication with the most complete data was included. The last search was conducted on January 20, 2019. The search strategy for the PubMed database was as follows: {[“liver” (MeSH Terms) OR “liver” (All Fields)] AND volume (All Fields)} AND calculation (All Fields). The searches of other databases were conducted using the same medical subject headings (MeSH) and keywords in various combinations.
Patient demographics and perioperative laboratory tests were extracted from the database, and differences between the groups were compared using the chi-square test followed by a post-hoc 2 × 2 Fisher’s exact test. The unpaired t-test was used for comparisons between the no liver dysfunction group (n = 78) and the liver dysfunction group (n = 8). The relationships among the various clinical parameters were evaluated using Spearman’s rank correlation coefficient. The intraclass correlation coefficient (ICC) was used to assess inter-rater reliability. All calculations were performed using the SPSS 20.0 software program (SPSS Inc., Chicago, IL, United States). All results are expressed as the mean together with minimum and maximum levels. P-values of < 0.05 were considered to be statistically significant.
We investigated the cases of patients who underwent hepatectomy for various malignancies, including HCC, at our institution between July 2009 and August 2011. Table 1 shows the clinical demographics of the patients in three groups; i.e., all patients (n = 86), the no liver dysfunction group (n = 78), and the liver dysfunction group (n = 8).
| Clinical variables / characteristics | Total values (n = 86) | No liver dysfunction (n = 78) | Liver dysfunction (n = 8) | P-values |
| Age (yr) | 67.0 ± 10.3 | 66.8 ± 10.5 | 68.7 ± 9.3 | NS |
| BSA (cm2) | 1.61 ± 0.18 | 1.60 ± 0.18 | 1.65 ± 0.18 | NS |
| Albumin (g/dL) | 4.0 ± 0.4 | 4.0 ± 0.4 | 3.8 ± 0.4 | NS |
| Bilirubin (mg/dL) | 0.7 ± 0.3 | 0.6 ± 0.3 | 0.8 ± 0.4 | NS |
| PT (%) | 93.4 ± 11.9 | 93.9 ± 12.1 | 88.4 ± 9.7 | NS |
| ICGR15 (%) | 9.0 ± 4.6 | 8.4 ± 4.3 | 14.9 ± 2.5 | < 0.001 |
| HH15 | 0.578 ± 0.079 | 0.575 ± 0.078 | 0.617 ± 0.010 | NS |
| LHL15 | 0.940 ± 0.027 | 0.940 ± 0.028 | 0.935 ± 0.024 | NS |
| ATIII (%) | 94.9 ± 17.2 | 96.0 ± 17.3 | 83.9 ± 13.4 | 0.036 |
| Intraoperative bleeding (mL) | 481.2 ± 468.5 | 455.8 ± 472.4 | 675.0 ± 430.0 | NS |
| Operative time (min) | 404.5 ± 135.2 | 390.8 ± 129.3 | 525.9 ± 140.9 | 0.014 |
| Sex (male:female) | 49:37 | 44:34 | 5:3 | NS |
| Background (HCC: meta or CCC) | 57:29 | 49:29 | 8:0 | 0.047 |
| Hr (0/S:1:2/3) | 53:17:16 | 49:16:13 | 4:1:3 | NS |
| Sapporo score | 13.2 ± 2.4 | 13.4 ± 2.5 | 11.0 ± 3.2 | 0.037 |
| MELD score | 7.6 ± 1.6 | 7.6 ± 1.7 | 7.8 ± 0.7 | 0.103 |
| Child-Pugh score | 3.2 ± 0.4 | 3.2 ± 0.4 | 3.4 ± 0.5 | 0.119 |
| Total liver volume (cc) | 1137.8 ± 222.9 | 1136.2 ± 228.5 | 1153.5 ± 221.4 | NS |
| Reduction in liver volume (%) | 19.0 ± 13.0 | 17.6 ± 12.1 | 32.8 ± 15.0 | 0.006 |
| Residual liver volume (%) | 85.1 ± 14.4 | 86.0 ± 13.7 | 75.4 ± 17.9 | 0.039 |
The ICGR15, serum ATIII level, operation time, the background of the malignancy, and the reduction in liver volume differed significantly between the no liver dysfunction group and liver dysfunction group (Table 1). The results of the linear regression analysis of the relationship between resectable liver volume and body surface area (BSA) are shown in Figure 1. The latter analysis resulted in the deve-lopment of the following formula for SLV: SLV (mL) = 822.7 × BSA - 183.2 (R2 = 0.419 and R = 0.644, P < 0.001). On the other hand, MELD score and Child-Pugh score did not correlate with resectable liver volume at all (Supplement Figure 1 and 2).
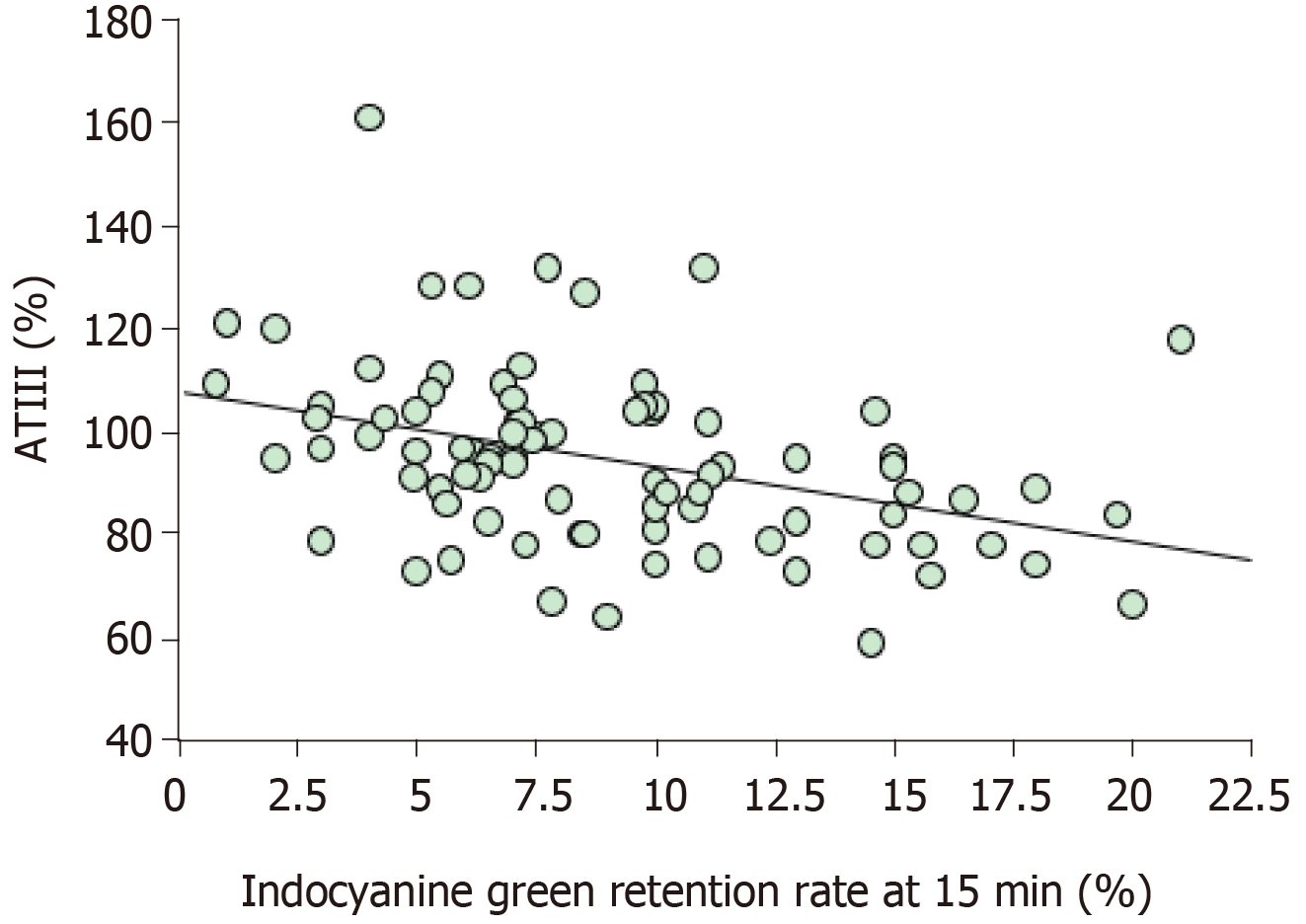
The results of the linear regression analysis of the serum ATIII level and ICGR15 are shown in Figure 2. The sum of the values for the serum ATIII level and ICGR15 was almost 100%, which is consistent with the findings of previous studies[20]. We have also previously reported Sapporo scores for liver resection for malignant tumors (Table 2). The Sapporo score consists of four clinical variables, including the ICGR15, serum ATIII level, HH15, and LHL15. Each factor is awarded 1 to 4 points. Thus, the maximum total score is 16 points, and the minimum total score is 4 points.
| Factors | Scores | |||
| 4 | 3 | 2 | 1 | |
| ICGR15 (%) | ≤ 10 | 10-19 | 20-29 | ≥ 30 |
| ATIII (%) | ≥ 90 | 80-89 | 70-79 | ≤ 69 |
| HH15 | ≤ 0.55 | 0.56-0.59 | 0.60-0.64 | ≥ 0.65 |
| LHL15 | ≥ 0.95 | 0.90-0.94 | 0.85-0.89 | ≤ 0.84 |
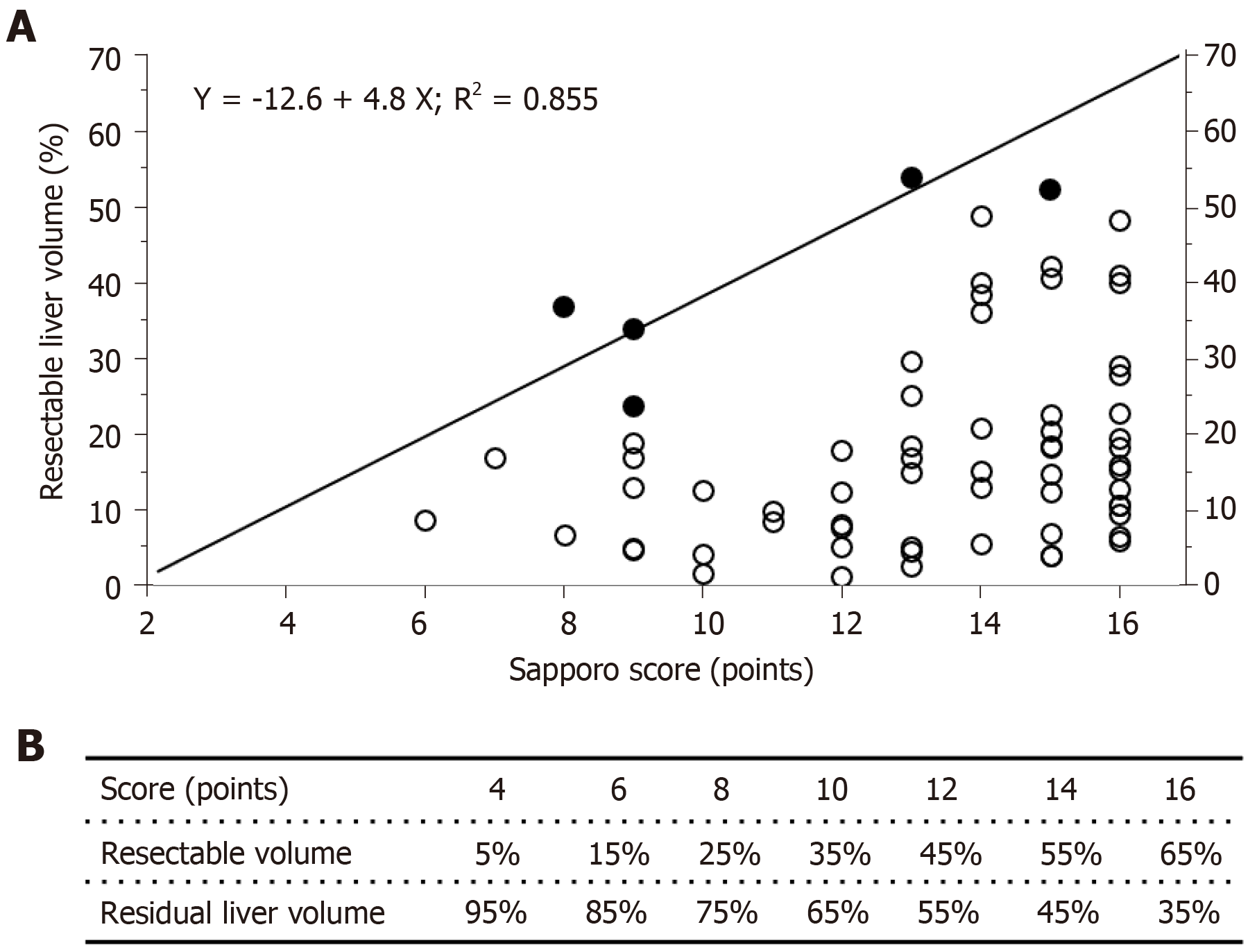
The results of the linear regression analysis of the reduction in liver volume and the Sapporo score are shown in Figure 3. The closed circles indicate the patients who exhibited liver dysfunction after hepatectomy, and the open circles indicate the patients who did not display liver dysfunction after hepatectomy. Linear regression analysis revealed a formula for predicting the risk of liver dysfunction after hepatectomy. The linear coefficient was almost 5, which meant that each extra Sapporo score point indicated that a further 5% of the total liver volume could be removed safely via liver resection. Therefore, the maximum Sapporo score (16 points) indicates that 65% of the total liver volume can be removed safely. On the other hand, the minimum score (4 points) only allows 5% of the total liver volume to be safely removed. The relationships between the Sapporo score, the resectable liver volume, and residual liver volume (RLV) are shown in Figure 3B.
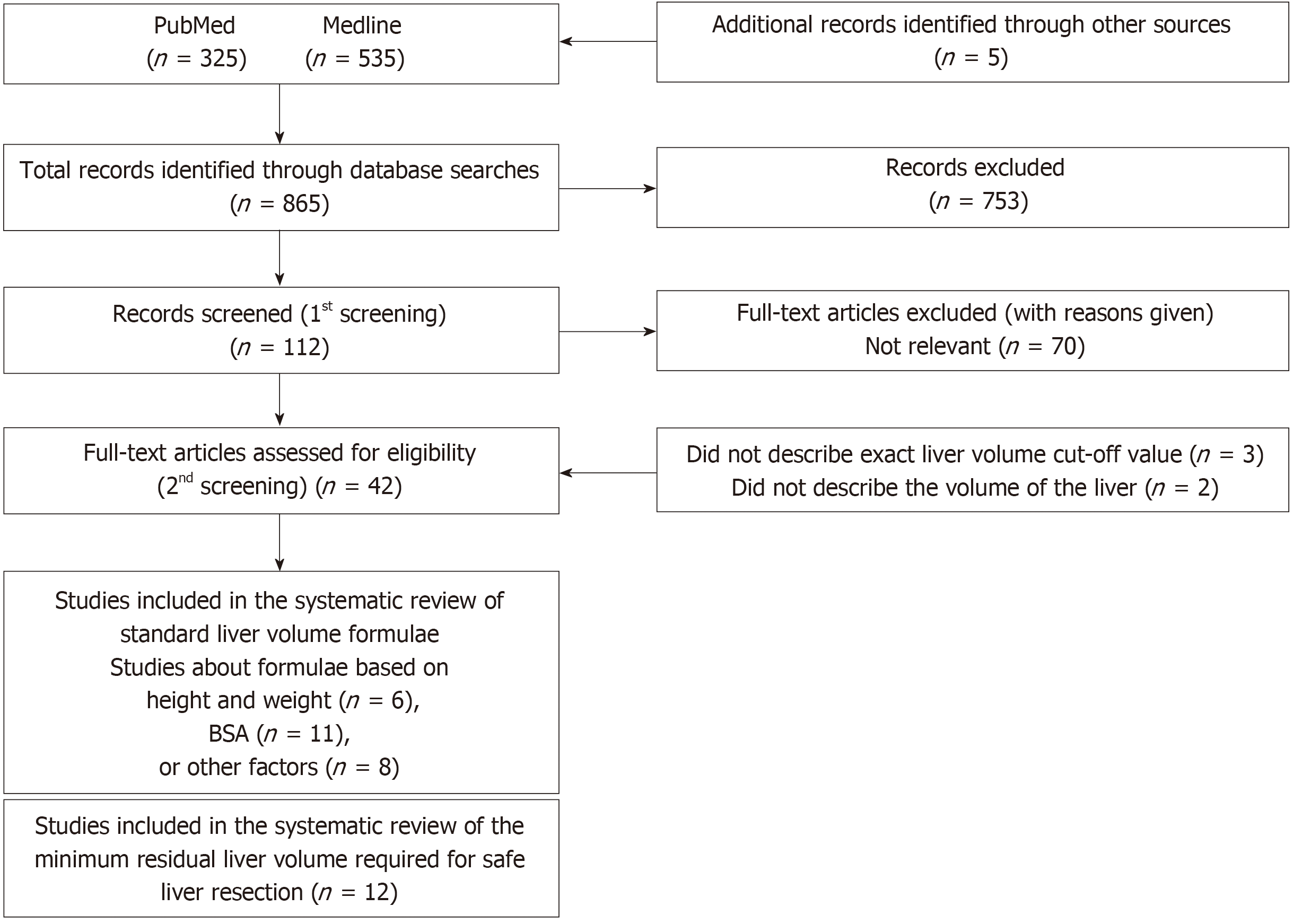
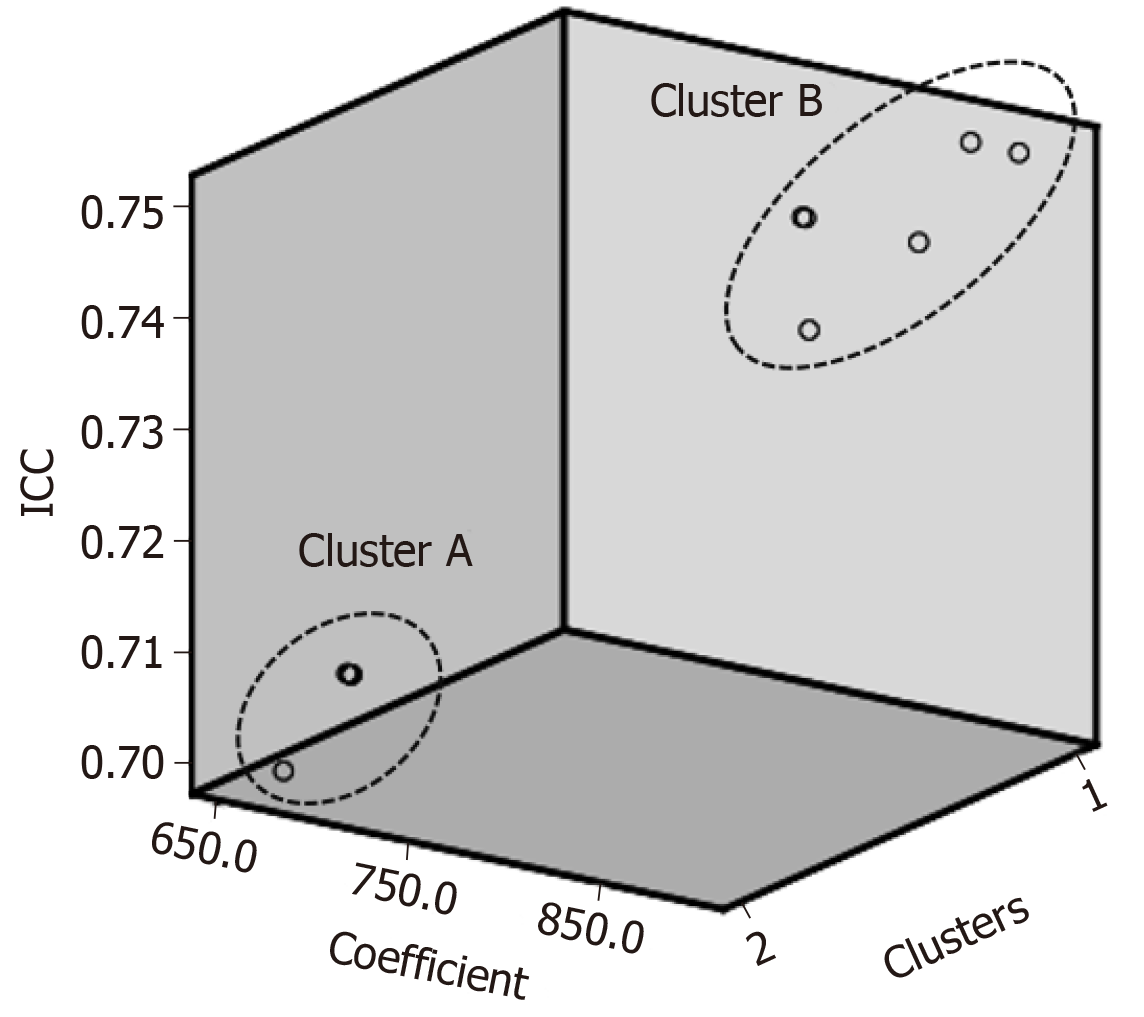
A PRISMA flow diagram is shown in Figure 4. Among the 25 studies about SLV formulae, three types of calculations were reported. The first group used height and weight as independent factors (Table 3)[22-28]; the second group used BSA (Table 4)[24,25,29-36]; and the third group used other variables including age, gender, race, and radiological findings (Table 5)[37-43]. Although the SLV formulae in the first and second groups included a variety of coefficient and constant values, they exhibited very similar ICC of between 0.70 and 0.78. On the other hand, in the third group the ICC of the SLV values obtained using age alone were very low (0.39 and -0.39, respectively). A combination of age and other variables gave ICC of between 0.66 and 0.79. If BSA were fixed at the mean value for the second group, the SLV formulae for the second group could be simplified as shown in Table 6. Although the previously reported SLV formulae included different coefficients and constant values, they could be grouped into two clusters (Figure 5). In cluster A, a simplified version of the SLV, the common SLV (cSLV), could be calculated as follows: cSLV (mL) = 710 × BSA, whereas in cluster B the cSLV could be calculated as follows: cSLV (mL) = 770 × BSA.
| Authors | Journals | Formulae | ICC | |
| Height and weight | Ogiu et al[22] | Health Phys, 1997 | LV = 576.9 × H + 8.9 × BW – 159.7 (males); LV = 674.3 × H + 6.5 × BW – 214.5 (females) | 0.73 |
| Lin et al[23] | Hepatogastroenterology, 1998 | LV = 133 × H + 12 × BW – 1530 | 0.78 | |
| Yu et al[24] | Liver Transpl, 2004 | LV = 21.585 × BW0.7322 × H0.225 | 0.77 | |
| Chandramohan et al[25] | Indian J Gastroenterol, 2007 | LV = 18.51 × BW + 191.80 | 0.77 | |
| Fu-Gui et al[26] | Transplant Proc, 2009 | LV = 11.508 × BW + 334.024 | 0.71 | |
| Poovathumkadavil et al[27] | Transplant Proc, 2010 | LV = 12.26 × BW + 555.65 | 0.72 | |
| Herden et al[28] | Transpl Int, 2013 | LV = −143.062973 + 4.274603051 × H + 14.78817631 × BW (Age: 0-1); LV = −20.2472281 + 3.339056437 × H + 13.11312561 × BW (Age: 1-16) | 0.76 |
| Authors | Journals | Formulae | ICC | |
| BSA | DeLand et al[29] | Radiology, 1968 | LV = 1020 × BSA – 220 | 0.77 |
| Urata et al[30] | Hepatology, 1995 | LV = 706.2 × BSA + 2.4 | 0.71 | |
| Murry et al[31] | Drug Metab Dispos, 1995 | LV = 710 × BSA | 0.71 | |
| Heinemann et al[32] | Liver Transpl Surg, 1999 | LV = 1072.8 × BSA – 345.7 | 0.78 | |
| Vauthey et al[33] | Liver Transpl, 2002 | LV = 1267.28 × BSA – 794.41 | 0.78 | |
| Yoshizumi et al[34] | Transplant Proc, 2003 | LV = 772 × BSA | 0.74 | |
| Yu et al[24] | Liver Transpl, 2004 | LV = 1145.4 × BSA − 506.1 (adults) | 0.78 | |
| Hashimoto et al[35] | J Gastroenterol Hepatol, 2006 | LV = 961.3 × BSA – 404.8 | 0.77 | |
| Chandramohan et al[25] | Indian J Gastroenterol, 2007 | LV = 1267.28 × BSA – 794.41 | 0.78 | |
| Saeki et al[36] | Pediatr Transplant, 2012 | LV = 689.9 × BSA − 24.7 | 0.70 | |
| Our study | LV = 822.7 × BSA – 183.2 | 0.74 |
| Authors | Journals | Formulae | ICC | |
| Others | Takahashi et al[37] | Clin Pharmacol Ther, 2000 | LV = 15 × (4.6 × Age + 19.8), Age: 1-18; LV = 15 × (0.31 × Age + 97.8), Age: 30-40; LV = 15 × (–0.91 × Age + 149), Age: ≥ 41 | 0.39 |
| Kanamori et al[38] | Int J Clin Pharmacol Ther, 2002 | LV = 67.3 × Age + 229.8 | −0.39 | |
| Choukèr et al[39] | Liver Transpl, 2004 | LV=452 + 16.34 × BW + 11.85 × Age – 166 × Gender (Age: 16–50, M: 0, F: 1); LV=1390 + 15.94 × BW – 12.86 × Age (Age: 51–70) | 0.79 | |
| Chan et al[40] | World J Gastroenterol, 2006 | LV = 218 + BW × 12.3 + Gender × 51 (F: 0, M: 1) | 0.74 | |
| Yuan et al[41] | Transplant Proc, 2008 | LV = 949.7 × BSA − 48.3 × Age − 247.4 (Age: 1: < 40, 2: 41–60, 3: > 60) | 0.77 | |
| Kokudo et al[42] | J Hepatol, 2015 | LV = 203.3 − 3.61 × Age + 58.7 × Thoracic width − 463.7 × Race (Asian: 1, Caucasian: 0) | 0.66 | |
| Ma et al[43] | Liver Transpl, 2017 | LV = (2 × Depth) + (10 × BW) + 190 | 0.75 |
| Authors | Mean age ± SD (range) | BSA | Simple formulae (tentative mean BSA = 1.61) | ICC | Clusters |
| DeLand et al[29] | ND | ND | LV = 883 × BSA | 0.75 | B |
| Urata et al[30] | 11.1 ± 8.8 | 1.078 ± 0.528 (0.248–1.935) | LV = 707 × BSA | 0.71 | A |
| Murry et al[31] | 9.7 (3.3–18.8) | Median: 1.37 (0.57–2.0) | LV = 710 × BSA | 0.71 | A |
| Heinemann et al[32] | 50.6±18.9 | ND | LV = 858 × BSA | 0.75 | B |
| Vauthey et al[33] | Mean: 54, Median: 56 (14-90) | Median: 1.82 (1.32–2.90) | LV = 770 × BSA | 0.74 | B |
| Yoshizumi et al[34] | 38.6 ± 20.6 (0–87) for males; 47.0 ± 19.7 (0–85) for females | 1.86 ± 0.36 (0.24-2.88); 1.68 ± 0.28 (0.28-2.38) | LV = 772 × BSA | 0.74 | B |
| Yu et al[24] | 42.4 ± 16.5 | 1.65 ± 0.26 | LV = 831 × BSA | 0.74 | B |
| Hashimoto et al[35] | (17-66) | 1.67 ± 0.18 (1.25-2.56) | LV = 710 × BSA | 0.71 | A |
| Chandramohan et al[25] | 46.5 (10-70) | Median: 1.60 (0.88-2.25) | LV = 774 × BSA | 0.73 | B |
| Saeki et al[36] | 5.8 (0 d-15) | ND | LV = 675 × BSA | 0.70 | A |
| Our study | 67.0 ± 10.3 | 1.61 ± 0.18 | LV = 709 × BSA | 0.71 | A |
The minimum RLV required for safe liver resection has been debated for several decades. Most studies that examined this issue involved the use of normal livers containing metastatic liver tumors or transplanted livers for volume estimation[44-48]. All of the studies except ours calculated minimum cut-off values based on pathological findings (Table 7)[16,17,49-57]. According to previous reports, the minimum RLV for normal livers ranged from 20%-40%[54-56], whereas that for damaged livers ranged from 30%-50%[16,52]. In contrast, the cut-off values obtained in the present study depended on liver function. According to the Sapporo score, the RLV cut-off values ranged from 35%-95%[20]. In addition, mortality rate ranged from 0.8% to 11% (Table 7).
| Authors | Publications | Functional assessments | Minimum residual LV | Mortality | ||
| NL | CH, liver injury | LC | ||||
| Shirabe et al[49] | J Am Coll Surg, 1999 | Pathology (HCC, HB, or HC) | 250 mL/m2 (40%) | 8.8% (180 d) | ||
| Shoup et al[50] | J Gastrointest Surg, 2003 | Pathology (NL, CRC metastasis alone) | 25% | - | - | ND |
| Schindl et al[51] | Gut, 2005 | Pathology (NL, 99% metastasis) | 26.6% | - | - | ND |
| Ferrero et al[17] | World J Surg, 2007 | Pathology (NL, liver injury) | 26.5% | 31% | - | 0.8% (60 d) |
| van den Esschert et al[52] | J Gastrointest Surg, 2009 | Pathology (CH, LC) | - | 40% | 50% | ND |
| Kishi et al[53] | Ann Surg, 2009 | Pathology (NL) | 20% | - | - | 2.0% (30 d) 4.7% (60 d) 6.0% (90 d) |
| Suda et al[54] | Am J Surg, 2009 | Pathology (NL, HCCa, GBCa, ICCa) | 40% | - | - | 8.1% (ND) |
| Vauthey et al[55] | HPB, 2010 | Pathology (NL, liver injury, LC) | 20% | 30% | 40% | ND |
| Gulielmi et al[56] | Dig Surg, 2012 | Pathology (NL, steatosis, LC) | 20% | 30% | 40% | ND |
| Hwang et al[16] | J Gastrointest Surg, 2015 | Pathology (CH, LC) | - | 35% | 30% | 0.8% (90 d) |
| Ribero et al[57] | J Am Coll Surg, 2016 | Pathology (NL, HCCa alone) | 30% | - | - | 11% (90 d) |
| Our series | Hepatogastroenterolo-gy (in press) | ATIII, ICG15, GSA | 35%-95% | 2.3% (90 d) | ||
We reviewed the previously described formulae for calculating SLV and the minimum RLV required for safe liver resection. Although various SLV formulae have been reported, some of them were similar[24,30,32-35]. Therefore, we simplified the formula for estimating SLV to produce the cSLV. Furthermore, we found that the minimum RLV required for a safe hepatectomy ranged from 25%-50% depending on the pathological background. The Sapporo score is the only liver function-based method for determining the minimum RLV.
Liver volume is obviously correlated with physical parameters[24,32-35]. However, the physiques of children and adults are markedly different[24]. In addition, the coefficients for the relationships among liver volume and physical parameters change during growth[24]. Yu et al[24] attempted to develop a non-linear or stepwise model for estimating liver volume, whereas the other reported models were linear models. Unfortunately, this elaborate model did not become popular. One possible explanation for this is that it might be too elaborate for estimating SLV, and the use of other simpler models does not result in favorable outcomes.
BSA-based models for calculating SLV are very simple and are widely used in the clinical setting. However, 25 different formulae for calculating BSA have been proposed[58]. The first formula for calculating BSA was reported in 1879 by Meeh et al[59]. Subsequently, the DuBios brothers developed a formula that included height and weight as variables[59,60]. This has remained the standard formula over the past century and we also used it for this study. However, these formulae do not produce precise estimates of BSA and provide no information regarding interindividual variability[58]. Therefore, SLV varies markedly depending on which BSA formula is used.
Since the variation in SLV is not as large as that in BSA, similar coefficient and constant values were used to calculate SLV in previous studies. We identified two clusters of SLV formulae, as shown in Figure 5, and created a simplified cSLV formula for each cluster. The cluster analysis actually identified three clusters, but two of the clusters were very similar and not significantly different (data not shown). Therefore, we combined them together as cluster B. The differences among the clusters related to age or BSA. The age and BSA of cluster A tended to be younger and smaller, respectively, than those of cluster B. Therefore, differences in the patients’ background data might have affected the coefficients used and the resultant ICC. Cluster B displayed ICC greater values than cluster A, although the exact reason for this was unclear. One possible explanation is that cSLV stabilized in elderly patients, and so the error range became smaller than that found in younger patients.
SLV is affected by aging; i.e., it was reported to be 4% of body weight at birth, but only 2%-2.7% of body weight in adults[30,61]. Therefore, age is an important factor when comparing the formulae used to calculate SLV. A study by Urata et al[30] involved young patients, whereas other studies involved adults[24,30,32-35]. Our study population was older than those employed in previous studies. However, the formulae produced in each study were very similar. Although the SLV is affected by aging, it might remain relatively constant in all patients.
Takahashi et al[37] and Kanamori et al[38] proposed that SLV can be assessed using age alone. However, their approach would not have been appropriate for our patient population, in which most patients were elderly. On the other hand, a combination of age and other variables provided ICC of between 0.66 and 0.79. Thus, it is likely that SLV is partially affected by aging. Although elaborate formulae were created in the third group, this did not result in better ICC compared with those seen in the other groups. Therefore, simple SLV formulas could be applied to patients who are > 10 years old.
The issue of the minimum RLV does not only involve the reduction in liver volume, but also several other factors. For example, bile duct reconstruction could be one of the predictors of short-term clinical outcomes[62,63]. The frequency of bile leakage is higher in cases involving biliary reconstruction after hepatectomy than in cases in which biliary reconstruction is not performed[64]. In addition, biliary reconstruction can cause intra-abdominal leakage followed by intra-abdominal infection[64,65]. Therefore, the minimum RLV might differ between cases that do and do not involve biliary reconstruction. Second, the background of the liver also plays an important role in determining clinical outcomes. The general question is how we could evaluate liver damage before surgery. Several liver function evaluation methods have been proposed, including methods based on serum protein levels, serum enzyme levels, the ICGR15, and radiological assessments[66-68]. However, none of them represent liver function perfectly. For example, ICGR15 has been used for several decades; however, it does not reflect liver function in patients that exhibit ICG intolerance or possess an arteriovenous shunt. Radiological evaluations are also affected by the systemic circulation, e.g., by dehydration and heart failure. Serum protein and enzyme levels are too stable to allow them to be used to evaluate liver function in the initial stages of liver damage, and they might not be valuable until the terminal stages of disease progression. Therefore, the Sapporo score is still the only method for evaluating liver function, regardless of the degree of disease progression.
The other factors that might affect postoperative liver function include the concordance rate of the removed segments and the blood supply[69]. The patency of veins is also considered to affect back-flow control[70,71]. Therefore, evaluations of liver function should take both biochemical and anatomical findings into account. Although the Sapporo score is a useful method for evaluating liver function, some technical issues need to be solved before it is used to assess liver function in the clinical setting.
In conclusion, we reviewed SLV formulae and the minimum RLV required for safe liver resection. Although several SLV formulae have been presented, we created two simple SLV formulae that could be applied to the clinical setting. The Sapporo score is the only liver function-based method for estimating the minimum RLV.
Various minimum residual liver volume (RLV) has been presented. In addition, many formulas of standard liver volume (SLV) were also established.
When we planned hepatectomy for malignant tumors, we had not proven which methods were the best reliable assessment to estimate minimum RLV and SLV.
Aim of this study was to review previous SLV formulae and the methods used to evaluate the minimum RLV, and explore the association between liver volume and mortality.
A systematic review was performed (No. CRD42019123642). We developed an SLV formula using data for 86 consecutive patients who underwent hepatectomy at our institution between July 2009 and August 2011.
Our formula: SLV (mL) = 822.7 × BSA − 183.2 (R2 = 0.419 and R = 0.644, P < 0.001). We retrieved 25 studies relating to SLV formulae and 12 studies about the RLV required for safe liver resection. The minimum RLV for normal and damaged livers ranged from 20%-40% and 30%-50%, respectively. The Sapporo score indicated that the minimum RLV ranges from 35%-95% depending on liver function.
We reviewed SLV formulae and the minimum RLV required for safe liver resection. Although several SLV formulae have been presented, we created two simple SLV formulae that could be applied to the clinical setting. The Sapporo score is the only liver function-based method for estimating the minimum RLV.
The Sapporo score should be validated by large study with prospective registration. The common SLV, which is cSLV (mL) = 710 or 770 × body surface area, needs to verify in the specific population.
We thank Sandy Tan and Thomas Hui for their help in preparing this manuscript and their valuable discussions.
PRISMA 2009 checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Shen ZY, Gumbs A, Lee HC S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 2. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3597] [Article Influence: 276.7] [Reference Citation Analysis (4)] |
| 3. | Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 799] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 4. | Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198-206; discussion 1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 615] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 5. | Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver Transpl. 2004;10:S39-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Wu CC, Cheng SB, Ho WM, Chen JT, Liu TJ, P'eng FK. Liver resection for hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2005;92:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Eguchi S, Kanematsu T, Arii S, Omata M, Kudo M, Sakamoto M, Takayasu K, Makuuchi M, Matsuyama Y, Monden M; Liver Cancer Study Group of Japan. Recurrence-free survival more than 10 years after liver resection for hepatocellular carcinoma. Br J Surg. 2011;98:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Rau HG, Schauer R, Helmberger T, Holzknecht N, von Rückmann B, Meyer L, Buttler E, Kessler M, Zahlmann G, Schuhmann D, Schildberg FW. Impact of virtual reality imaging on hepatic liver tumor resection: calculation of risk. Langenbecks Arch Surg. 2000;385:162-170. [PubMed] |
| 9. | Wigmore SJ, Redhead DN, Yan XJ, Casey J, Madhavan K, Dejong CH, Currie EJ, Garden OJ. Virtual hepatic resection using three-dimensional reconstruction of helical computed tomography angioportograms. Ann Surg. 2001;233:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 11. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5737] [Article Influence: 110.3] [Reference Citation Analysis (2)] |
| 12. | Kudo M, Kubo S, Takayasu K, Sakamoto M, Tanaka M, Ikai I, Furuse J, Nakamura K, Makuuchi M; Liver Cancer Study Group of Japan (Committee for Response Evaluation Criteria in Cancer of the Liver, Liver Cancer Study Group of Japan). Response Evaluation Criteria in Cancer of the Liver (RECICL) proposed by the Liver Cancer Study Group of Japan (2009 Revised Version). Hepatol Res. 2010;40:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Mizuguchi T, Kawamoto M, Meguro M, Nakamura Y, Harada K, Kukita K, Hirata K. Prognostic impact of preoperative the branched-chain amino acid to the tyrosine ratio in hepatocellular carcinoma patients after initial hepatectomy. J Gastrointest Surg. 2011;15:1433-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1521] [Cited by in RCA: 2017] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 15. | Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4165] [Cited by in RCA: 5298] [Article Influence: 529.8] [Reference Citation Analysis (1)] |
| 16. | Hwang S, Ha TY, Song GW, Jung DH, Ahn CS, Moon DB, Kim KH, Lee YJ, Lee SG. Quantified Risk Assessment for Major Hepatectomy via the Indocyanine Green Clearance Rate and Liver Volumetry Combined with Standard Liver Volume. J Gastrointest Surg. 2015;19:1305-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Ferrero A, Viganò L, Polastri R, Muratore A, Eminefendic H, Regge D, Capussotti L. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg. 2007;31:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Yamanaka J, Okada T, Saito S, Kondo Y, Yoshida Y, Suzumura K, Hirano T, Iimuro Y, Fujimoto J. Minimally invasive laparoscopic liver resection: 3D MDCT simulation for preoperative planning. J Hepatobiliary Pancreat Surg. 2009;16:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Hori M, Suzuki K, Epstein ML, Baron RL. Computed tomography liver volumetry using 3-dimensional image data in living donor liver transplantation: effects of the slice thickness on the volume calculation. Liver Transpl. 2011;17:1427-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Harada K, Mizuguchi T, Kawamoto M, Meguro M, Ota S, Sasaki S, Miyanishi K, Hatakenaka M, Shinomura Y, Kato J, Hirata K. Prediction of postoperative liver failure and evaluation of modified criteria for liver resection with computed volume analysis. Hepatogastroenterology. 2014;In press. |
| 21. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828-discussion 829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 823] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 22. | Ogiu N, Nakamura Y, Ijiri I, Hiraiwa K, Ogiu T. A statistical analysis of the internal organ weights of normal Japanese people. Health Phys. 1997;72:368-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Lin XZ, Sun YN, Liu YH, Sheu BS, Cheng BN, Chen CY, Tsai HM, Shen CL. Liver volume in patients with or without chronic liver diseases. Hepatogastroenterology. 1998;45:1069-1074. [PubMed] |
| 24. | Yu HC, You H, Lee H, Jin ZW, Moon JI, Cho BH. Estimation of standard liver volume for liver transplantation in the Korean population. Liver Transpl. 2004;10:779-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Chandramohan A, Eapen A, Govil S, Govil S, Jeyaseelan V. Determining standard liver volume: assessment of existing formulae in Indian population. Indian J Gastroenterol. 2007;26:22-25. [PubMed] |
| 26. | Fu-Gui L, Lu-Nan Y, Bo L, Yong Z, Tian-Fu W, Ming-Qing X, Wen-Tao W, Zhe-Yu C. Estimation of standard liver volume in Chinese adult living donors. Transplant Proc. 2009;41:4052-4056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Poovathumkadavil A, Leung KF, Al Ghamdi HM, Othman Iel H, Meshikhes AW. Standard formula for liver volume in Middle Eastern Arabic adults. Transplant Proc. 2010;42:3600-3605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Herden U, Wischhusen F, Heinemann A, Ganschow R, Grabhorn E, Vettorazzi E, Nashan B, Fischer L. A formula to calculate the standard liver volume in children and its application in pediatric liver transplantation. Transpl Int. 2013;26:1217-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 29. | DeLand FH, North WA. Relationship between liver size and body size. Radiology. 1968;91:1195-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, Momose Y, Komiyama A, Makuuchi M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 704] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 31. | Murry DJ, Crom WR, Reddick WE, Bhargava R, Evans WE. Liver volume as a determinant of drug clearance in children and adolescents. Drug Metab Dispos. 1995;23:1110-1116. [PubMed] |
| 32. | Heinemann A, Wischhusen F, Püschel K, Rogiers X. Standard liver volume in the Caucasian population. Liver Transpl Surg. 1999;5:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 194] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, Lerut J, Materne R, Wang X, Encarnacion A, Herron D, Mathey C, Ferrari G, Charnsangavej C, Do KA, Denys A. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 465] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 34. | Yoshizumi T, Gondolesi GE, Bodian CA, Jeon H, Schwartz ME, Fishbein TM, Miller CM, Emre S. A simple new formula to assess liver weight. Transplant Proc. 2003;35:1415-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Hashimoto T, Sugawara Y, Tamura S, Hasegawa K, Kishi Y, Kokudo N, Makuuchi M. Estimation of standard liver volume in Japanese living liver donors. J Gastroenterol Hepatol. 2006;21:1710-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Saeki I, Tokunaga S, Matsuura T, Hayashida M, Yanagi Y, Taguchi T. A formula for determining the standard liver volume in children: a special reference for neonates and infants. Pediatr Transplant. 2012;16:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Takahashi H, Ishikawa S, Nomoto S, Nishigaki Y, Ando F, Kashima T, Kimura S, Kanamori M, Echizen H. Developmental changes in pharmacokinetics and pharmacodynamics of warfarin enantiomers in Japanese children. Clin Pharmacol Ther. 2000;68:541-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Kanamori M, Takahashi H, Echizen H. Developmental changes in the liver weight- and body weight-normalized clearance of theophylline, phenytoin and cyclosporine in children. Int J Clin Pharmacol Ther. 2002;40:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Choukèr A, Martignoni A, Dugas M, Eisenmenger W, Schauer R, Kaufmann I, Schelling G, Löhe F, Jauch KW, Peter K, Thiel M. Estimation of liver size for liver transplantation: the impact of age and gender. Liver Transpl. 2004;10:678-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Chan SC, Liu CL, Lo CM, Lam BK, Lee EW, Wong Y, Fan ST. Estimating liver weight of adults by body weight and gender. World J Gastroenterol. 2006;12:2217-2222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Yuan D, Lu T, Wei YG, Li B, Yan LN, Zeng Y, Wen TF, Zhao JC. Estimation of standard liver volume for liver transplantation in the Chinese population. Transplant Proc. 2008;40:3536-3540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Kokudo T, Hasegawa K, Uldry E, Matsuyama Y, Kaneko J, Akamatsu N, Aoki T, Sakamoto Y, Demartines N, Sugawara Y, Kokudo N, Halkic N. A new formula for calculating standard liver volume for living donor liver transplantation without using body weight. J Hepatol. 2015;63:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Ma KW, Chok KSH, Chan ACY, Tam HSC, Dai WC, Cheung TT, Fung JYY, Lo CM. A new formula for estimation of standard liver volume using computed tomography-measured body thickness. Liver Transpl. 2017;23:1113-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Vibert E, Pittau G, Gelli M, Cunha AS, Jamot L, Faivre J, Castro Benitez C, Castaing D, Adam R. Actual incidence and long-term consequences of posthepatectomy liver failure after hepatectomy for colorectal liver metastases. Surgery. 2014;155:94-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Calatayud D, Sánchez Cabús S, Sampson J, Resendiz A, Molina V, Fondevila C, Fuster J, García-Valdecasas JC. Hepatic resection: a safe and effective surgery. Cir Esp. 2017;95:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 46. | Olthof PB, Tomassini F, Huespe PE, Truant S, Pruvot FR, Troisi RI, Castro C, Schadde E, Axelsson R, Sparrelid E, Bennink RJ, Adam R, van Gulik TM, de Santibanes E. Hepatobiliary scintigraphy to evaluate liver function in associating liver partition and portal vein ligation for staged hepatectomy: Liver volume overestimates liver function. Surgery. 2017;162:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 47. | Pietrasz D, Fuks D, Subar D, Donatelli G, Ferretti C, Lamer C, Portigliotti L, Ward M, Cowan J, Nomi T, Beaussier M, Gayet B. Laparoscopic extended liver resection: are postoperative outcomes different? Surg Endosc. 2018;32:4833-4840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Watanabe Y, Kuboki S, Shimizu H, Ohtsuka M, Yoshitomi H, Furukawa K, Miyazaki M. A New Proposal of Criteria for the Future Remnant Liver Volume in Older Patients Undergoing Major Hepatectomy for Biliary Tract Cancer. Ann Surg. 2018;267:338-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, Sugimachi K. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 326] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 50. | Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, Tuorto S, Blumgart LH, Fong Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 340] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 51. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ; Edinburgh Liver Surgery and Transplantation Experimental Research Group (eLISTER). The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 52. | van den Esschert JW, de Graaf W, van Lienden KP, Busch OR, Heger M, van Delden OM, Gouma DJ, Bennink RJ, Laméris JS, van Gulik TM. Volumetric and functional recovery of the remnant liver after major liver resection with prior portal vein embolization : recovery after PVE and liver resection. J Gastrointest Surg. 2009;13:1464-1469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, Curley SA, Vauthey JN. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 364] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 54. | Suda K, Ohtsuka M, Ambiru S, Kimura F, Shimizu H, Yoshidome H, Miyazaki M. Risk factors of liver dysfunction after extended hepatic resection in biliary tract malignancies. Am J Surg. 2009;197:752-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Vauthey JN, Dixon E, Abdalla EK, Helton WS, Pawlik TM, Taouli B, Brouquet A, Adams RB; American Hepato-Pancreato-Biliary Association; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract. Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford). 2010;12:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 56. | Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 248] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 57. | Ribero D, Zimmitti G, Aloia TA, Shindoh J, Fabio F, Amisano M, Passot G, Ferrero A, Vauthey JN. Preoperative Cholangitis and Future Liver Remnant Volume Determine the Risk of Liver Failure in Patients Undergoing Resection for Hilar Cholangiocarcinoma. J Am Coll Surg. 2016;223:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 58. | Redlarski G, Palkowski A, Krawczuk M. Body surface area formulae: an alarming ambiguity. Sci Rep. 2016;6:27966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 59. | Meeh K. Oberflächenmessungen des menschlichen Körpers. Ztschr f Biol. 1879;1:458-425. |
| 60. | Du Bois D, Du Bois E. A formula to estimate the approximate surface area if height and weight are known. Arch Intern Med. 1916;17:863–871. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3259] [Cited by in RCA: 3135] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 61. | Balistreri W. Nelson textbook of pediatrics. Philadelphia: Saunders 1992; 1001-1004. |
| 62. | Kadaba RS, Bowers KA, Khorsandi S, Hutchins RR, Abraham AT, Sarker SJ, Bhattacharya S, Kocher HM. Complications of biliary-enteric anastomoses. Ann R Coll Surg Engl. 2017;99:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 63. | Otto W, Sierdziński J, Smaga J, Dudek K, Zieniewicz K. Long-term effects and quality of life following definitive bile duct reconstruction. Medicine (Baltimore). 2018;97:e12684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Martin AN, Narayanan S, Turrentine FE, Bauer TW, Adams RB, Stukenborg GJ, Zaydfudim VM. Clinical Factors and Postoperative Impact of Bile Leak After Liver Resection. J Gastrointest Surg. 2018;22:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 65. | Jin S, Fu Q, Wuyun G, Wuyun T. Management of post-hepatectomy complications. World J Gastroenterol. 2013;19:7983-7991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 172] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (3)] |
| 66. | Mizuguchi T, Kawamoto M, Meguro M, Son S, Nakamura Y, Harada K, Shibata T, Ota S, Hirata K. Serum antithrombin III level is well correlated with multiple indicators for assessment of liver function and diagnostic accuracy for predicting postoperative liver failure in hepatocellular carcinoma patients. Hepatogastroenterology. 2012;59:551-557. [PubMed] |
| 67. | Harada K, Mizuguchi T, Katagiri Y, Kawamoto M, Nakamura Y, Meguro M, Ota S, Sasaki S, Miyanishi K, Sonoda T, Mori M, Shinomura Y, Kato J, Hirata K. Area between the hepatic and heart curves of (99m)Tc-galactosyl-human serum albumin scintigraphy represents liver function and disease progression for preoperative evaluation in hepatocellular carcinoma patients. J Hepatobiliary Pancreat Sci. 2012;19:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Ishii M, Mizuguchi T, Harada K, Ota S, Meguro M, Ueki T, Nishidate T, Okita K, Hirata K. Comprehensive review of post-liver resection surgical complications and a new universal classification and grading system. World J Hepatol. 2014;6:745-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | Sanjeevi S, Sparrelid E, Gilg S, Jonas E, Isaksson B. Arterial ischemia in the deportalized liver following associating liver partition and portal vein ligation for staged hepatectomy. World J Hepatol. 2015;7:2492-2496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 70. | Balzan SM, Gava VG, Magalhaes MA, Dotto ML. Outflow modulation to target liver regeneration: something old, something new. Eur J Surg Oncol. 2014;40:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 71. | Radtke A, Sgourakis G, Molmenti EP, Beckebaum S, Cicinnati VR, Schmidt H, Peitgen HO, Broelsch CE, Malagó M, Schroeder T. Risk of venous congestion in live donors of extended right liver graft. World J Gastroenterol. 2015;21:6008-6017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |