Published online Apr 26, 2017. doi: 10.13105/wjma.v5.i2.14
Peer-review started: October 9, 2016
First decision: December 29, 2016
Revised: January 10, 2017
Accepted: February 28, 2017
Article in press: March 2, 2017
Published online: April 26, 2017
To review evidence relating passive smoking to heart disease risk in never smokers.
Epidemiological studies were identified providing estimates of relative risk (RR) of ischaemic heart disease and 95%CI for never smokers for various indices of exposure to environmental tobacco smoke (ETS). “Never smokers” could include those with a minimal smoking experience. The database set up included the RRs and other study details. Unadjusted and confounder-adjusted RRs were entered, derived where necessary using standard methods. The fixed-effect and random-effects meta-analyses conducted for each exposure index included tests for heterogeneity and publication bias. For the main index (ever smoking by the spouse or nearest equivalent, and preferring adjusted to unadjusted data), analyses investigated variation in the RR by sex, continent, period of publication, number of cases, study design, extent of confounder adjustment, availability of dose-response results and biomarker data, use of proxy respondents, definitions of exposure and of never smoker, and aspects of disease definition. Sensitivity analyses were also run, preferring current to ever smoking, or unadjusted to adjusted estimates, or excluding certain studies.
Fifty-eight studies were identified, 20 in North America, 19 in Europe, 11 in Asia, seven in other countries, and one in 52 countries. Twenty-six were prospective, 22 case-control and 10 cross-sectional. Thirteen included 100 cases or fewer, and 11 more than 1000. For the main index, 75 heterogeneous (P < 0.001) RR estimates gave a combined random-effects RR of 1.18 (95%CI: 1.12-1.24), which was little affected by preferring unadjusted to adjusted RRs, or RRs for current ETS exposure to those for ever exposure. Estimates for each level of each factor considered consistently exceeded 1.00. However, univariate analyses revealed significant (P < 0.001) variation for some factors. Thus RRs were lower for males, and in North American, larger and prospective studies, and also where the RR was for spousal smoking, fatal cases, or specifically for IHD. For case-control studies RRs were lower if hospital/diseased controls were used. RRs were higher when diagnosis was based on medical data rather than death certificates or self-report, and where the never smoker definition allowed subjects to smoke products other than cigarettes or have a limited smoking history. The association with spousal smoking specifically (1.06, 1.01-1.12, n = 34) was less clear in analyses restricted to married subjects (1.03, 0.99-1.07, n = 23). In stepwise regression analyses only those associations with source of diagnosis, study size, and whether the spouse was the index, were independently predictive (at P < 0.05) of heart disease risk. A significant association was also evident with household exposure (1.19, 1.13-1.25, n = 37). For those 23 studies providing dose-response results for spouse or household exposure, 11 showed a significant (P < 0.05) positive trend including the unexposed group, and two excluding it. Based on fewer studies, a positive, but non-significant (P > 0.05) association was found for workplace exposure (RR = 1.08, 95%CI: 0.99-1.19), childhood exposure (1.12, 0.95-1.31), and biomarker based exposure indices (1.15, 0.94-1.40). However, there was a significant association with total exposure (1.23, 1.12-1.35). Some significant positive dose-response trends were also seen for these exposure indices, particularly total exposure, with no significant negative trends seen. The evidence suffers from various weaknesses and biases. Publication bias may explain the large RR (1.66, 1.30-2.11) for the main exposure index for smaller studies (1-99 cases), while recall bias may explain the higher RRs seen in case-control and cross-sectional than in prospective studies. Some bias may also derive from including occasional smokers among the “never smokers”, and from misreporting smoking status. Errors in determining ETS exposure, and failing to update exposure data in long term prospective studies, also contribute to the uncertainty. The tendency for RRs to increase as more factors are adjusted for, argues against the association being due to uncontrolled confounding.
The increased risk and dose-response for various exposure indices suggests ETS slightly increases heart disease risk. However heterogeneity, study limitations and possible biases preclude definitive conclusions.
Core tip: We present an up-to-date meta-analysis of the evidence relating environmental tobacco smoke (ETS) exposure to heart disease risk in never smokers. An association is evident for smoking by the spouse (or nearest equivalent) with the relative risk estimated as 1.18 (95%CI: 1.12-1.24), and also with some other indices of ETS exposure. Though the findings suggest a causal relationship, data limitations and bias limit interpretation.
- Citation: Lee PN, Forey BA, Hamling JS, Thornton AJ. Environmental tobacco smoke exposure and heart disease: A systematic review. World J Meta-Anal 2017; 5(2): 14-40
- URL: https://www.wjgnet.com/2308-3840/full/v5/i2/14.htm
- DOI: https://dx.doi.org/10.13105/wjma.v5.i2.14
This review concerns studies of environmental tobacco smoke (ETS) and heart disease in lifelong non-smokers (“never smokers”). In the 1990s some reviewers[1-4] concluded that exposure of non-smokers to ETS increases risk of heart disease, based partly on meta-analyses of epidemiological data from between 12 and 19 studies which reported statistically significant overall increases of about 25%, and partly on evidence from experimental and clinical studies. Their conclusions were accepted by some major bodies[5-8], and supported by some other reviewers[9-13]. However, other reviewers[14-18] disagreed, pointing to omission of relevant studies, inclusion of inappropriate estimates, heterogeneity of findings, study weaknesses and various sources of bias, as well as limitations in the experimental and clinical evidence.
Since then, the number of relevant epidemiological studies has increased, with over 50 now published. However, no recent comprehensive meta-analysis has been conducted, one published in 2015[13] including fewer studies than in some earlier reviews.
Our main objective is to present an updated meta-analysis of the epidemiological data, although we also briefly discuss the experimental evidence, and studies of smoking bans.
Attention is restricted to epidemiological prospective, case-control or cross-sectional studies providing relative risk (RR) estimates for never smokers for one or more of these ETS exposure indices: Spouse (including cohabiting partner), other at home exposure, at work, in adulthood, in childhood, in total, and biomarker based. We use the term “relative risk” to include estimates of it, such as the odds ratio or hazard ratio. Results must be available for a disease definition sufficiently close to ischaemic heart disease (IHD) as currently defined. Studies using a near equivalent definition of “never smokers” are accepted when results for stricter definitions are unavailable. Thus, never smokers may include occasional smokers, those with a minimal lifetime duration of smoking or number smoked, or those who quit at least 5 years ago.
At intervals until July 2016 potentially relevant papers were regularly sought from Medline searches, from extensive in-house files accumulated over many years and from references cited in papers obtained. At the end of the process no paper examined cited a possibly relevant paper not previously examined. The latest search used the terms [“tobacco smoke pollution” (MeSH terms)] AND {[“heart diseases”(MeSH Terms)] OR [“cardiovascular diseases” (MeSH Terms)] OR [“myocardial infarction” (MeSH Terms)]} AND (“2012/0101”[Date-MeSH]:”3000”[Date-MeSH]), restricted to humans, and published in the last 5 years.
Relevant publications were separated into studies, noting multiple papers per study or multiple studies per paper, and any study overlaps.
Details were extracted on study author, publication year, study location and design, sexes included, number of cases, potential confounding variables considered, and definitions of disease and of never smoker. RR estimates, together with associated 95%CIs were obtained, where available, for ETS exposure at home, at work, in childhood, and in total, and using biomarker based estimates (cotinine or COHb). Separate estimates were extracted or calculated for fatal, non-fatal and overall outcomes and for both unadjusted (or for prospective studies, age-adjusted) and covariate-adjusted RRs. If a study provided more than one adjusted estimate, we used that adjusted for most covariates.
Where studies report RRs/CIs only by level of exposure, those for the overall unexposed/exposed comparisons were estimated[19,20]. These methods were also used to estimate significance of dose-related trends, if not given in the source. Similar methods were used to estimate RRs and CIs excluding stroke from a broader circulatory disease definition.
Pre-planned fixed-effect and random-effects meta-analyses were conducted using standard methods[21]. Heterogeneity between RR estimates was assessed by the heterogeneity χ2, the ratio of which to its degrees of freedom, H, relates to the I2 statistic[22] by I2 = 100 (H-1)/H. Publication bias tests were also carried out[23].
For our main analyses, we aimed to produce an exposure index most closely equivalent to “spouse ever smoked”, since spousal smoking is the traditional index for studying ETS effects, women married to a smoker having a markedly higher ETS exposure, as measured by cotinine, than women married to a non-smoker[24]. Thus, results (sex-specific if available, otherwise combined sex) were selected in the following order of preference for: Exposure (spouse, household, total), time of exposure (ever, during marriage, current, in the past, in the last 10 years, in adulthood), disease type (fatal or non-fatal, fatal only, non-fatal only), disease definition (circulatory disease minus stroke, overall circulatory disease), and definition of no ETS exposure (unexposed to the specific ETS exposure, unexposed to any ETS, low exposure to the specific ETS exposure, never exposed to the specific ETS exposure, unexposed to ETS at home and at work). In addition, results selected were those adjusted for the most confounders for which results were given. This approach of selecting the most relevant result allowed the meta-analyses to include results from each study. Apart from conducting meta-analyses based on all selected estimates, additional meta-analyses using the same set of estimates, investigated variation in RR by the factors sex, continent, publication period, number of cases, study type, number of confounders considered in the study, availability of dose-response results, whether the spouse was the index, and whether (where the spouse was the index), analyses excluded unmarried subjects. Variation was also studied by fatality of cases, definition of disease, whether biomarker data was used to exclude smokers, use of proxy respondents, type of control used, source of diagnosis, and never smoker definition.
Sensitivity analyses repeated the complete set of meta-analyses described above for the main index of exposure with the order of preference for time of exposure revised to favour current rather than ever exposure (current, during marriage, ever, in the past, in the last 10 years, in adulthood), and also preferring unadjusted (or least adjusted) estimates. Further sensitivity analyses were carried out omitting results from: (1) studies by Layard[25] and LeVois et al[26]; (2) a study by Enstrom et al[27]; or (3) all three studies. These studies have been criticised (see discussion).
For the main exposure index stepwise regression analysis using forward selection[28] was also used to determine factors independently predicting risk of heart disease.
Similar meta-analyses were also conducted for other indices with sufficient data (household, workplace, childhood, total, biomarker based), though the meta-analyses by subset were more limited.
Results of meta-analyses are displayed in forest plots. Within each plot, study estimates are listed in increasing order of RR. For the main index, the estimates are grouped by location. The estimates are shown both as numbers and in graphical form logarithmically. In the latter representation an RR is shown as a square with area proportional to its inverse-variance weight. Arrows warn if a CI extends outside the range of the plot. Random-effects estimates are also presented, overall and by location, shown by a diamond whose width indicates the 95%CI.
Fifty-eight studies met the inclusion criteria. These come from 57 publications[25-27,29-82], one publication[66] describing results from two studies. Table 1 gives study details including author, reference(s), publication year, location, design, sexes included, disease definition and fatality, and numbers of cases in never smokers. The studies are listed in chronological order of publication and given consecutive study numbers. Minor overlap between cases in studies 16 and 30, was ignored. Table 2 gives variables adjusted for and never smoker definitions. Supplementary File 1 describes why other publications which might be thought possibly relevant are not included.
| Study No. | Ref.1 | Year2 | Location | Type3 | Sexes included4 | Disease fatality5 | Disease definition6 | No. of cases7 |
| 1 | Hirayama[29] | 1984 | Japan | P16 | F | F | IHD | 494 |
| 2 | Garland et al[30] | 1985 | United States/California | P10 | F | F | IHD | 19 |
| 3 | Lee et al[31] | 1986 | England | CC | M, F | NF | IHD | 118 |
| 4 | Martin et al[32] | 1986 | United States/Utah | CS | F | NF | PHA | 23 |
| 5 | Svendsen et al[33] | 1987 | United States | P9 | M | F + NF | IHD | 69 |
| 6 | Butler[34] | 1988 | United States/California | P6 | F | F | IHD | 808 |
| 7 | Palmer et al[35] | 1988 | United States/Not known | CC | F | NF | MI | 336 |
| 8 | Hole et al[36] | 1989 | Scotland | P12 | M, F | F, NF | IHD, A/E | 120 |
| 9 | Jackson[37] | 1989 | New Zealand | CC | M, F | F + NF | IHD + MI | 303 |
| 10 | Sandler et al[38] | 1989 | United States/Maryland | P12 | M, F | F | AHD | 1358 |
| 11 | Humble et al[39] | 1990 | United States/Georgia | P20 | F | F | CVD | 76 |
| 12 | Dobson et al[40] | 1991 | Australia | CC | M, F | F + NF | IHD + MI | 343 |
| 13 | Gardiner et al[41] | 1992 | Scotland | CC | M + F | F + NF | IHD | 12 |
| 14 | La Vecchia et al[42] | 1993 | Italy | CC | M, F | NF | FMI | 113 |
| 15 | Layard[25] | 1995 | United States | CC | M, F | F | IHD | 1389 |
| 169 | Le Vois et al[26] (CPS I) | 1995 | United States | P13 | M, F | F | AHD | 14891 |
| 17 | Mannino et al[43] | 1995 | United States | CS | M + F | NF | CVD | ? |
| 18 | Muscat et al[44] | 1995 | United States/4 cities | CC | M, F | NF | NMI | 114 |
| 19 | Tunstall-Pedoe et al[45] | 1995 | Scotland | CS | M + F | NF | IHD | 428 |
| 20 | Steenland et al[46] | 1996 | United States | P7 | M, F | F | IHD | 3819 |
| 21 | Janghorbani et al[47] | 1997 | Iran | CC | F | NF | IHD | 200 |
| 22 | Kawachi et al[48] | 1997 | United States | P10 | F | F + NF | IHD + MI | 152 |
| 23 | Ciruzzi et al[49] | 1998 | Argentina | CC | M, F | NF | FMI | 336 |
| 24 | McElduff et al[50] | 1998 | Australia | CC | M, F | F + NF | MI | 283 |
| 25 | Spencer et al[51] | 1999 | Australia | CC | M | NF | FMIS | 91 |
| 26 | He et al[52] | 2000 | China/Xi’an | CC | F | NF | MI/CS | 115 |
| 27 | Iribarren et al[53] | 2001 | United States | CS | M, F | NF | HD | 4801 |
| 28 | Rosenlund et al[54] | 2001 | Sweden | CC | M, F | NF | FMI | 334 |
| 29 | Pitsavos et al[55] | 2002 | Greece | CC | M + F | NF | FMI/UA | 279 |
| 309 | Enstrom et al[27] | 2003 | United States/California | P39 | M, F | F | IHD | 5932 |
| 31 | Chen et al[56] | 2004 | Scotland | CS | M + F | NF | IHD | 385 |
| 32 | Nishtar et al5710 | 2004 | Pakistan | CC | M + F | NF | CAD | ? |
| 3311 | Whincup et al[58] | 2004 | Great Britain | P21 | M | F + NF | IHD | 111 |
| 34 | McGhee et al[59] | 2005 | Hong Kong | CC | M, F | F | IHD | 584 |
| 35 | Qureshi et al[60] | 2005 | United States | P11 | F | F + NF | CVD | 328 |
| CVD-Stroke | 219 | |||||||
| 36 | Hedblad et al[61] | 2006 | Sweden | P19 | M | F + NF | IHD + MI, FMI | 91 |
| 37 | Stranges et al[62] | 2006 | United States | CC | M, F | NF | FMI | 284 |
| 38 | Teo et al[63] | 2006 | 52 countries | CC | M + F | NF | FMI | 6280 |
| 39 | Wen et al[64] | 2006 | China/Not known | P6 | F | F | CVD | 272 |
| CVD-Stroke | 115 | |||||||
| 40 | Eisner et al[65] | 2007 | United States | P8 | M, F | F | CVD | 1057 |
| 41 | Hill et al[66] | 2007 | New Zealand | P3 | M, F | F | IHD | 2571 |
| 42 | Hill et al[66] | 2007 | New Zealand | P3 | M, F | F | IHD | 1680 |
| 43 | He et al[67] | 2008 | China/Beijing | CS | F | NF | IHD | 431 |
| 44 | Sulo et al[68] | 2008 | Albania | CC | M + F | NF | ACS | 169 |
| 45 | Vozoris et al[69] | 2008 | Canada | CS | M + F | NF | HD | 1773 |
| 46 | Ding et al[70] | 2009 | Hong Kong | CC | F | NF | IHD | 314 |
| 47 | Gallo et al[71] | 2010 | Europe | P? | M, F | F | CVD12 | 399 |
| M + F | IHD | 81 | ||||||
| 48 | Hamer et al[72] | 2010 | England, Scotland | P7 | M + F | F | CVD | 96 |
| 4911 | Jefferis et al[73] | 2010 | Great Britain | P11 | M + F | F + NF | FMI | 74 |
| 50 | Peineman et al[74] | 2011 | Germany | CS | M + F | NF | IHD | 128 |
| 51 | Chen[75] | 2012 | China/4 provinces | CS | M + F | NF | IHD | 405 |
| MI | 171 | |||||||
| 52 | He et al[76] | 2012 | China/Xi’an | P26 | M, F | F | IHD | 41 |
| 53 | Clark et al[77] | 2013 | Singapore | P16 | M, F | F | IHD | 311 |
| 54 | Iversen et al[78] | 2013 | Norway | P11 | M, F | F + NF | FMI | 326 |
| 55 | Kastorini et al[79] | 2013 | Greece | CC | M + F | NF | ACS | 52 |
| 56 | Rostron[80] | 2013 | United States | P11 | M + F | F | IHD | ? |
| 57 | Batty et al[81]13 | 2014 | United Kingdom | P17 | M, F | F | CVD | 98 |
| 58 | Shiue[82] | 2014 | Scotland | CS | M + F | NF | MI | 255 |
| Study No. | Ref.1 | Variables adjusted for2 | Definition of never smokers3 |
| 1 | Hirayama[29] | Sex, age, marital status | Never cigarettes |
| 2 | Garland et al[30] | Sex, age, marital status, blood pressure, cholesterol, obesity | Never cigarettes |
| 3 | Lee et al[31] | Sex, age, marital status | Never NOS |
| 4 | Martin et al[32] | Sex, marital status, blood pressure, obesity, alcohol, diabetes, family history of heart disease, exercise | Never NOS |
| 5 | Svendsen et al[33] | Sex, age, marital status, blood pressure, cholesterol, social class, obesity, alcohol | Never any product |
| 6 | Butler[34] | Sex, age, marital status | Never cigarettes |
| 7 | Palmer et al[35] | Sex, marital status | Never NOS |
| 8 | Hole et al[36] | Sex, age, blood pressure, cholesterol, social class, obesity | Never NOS |
| 9 | Jackson[37] | Sex, age, social class, obesity, family history of heart disease | Never NOS |
| 10 | Sandler et al[38] | Sex, age, social class, personal history of heart disease | Never any product |
| 11 | Humble et al[39] | Sex, age, marital status, blood pressure, cholesterol, obesity | Never NOS |
| 12 | Dobson et al[40] | Sex, age, social class, obesity, personal history of heart disease | Never cigarettes |
| 13 | Gardiner et al[41] | Sex, age, hospital admission date | Never any product |
| 14 | La Vecchia et al[42] | Sex, age, marital status, blood pressure, cholesterol, social class, obesity, diabetes, family history of heart disease, coffee | Never NOS |
| 15 | Layard[25] | Sex, age, marital status, race | Never 100 cigarettes in lifetime |
| 16 | Le Vois et al[26] (CPS I) | Sex, age, marital status, race | Never NOS |
| 17 | Mannino et al[43] | Sex, age, social class, race, housing | Never NOS |
| 18 | Muscat et al[44] | Sex, age, blood pressure, social class, race | Never one cigarette, pipe or cigar per day for more than a year |
| 19 | Tunstall-Pedoe et al[45] | Age, blood pressure, cholesterol, housing | Never any product and cotinine < 17.5 mg/mL |
| 20 | Steenland et al[46] | Sex, age, marital status, blood pressure, social class, obesity, alcohol, diabetes, exercise, personal history of heart disease, occupation, oestrogen use, aspirin use, diuretic use and personal history of arthritis | Never any product daily for as long as a year (men), never cigarettes (women) |
| 21 | Janghorbani et al[47] | Sex, age, marital status | Never any product |
| 22 | Kawachi et al[48] | Sex, age, blood pressure, cholesterol, obesity, alcohol, diabetes, family history of heart disease, exercise, occupation, oestrogen use, oral contraceptive use, saturated fat intake, vitamin E intake, menopausal status and use of postmenopausal hormones | Never NOS |
| 23 | Ciruzzi et al[49] | Sex, age, blood pressure, cholesterol, social class, obesity, diabetes, family history of heart disease, exercise | Never NOS |
| 24 | McElduff et al[50] | Sex, age, social class, obesity, family history of heart disease | Never cigarettes or quit at least 10 yr ago, and not current other products |
| 25 | Spencer et al[51] | Sex, age | Never NOS |
| 26 | He et al[52] | Sex, age, blood pressure, cholesterol, family history of heart disease, personality type | Never NOS |
| 27 | Iribarren et al[53] | Sex, age, marital status, cholesterol, social class, obesity, alcohol, diabetes, race, exercise, personality type | Never any product |
| 28 | Rosenlund et al[54] | Sex, age, blood pressure, cholesterol, social class, obesity, diabetes, occupation | Never any product regularly for at least a year |
| 29 | Pitsavos et al[55] | Sex, age, blood pressure, cholesterol, obesity, alcohol, diabetes, exercise and family history of heart disease | Never cigarettes |
| 30 | Enstrom et al[27] | Sex, age, marital status, social class, obesity, race, exercise, housing, fruit or fruit juice intake and health status | Never any product4 |
| 31 | Chen et al[56] | Sex, age, blood pressure, cholesterol, social class, obesity, alcohol, family history of heart disease, employment status, dietary vitamin C and fibre | Never NOS and cotinine < 17.5 mg/mL |
| 32 | Nishtar et al[57] | Sex, age, matched pair (conditional logistic regression was used) | Never NOS |
| 33 | Whincup et al[58] | Sex, age, blood pressure, cholesterol, social class, obesity, alcohol, diabetes, exercise, personal history of heart disease, town of residence, FEV1, height, triglycerides and white cell count | Never any product and cotinine < 14.1 mg/mL |
| 34 | McGhee et al[59] | Sex, age, marital status, social class | Never NOS |
| 35 | Qureshi et al[60] | Sex, age, marital status, blood pressure, cholesterol, obesity, alcohol, diabetes, race | Never NOS |
| 36 | Hedblad et al[61] | Sex, blood pressure, cholesterol, obesity, alcohol, diabetes, exercise, personal history of heart disease, triglycerides and FEV1 | Never one cigarette per day |
| 37 | Stranges et al[62] | Sex, blood pressure, cholesterol, social class, obesity, alcohol, diabetes, race, exercise | Never 100 cigarettes in lifetime |
| 38 | Teo et al[63] | Sex, age, alcohol, exercise, region, consumption of fruits and vegetables | Never any product regularly |
| 39 | Wen et al[64] | Sex, age, social class, obesity, exercise, occupation, intake of meats, vegetables and fruit | Never NOS |
| 40 | Eisner et al[65] | Sex, age, marital status, social class | Never cigarettes or quit at least 20 yr ago, and < 10 pack-years |
| 41, 42 | Hill et al[66] | Sex, age, marital status, social class, race, occupation | Never NOS |
| 43 | He et al[67] | Sex, age, marital status, blood pressure, cholesterol, social class, obesity, alcohol, diabetes, family history of heart disease, exercise, triglycerides, family history of stroke | Never 100 cigarettes in lifetime |
| 44 | Sulo et al[68] | Sex, age, blood pressure, social class, obesity, diabetes, family history of heart disease, race, exercise, occupation, financial loss in pyramid schemes, emigration of spouse and/or offspring, religious observance | Never cigarettes |
| 45 | Vozoris et al[69] | Sex, age, social class, province, immigration status, presence of children younger than 12 yr in household | Never cigarettes |
| 46 | Ding et al[70] | Sex, age, blood pressure, cholesterol, social class, alcohol, diabetes, family history of heart disease, exercise, oestrogen use, history of stroke, history of gout | Never NOS |
| 47 | Gallo et al[71] | Sex, age, social class, obesity, exercise, study centre | Never NOS |
| 48 | Hamer et al[72] | Sex, age, blood pressure, cholesterol, social class, exercise, personality type, survey location, log C-reactive protein, fibrinogen | Never NOS |
| 49 | Jefferis et al[73] | Sex, age, blood pressure, cholesterol, social class, obesity, alcohol, diabetes, exercise, region, triglycerides, FEV1, C-reactive protein, interleukin 6, white cell count | Never any product or quit at least 5 yr ago, and cotinine < 15 mg/mL |
| 50 | Peinemann et al[74] | None | Never NOS |
| 51 | Chen[75] | None | Never cigarettes |
| 52 | He et al[76] | Sex, age, marital status, blood pressure, cholesterol, social class, obesity, alcohol, occupation, triglycerides | Never 100 cigarettes in lifetime |
| 53 | Clark et al[77] | Sex, age, social class, obesity, dialect, dietary fibre intake | Never NOS |
| 54 | Iversen et al[78] | Sex, age, blood pressure, cholesterol, obesity, exercise, living with a smoker (for analysis of hours spent in smoke-filled rooms), hours spent in smoke-filled rooms (for analysis of living with a smoker) | Never cigarettes |
| 55 | Kastorini et al[79] | Sex, age, blood pressure, cholesterol, obesity, diabetes, family history of heart disease, exercise, personality type, Mediterranean Diet Score | Never one cigarette a day |
| 56 | Rostron[80] | Sex, age, race, social class, alcohol, blood pressure, obesity, personal history of heart disease | Never 100 cigarettes in lifetime |
| 57 | Batty et al[81] | Sex, age, social class, alcohol, diabetes, exercise, personal history of heart disease, personal history of cancer | Never NOS |
| 58 | Shiue[82] | Sex, age, race, social class, alcohol, survey weighting, exercise, blood pressure, obesity | Never any product |
Of the 58 studies, 10 were published in the 1980s, 15 in the 1990s, 21 between 2000 and 2009 and 12 more recently. Twenty studies were in North America (19 United States, one Canada), 19 in Europe (10 United Kingdom, two Sweden, two Greece, one each in Albania, Germany, Italy and Norway and one in multiple countries), 11 in Asia (two Hong Kong, five in the rest of China, and one each in Iran, Japan, Pakistan and Singapore) and eight in other countries (three in each of Australia and New Zealand, one in Argentina, and one in 52 countries worldwide).
Twenty six studies were prospective, with lengths of follow-up from three to 39 years, while 22 were case-control, and 10 cross-sectional. Thirteen studies were of females, and four of males. The rest included both sexes, though some did not report sex-specific results. Twenty studies considered only fatal cases and 26 only non-fatal cases, the other 12 including both. As shown in Table 1, although IHD specifically was the disease definition used in almost half the studies, various other definitions were used. The studies varied considerably in size, with 13 of < 100 cases and 11 of > 1000 cases, the largest being of 14891, 6280 and 5932 cases.
As Table 2 shows, two studies only provided unadjusted results. While in a number of the mainly earlier studies there was quite limited adjustment, many studies adjusted for numerous variables. Apart from sex and age, variables adjusted for in > 10 studies included marital status, blood pressure (or hypertension), cholesterol, social class (or similar variables based on education or income), obesity (or weight), alcohol consumption, diabetes, family history of heart disease (or hypertension), race and exercise.
Thirty-five studies were of never smokers, though only nine of these clarified that subjects never smoked cigarettes, pipes or cigars. Nine studies were of never cigarette smokers, 11 allowed a minimal smoking history, such as smoking less than one cigarette a day or fewer than 100 cigarettes in life, while three studies allowed those who quit smoking some time ago. Four studies excluded subjects with cotinine levels indicative of current smoking.
Our main analyses use an index as close as possible to ever smoking by the spouse. Four studies were not included in the main index analyses, one (study 40) only reporting risk per 10 years living or working with a smoker, and three (studies 33, 36 and 48) providing results only for a biochemical index. Table 3, supported by Figure 1, presents RRs for the main index, and also gives details of ETS exposure, the definitions of the unexposed group being given in Supplementary File 2. RRs for the sensitivity analysis preferring current exposure are also in Table 3, nine studies providing RRs and 95%CIs for both ever and current exposure. RRs for the sensitivity analysis preferring unadjusted to adjusted results are given in Supplementary file 2. Studies 7, 17 and 25 only provided incomplete estimates that could not be included in meta-analyses. Similarly, the result for current exposure from study 4 could not be included in the sensitivity analysis. Otherwise, for each study/sex combination, the RR estimate listed first in Table 3 is that used in the main analysis. Exposure was based on spousal smoking for 24 studies, on at home exposure for 17, and on exposure from multiple sources, including outside the home, for 10. Table 4 presents results of meta-analyses, fuller details being given in Supplementary File 2. Table 5 presents dose-response data, separately for spousal and household exposure.
| Exposure index | ||||||
| Study No.1 | Author2 | Sex | Source3 | Timing4 | Fatality5 | Relative risk (95%CI)6 |
| Results used in the main analysis7 | ||||||
| 1 | Hirayama[29] | F | S | E | F | 1.16 (0.94-1.43)8 |
| 2 | Garland et al[30] | F | S | E | F | 2.70 (0.63-11.58) |
| 3 | Lee et al[31] | M | S | M | NF | 1.24 (0.58-2.67) |
| F | S | M | NF | 0.93 (0.53-1.64) | ||
| 4 | Martin et al[32] | F | S | E | NF | 2.60 (1.20-5.70)9 |
| 5 | Svendsen et al[33] | M | S | C | F + NF | 1.61 (0.96-2.71) |
| 6 | Butler[34] | F | S | E | F | 1.07 (0.65-1.75) |
| 7 | Palmer et al[35] | F | S | E | NF | 1.20 |
| 8 | Hole et al[36] | M | H10 | E | F | 1.73 (1.01-2.96)11 |
| F | H10 | E | F | 1.65 (0.79-3.46)11 | ||
| 9 | Jackson[37] | M | H | C | F + NF | 1.06 (0.39-2.91) |
| F | H | C | F + NF | 3.74 (1.15-12.19) | ||
| 10 | Sandler et al[38] | M | H | C | F | 1.31 (1.05-1.64) |
| F | H | C | F | 1.19 (1.04-1.36) | ||
| 11 | Humble et al[39] | F | S | C(N) | F | 1.59 (0.99-2.57) |
| 12 | Dobson et al[40] | M | H | C | F + NF | 0.97 (0.50-1.86) |
| F | H | C | F + NF | 2.46 (1.47-4.13) | ||
| 13 | Gardiner et al[41] | M + F | S | M | F + NF | 0.57 (0.19-1.74) |
| 14 | La Vecchia et al[42] | M | S | E | NF | 1.09 (0.47-2.53) |
| F | S | E | NF | 1.27 (0.52-3.09) | ||
| 15 | Layard[25] | M | S | E | F | 0.97 (0.73-1.28) |
| F | S | E | F | 0.99 (0.84-1.16) | ||
| 16 | Le Vois et al[26] (CPS I) | M | S | E | F | 0.97 (0.90-1.05) |
| F | S | E | F | 1.03 (0.98-1.08) | ||
| 17 | Mannino et al[43] | M + F | H | C | NF | 1.12 |
| 18 | Muscat et al[44] | M | S | E | NF | 1.38 (0.70-2.75) |
| F | S | E | NF | 1.33 (0.59-2.99) | ||
| 19 | Tunstall-Pedoe et al[45] | M + F | T | C | NF | 1.34 (1.07-1.67) |
| 20 | Steenland et al[46] | M | S | E | F | 1.09 (0.98-1.21) |
| F | S | E | F | 1.04 (0.93-1.16) | ||
| 21 | Janghorbani et al[47] | F | S | E | NF | 1.38 (0.95-2.01) |
| 22 | Kawachi et al[48] | F | H | C | F + NF | 1.53 (0.81-2.90)9 |
| 23 | Ciruzzi et al[49] | M | S | C | NF | 1.18 (0.55-2.52) |
| F | S | C | NF | 1.73 (0.89-3.36) | ||
| 24 | McElduff et al[50] | M | T | C | F + NF | 0.82 (0.55-1.22) |
| F | T | C | F + NF | 2.15 (1.18-3.92) | ||
| 25 | Spencer et al[51] | M | H | E | NF | No significant association |
| 26 | He et al[52] | F | S | E | NF | 1.60 (0.94-2.90) |
| 27 | Iribarren et al[53] | M | H | C | NF | 1.13 (1.00-1.27) |
| F | H | C | NF | 1.20 (1.09-1.30) | ||
| 28 | Rosenlund et al[54] | M | S | E | NF | 0.96 (0.64-1.44) |
| F | S | E | NF | 1.53 (0.95-2.44) | ||
| 29 | Pitsavos et al[55] | M + F | H | C | NF | 1.33 (0.89-1.99) |
| 30 | Enstrom et al[27] | M | S | E | F | 0.93 (0.83-1.04) |
| F | S | E | F | 0.99 (0.92-1.08) | ||
| 31 | Chen et al[56] | M + F | H | C | NF | 1.20 (0.70-2.20) |
| 32 | Nishtar et al[57] | M + F | S | E | NF | 2.38 (1.04-5.42) |
| 34 | McGhee et al[59] | M | H | P | F | 1.30 (0.88-1.93) |
| F | H | P | F | 1.39 (0.95-2.04) | ||
| 35 | Qureshi et al[60] | F | S | E | F + NF | 1.05 (0.81-1.38)12 |
| 37 | Stranges et al[62] | M | H | E | NF | 0.98 (0.65-1.50) |
| F | H | E | NF | 1.30 (0.67-2.51) | ||
| 38 | Teo et al[63] | M + F | T | C | NF | 1.37 (1.27-1.48) |
| 39 | Wen et al[64] | F | S | M | F | 0.99 (0.72-1.37)13 |
| 41 | Hill et al[66] | M | H | C | F | 1.04 (0.88-1.23) |
| F | H | C | F | 0.98 (0.83-1.17) | ||
| 42 | Hill et al[66] | M | H | C | F | 1.18 (0.96-1.44) |
| F | H | C | F | 1.27 (0.98-1.66) | ||
| 43 | He et al[67] | F | T | T | NF | 1.69 (1.31-2.18) |
| 44 | Sulo et al[68] | M | S | C | NF | 1.68 (0.81-3.47) |
| F | S | C | NF | 1.19 (0.25-5.64) | ||
| 45 | Vozoris et al[69] | M + F | T | C | NF | 1.00 (0.80-1.20) |
| 46 | Ding et al[70] | F | H | E | NF | 1.52 (1.01-2.27) |
| 47 | Gallo et al[71] | M + F | S | C | F | 1.99 (0.92-4.29)14 |
| 49 | Jefferis et al[73] | M + F | S | C | F + NF | 2.41 (1.04-5.59) |
| 50 | Peinemann et al[74] | M + F | T | C | NF | 1.27 (0.84-1.92) |
| 51 | Chen[75] | M + F | T | E | NF | 1.16 (0.93-1.45)15 |
| 52 | He et al[76] | M | T | E | F | 2.24 (0.76-6.59) |
| F | T | E | F | 2.10 (0.69-6.33) | ||
| 53 | Clark et al[77] | M | H | C | F | 1.98 (1.00-3.93) |
| F | H | C | F | 0.94 (0.67-1.32) | ||
| 54 | Iversen et al[78] | M | H | A | F + NF | 0.91 (0.61-1.35) |
| F | H | A | F + NF | 1.42 (1.06-1.90) | ||
| 55 | Kastorini et al[79] | M + F | T | E | NF | 4.33 (1.52-12.38) |
| 56 | Rostron[80] | M + F | H | C | F | 0.82 (0.39-1.70) |
| 57 | Batty et al[81] | M | H | C | F | 1.26 (0.37-4.31) |
| F | H | C | F | 1.12 (0.55-2.28) | ||
| 58 | Shiue[82] | M + F | T | C | NF | 1.47 (0.96-2.24) |
| Alternative result used in the analysis of spouse a current smoker | ||||||
| 2 | Garland et al[30] | F | S | C(N) | F | 2.25 (0.32-15.74) |
| 4 | Martin et al[32] | F | S | C | NF | 3.40 |
| 6 | Butler[34] | F | S | C(N) | F | 1.40 (0.51-3.84) |
| 14 | La Vecchia et al[42] | M | S | C(N) | NF | 1.09 (0.39-3.01) |
| F | S | C(N) | NF | 1.36 (0.46-4.05) | ||
| 16 | Le Vois et al[26] (CPS I) | M | S | C(N) | F | 0.98 (0.91-1.06) |
| F | S | C(N) | F | 1.04 (0.99-1.09) | ||
| 20 | Steenland et al[46] | M | S | C(N) | F | 1.22 (1.07-1.40) |
| F | S | C(N) | F | 1.10 (0.96-1.27) | ||
| 28 | Rosenlund et al[54] | M | S | C(N) | NF | 0.98 (0.57-1.69) |
| F | S | C(N) | NF | 2.59 (1.27-5.29) | ||
| 30 | Enstrom et al[27] | M | S | C(N) | F | 0.92 (0.80-1.05) |
| F | S | C(N) | F | 0.97 (0.89-1.06) | ||
| 37 | Stranges et al[62] | M | H | C | NF | 0.71 (0.40-1.23) |
| F | H | C | NF | 0.94 (0.48-1.82) | ||
| 39 | Wen et al[64] | F | S | C | F | 1.19 (0.84-1.67)16 |
| Additional household exposure results | ||||||
| 18 | Muscat et al[44] | M | H | E | NF | 1.40 (0.70-2.81) |
| F | H | E | NF | 1.55 (0.55-4.37) | ||
| 20 | Steenland et al[46] | M | H | C(N) | F | 1.15 (1.01-1.32) |
| F | H | C(N) | F | 1.07 (0.98-1.17) | ||
| 21 | Janghorbani et al[47] | F | H | E | NF | 1.34 (0.94-1.91) |
| 23 | Ciruzzi et al[49] | M | H17 | C | NF | 1.89 (1.13-3.18) |
| F | H17 | C | NF | 1.54 (0.95-2.51) | ||
| 47 | Gallo et al[71] | M + F | H | C | F | 1.31 (0.83-2.08)18 |
| Fixed-effect | Random-effects | Publication bias | Heterogeneity2 | ||||
| Subgroup | n3 | Relative risk (95%CI) | Relative risk (95%CI) | P4 value | χ2 | DF5 | P6 value |
| Main analyses7 | |||||||
| All | 75 | 1.10 (1.08-1.13) | 1.18 (1.12-1.24) | < 0.001 | 176.45 | 74 | < 0.001 |
| By sex | |||||||
| Combined | 14 | 1.32 (1.24-1.40) | 1.30 (1.14-1.47) | NS | 23.54 | 13 | < 0.05 |
| Males | 25 | 1.04 (1.00-1.09) | 1.07 (1.01-1.15) | < 0.05 | 32.90 | 24 | NS |
| Females | 36 | 1.09 (1.06-1.12) | 1.20 (1.12-1.29) | < 0.001 | 81.04 | 35 | < 0.001 |
| Between sexes | 38.98 | 2 | < 0.001 | ||||
| By continent | |||||||
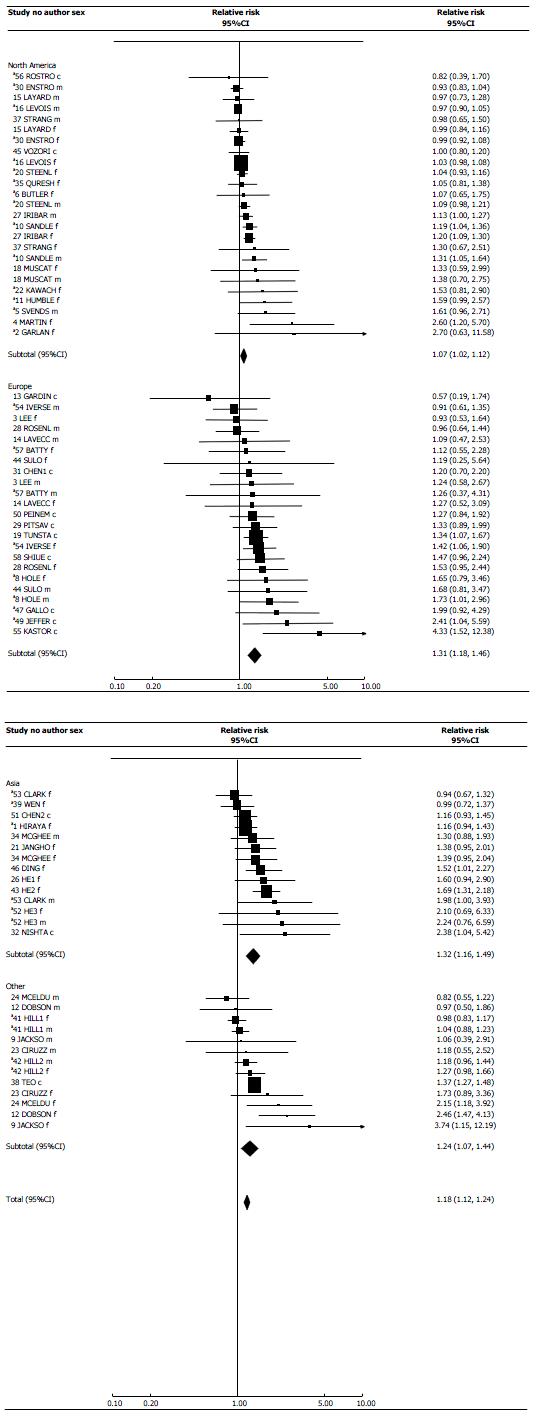
| North America | 25 | 1.05 (1.02-1.08) | 1.07 (1.02-1.12) | < 0.05 | 45.67 | 24 | < 0.01 |
| Europe | 23 | 1.31 (1.18-1.46) | 1.31 (1.18-1.46) | NS | 20.63 | 22 | NS |
| Asia | 14 | 1.29 (1.17-1.42) | 1.32 (1.16-1.49) | < 0.05 | 18.94 | 13 | NS |
| Other | 13 | 1.26 (1.19-1.33) | 1.24 (1.07-1.44) | NS | 37.12 | 12 | < 0.001 |
| Between continents | 54.09 | 3 | < 0.001 | ||||
| By publication period | |||||||
| 1984-1991 | 16 | 1.28 (1.17-1.39) | 1.35 (1.18-1.54) | < 0.05 | 21.29 | 15 | NS |
| 1992-1998 | 18 | 1.04 (1.00-1.07) | 1.06 (1.00-1.12) | < 0.1 | 24.86 | 17 | < 0.1 |
| 1999-2005 | 13 | 1.08 (1.03-1.13) | 1.13 (1.02-1.24) | < 0.1 | 28.86 | 12 | < 0.01 |
| 2006-2009 | 13 | 1.24 (1.17-1.31) | 1.19 (1.06-1.34) | NS | 32.96 | 12 | < 0.001 |
| 2010-2016 | 15 | 1.26 (1.11-1.41) | 1.31 (1.11-1.55) | < 0.05 | 21.07 | 14 | < 0.1 |
| Between periods | 47.42 | 4 | < 0.001 | ||||
| By number of heart disease cases8 | |||||||
| 1-99 | 13 | 1.62 (1.32-1.99) | 1.66 (1.30-2.11) | NS | 14.83 | 12 | NS |
| 100-199 | 14 | 1.33 (1.11-1.58) | 1.33 (1.11-1.58) | NS | 5.78 | 13 | NS |
| 200-999 | 30 | 1.26 (1.17-1.35) | 1.27 (1.16-1.39) | NS | 44.09 | 29 | < 0.05 |
| 1000+ | 18 | 1.08 (1.05-1.10) | 1.08 (1.02-1.15) | NS | 76.70 | 17 | < 0.001 |
| Between numbers | 35.06 | 3 | < 0.001 | ||||
| By study design | |||||||
| Case-control | 32 | 1.29 (1.21-1.36) | 1.28 (1.15-1.42) | NS | 52.18 | 31 | < 0.05 |
| Prospective | 33 | 1.04 (1.01-1.07) | 1.09 (1.03-1.14) | < 0.001 | 55.43 | 32 | < 0.01 |
| Cross-sectional | 10 | 1.20 (1.14-1.28) | 1.24 (1.12-1.37) | NS | 16.78 | 9 | < 0.1 |
| Between types | 52.06 | 2 | < 0.001 | ||||
| By number of confounders considered in the study | |||||||
| 0-2 | 15 | 1.03 (0.99-1.07) | 1.05 (0.92-1.12) | < 0.1 | 17.51 | 14 | NS |
| 3-4 | 10 | 1.27 (1.16-1.39) | 1.32 (1.13-1.55) | NS | 16.65 | 9 | < 0.1 |
| 5-9 | 38 | 1.13 (1.09-1.18) | 1.19 (1.09-1.30) | < 0.05 | 94.55 | 37 | < 0.001 |
| 10+ | 12 | 1.16 (1.10-1.22) | 1.21 (1.10-1.32) | < 0.05 | 21.01 | 11 | < 0.05 |
| Between groups | 26.72 | 3 | < 0.01 | ||||
| By results available in the study on dose-response | |||||||
| No | 24 | 1.15 (1.08-1.22) | 1.19 (1.08-1.32) | < 0.05 | 44.81 | 23 | < 0.01 |
| Yes | 51 | 1.10 (1.07-1.12) | 1.18 (1.11-1.25) | < 0.01 | 129.74 | 50 | < 0.001 |
| Between groups | 1.90 | 1 | NS | ||||
| By spouse the index | |||||||
| Yes | 34 | 1.03 (1.00-1.06) | 1.06 (1.01-1.12) | < 0.001 | 47.62 | 33 | < 0.05 |
| No | 41 | 1.23 (1.19-1.28) | 1.24 (1.16-1.32) | NS | 72.59 | 40 | < 0.01 |
| Between groups | 56.24 | 1 | < 0.001 | ||||
| Spouse the index, by whether unmarried subjects were excluded | |||||||
| Yes | 23 | 1.02 (0.99-1.05) | 1.03 (0.99-1.07) | < 0.05 | 27.88 | 22 | NS |
| No | 11 | 1.30 (1.10-1.54) | 1.35 (1.11-1.63) | < 0.01 | 12.00 | 10 | NS |
| Between groups | 7.74 | 1 | < 0.01 | ||||
| By heart disease fatality considered | |||||||
| Fatal | 31 | 1.04 (1.01-1.07) | 1.07 (1.02-1.12) | < 0.001 | 46.74 | 30 | < 0.05 |
| Non-fatal | 31 | 1.27 (1.22-1.33) | 1.27 (1.19-1.36) | NS | 39.58 | 30 | NS |
| Both | 13 | 1.25 (1.10-1.43) | 1.34 (1.06-1.68) | NS | 28.43 | 12 | < 0.01 |
| Between groups | 61.70 | 2 | < 0.001 | ||||
| By heart disease definition | |||||||
| IHD | 32 | 1.06 (1.03-1.11) | 1.12 (1.05-1.19) | < 0.001 | 56.92 | 31 | < 0.01 |
| MI | 18 | 1.34 (1.25-1.43) | 1.29 (1.14-1.46) | NS | 23.10 | 17 | NS |
| Other/Mixed | 25 | 1.08 (1.05-1.12) | 1.20 (1.10-1.30) | < 0.001 | 58.29 | 24 | < 0.001 |
| Between definitions | 38.14 | 2 | < 0.001 | ||||
| By use of biomarker data to exclude smokers | |||||||
| Yes | 6 | 1.30 (1.08-1.57) | 1.30 (1.08-1.57) | NS | 3.89 | 5 | NS |
| No | 69 | 1.10 (1.08-1.13) | 1.18 (1.12-1.24) | < 0.001 | 169.45 | 68 | < 0.001 |
| Between groups | 3.12 | 1 | < 0.1 | ||||
| By any use of proxy respondents | |||||||
| Yes | 11 | 1.10 (0.99-1.23) | 1.23 (0.98-1.53) | NS | 26.38 | 10 | < 0.01 |
| No | 64 | 1.10 (1.08-1.13) | 1.18 (1.12-1.24) | < 0.001 | 150.07 | 63 | < 0.001 |
| Between groups | 0.00 | 1 | NS | ||||
| By type of control | |||||||
| Healthy | 15 | 1.30 (1.13-1.50) | 1.38 (1.12-1.70) | < 0.1 | 27.67 | 14 | < 0.05 |
| Diseased/hospital | 15 | 1.12 (1.01-1.24) | 1.14 (1.01-1.28) | < 0.1 | 14.72 | 14 | NS |
| Both | 2 | 1.37 (1.27-1.48) | 1.37 (1.27-1.48) | NC | 0.29 | 1 | NS |
| Prospective/cross-sectional | 43 | 1.07 (1.05-1.10) | 1.13 (1.08-1.19) | < 0.001 | 91.01 | 42 | < 0.001 |
| Between types | 42.78 | 3 | < 0.001 | ||||
| Between types, excluding prospective/cross-sectional | 9.51 | 2 | < 0.01 | ||||
| By source of diagnosis | |||||||
| Death certificate only | 27 | 1.04 (1.01-1.07) | 1.06 (1.02-1.11) | < 0.01 | 41.57 | 26 | < 0.05 |
| Medical data used | 41 | 1.35 (1.28-1.43) | 1.34 (1.23-1.46) | NS | 51.49 | 40 | NS |
| Self-report only | 7 | 1.17 (1.10-1.24) | 1.17 (1.07-1.27) | NS | 8.11 | 6 | NS |
| Between sources | 75.29 | 2 | < 0.001 | ||||
| By definition of never smoker | |||||||
| Never any product | 11 | 1.10 (1.05-1.15) | 1.15 (1.05-1.27) | NS | 32.42 | 10 | < 0.001 |
| Never, product unstated | 33 | 1.05 (1.02-1.09) | 1.15 (1.07-1.24) | < 0.001 | 49.99 | 32 | < 0.05 |
| Never cigarettes | 12 | 1.17 (1.06-1.30) | 1.21 (1.05-1.38) | NS | 16.54 | 11 | NS |
| Other | 19 | 1.20 (1.14-1.25) | 1.21 (1.07-1.37) | NS | 57.89 | 18 | < 0.001 |
| Between definitions | 19.62 | 3 | < 0.001 | ||||
| Sensitivity analyses | |||||||
| Preferring unadjusted to adjusted estimates | 75 | 1.06 (1.04-1.08) | 1.16 (1.09-1.24) | < 0.01 | 321.31 | 74 | < 0.001 |
| Preferring current to ever exposure | 75 | 1.12 (1.09-1.14) | 1.19 (1.13-1.26) | < 0.001 | 176.96 | 74 | < 0.001 |
| Excluding studies 15 and 16 | 71 | 1.16 (1.12-1.19) | 1.21 (1.15-1.28) | < 0.01 | 144.97 | 70 | < 0.001 |
| Excluding study 30 | 73 | 1.12 (1.10-1.15) | 1.20 (1.14-1.26) | < 0.001 | 158.21 | 72 | < 0.001 |
| Excluding studies 15, 16 and 30 | 69 | 1.20 (1.17-1.24) | 1.23 (1.17-1.29) | < 0.05 | 109.86 | 68 | < 0.001 |
| Study No. | Ref.1 | Sex | Exposure grouping | Relative risks by grouping2 | Significance (trend)3 |
| Smoking by the spouse | |||||
| 1 | Hirayama[29] | F | 0, 1-19, 20+ (cigs/d) | 1.00, 1.10, 1.314 | + |
| 5 | Svendsen et al[33] | M | 0, 1-19, 20+ (cigs/d) | 1.00, 1.20, 1.75 | |
| 14 | La Vecchia et al[42] | M + F | 0, 1-14, 15+ (cigs/d) | 1.00, 1.13, 1.30 | |
| 15 | Layard[25] | M | 0, 1-14, 15-34, 35+ (cigs/d) | 1.00, 0.76, 1.07, 0.92 | |
| F | 0, 1-14, 15-34, 35+ (cigs/d) | 1.00, 0.85, 1.15, 1.06 | |||
| 16 | Le Vois et al[26] (CPS I) | M | 0, 1-19, 20-39, 40+ (cigs/d) | 1.00, 0.99, 0.98, 0.72 | |
| F | 0, 1-19, 20-39, 40+ (cigs/d) | 1.00, 1.04, 1.06, 0.95 | |||
| 20 | Steenland et al[46] | M | 0, 1-19, 20, 21+ (cigs/d) | 1.00, 1.33, 1.17, 1.09 | |
| F | 0, 1-19, 20, 21-39, 40+ (cigs/d) | 1.00, 1.15, 1.07, 0.99, 1.04 | |||
| M | 0, 1-12, 13-21, 22-29, 30+ (year) | 1.00, 1.14, 1.13, 1.14, 1.25 | |||
| F | 0, 1-14, 15-25, 26-33, 34+ (year) | 1.00, 0.84, 0.99, 1.20, 1.20 | |||
| M | 0, 1-5, 6-14, 15-27, 28+ (pack year) | 1.00, 1.25, 1.33, 1.13, 1.00 | |||
| F | 0, 1-12, 13-25, 26-33, 34+ (pack year) | 1.00, 0.83, 1.12, 1.09, 1.26 | |||
| 21 | Janghorbani et al[47] | F | 0, 1-30, 31+ (year) | 1.00, 1.74, 0.85 | |
| F | 0, 1-19, 20+ (cigs/d) | 1.00, 1.76, 1.11 | |||
| F | 0, 1-10, 11+ (pack year) | 1.00, 1.95, 1.17 | |||
| 23 | Ciruzzi et al[49] | F | 0, 1-20, 21+ (cigs/d) | 1.00, 0.82, 3.00 | |
| 26 | He et al[52] | F | 0, 1-10, 11-20, 21+ (cigs/d) | 1.00, 0.93, 1.40, 3.20 | + |
| 0-5, 6-15, 16-30, 31+ (year) | 1.00, 0.80, 2.10, 2.30 | + | |||
| 0, 1-399, 400-799, 800+ (cigs/day × year) | 1.00, 1.20, 1.90, 3.60 | + | |||
| 28 | Rosenlund et al[54] | M + F | 0, 1-19, 20+ (cigs/d) | 1.00, 1.02, 1.58 | |
| M + F | 0, 1-32, 33+ (year) | 1.00, 1.11, 1.25 | |||
| M + F | 0, 1-20, 21+ (pack-year) | 1.00, 1.09, 1.33 | |||
| 30 | Enstrom et al[27] | M | 0, 1-9, 10-19, 20, 21-39, 40+ (cigs/d) | 1.00, 0.98, 0.82, 0.89, 1.13, 1.24 | |
| F | 0, 1-9, 10-19, 20, 21-39, 40+ (cigs/d) | 1.00, 1.03, 0.99, 1.02, 0.88, 0.80 | |||
| 39 | Wen et al[64] | F | 0, < 8.8, 8.8-17.9, 18.0+ (pack-year) | 1.00, 1.10, 1.12, 1.225 | |
| 47 | Gallo et al[71] | M + F | 0, 0.5, 1.0, 1.5+ (packs/d) | 1.00, 1.87, 1.89, 2.466 | |
| Smoking by household members | |||||
| 8 | Hole et al[36] | F | 0, 1-14, 15+ (cigs/d) | 1.00, 2.09, 4.12 | + |
| 9 | Jackson[37] | M | None, low, high (exposure) | 1.00, 1.30, 0.90 | |
| F | None, low, high (exposure) | 1.00, 2.10, 7.50 | + | ||
| 18 | Muscat et al[44] | M | None, 1-20, 21-30, 31+ (year) | 1.0, 1.7, 1.5, 1.1 | |
| F | None, 1-20, 21-30, 31+ (year) | 1.0, 2.0, 0.9, 1.7 | |||
| 22 | Kawachi et al[48] | F | None, occasional, regular | 1.00, 1.19, 2.11 | + |
| F | < 1, 1-9, 10-19, 20-29, 30+ (year) | 1.00, 1.19, 1.54, 1.11, 1.50 | |||
| 27 | Iribarren et al[53] | M | 0, 1-9, 10-39, 40+ (h/wk) | 1.00, 1.12, 1.26, 1.20 | + |
| F | 0, 1-9, 10-39, 40+ (h/wk) | 1.00, 1.21, 1.31, 1.36 | + | ||
| 29 | Pitsavos et al[55] | M + F | 0, 1-4, 5-9, 10-19, 20-29, 30-39, 40+ (years living with a regular smoker) | 1.00, 1.07, 1.16, 1.39, 1.75, 2.20, 3.09 | + |
| 34 | McGhee et al[59] | M + F | 0, 1, 2+ (smokers in the home) | 1.00, 1.26, 1.68 | + |
| 40 | Eisner et al[65] | M + F | Per 10 years exposure | 1.10 | |
| 46 | Ding et al[70] | F | 0, < 1, 1+ (packs/d) | 1.00, 1.14, 1.69 | + |
| 0, < 5, 5+, (year) | 1.00, 1.26, 1.52 | + | |||
| 0, < 4, 4+, (h/d) | 1.00, 1.28, 1.82 | + | |||
| 0, < 5, 5+, (pack-year) | 1.00, 1.44, 1.53 | + | |||
| 0, < 20, 20+ (h-year) | 1.00, 1.22, 1.61 | + | |||
| 47 | Gallo et al[71] | M + F | 0, < 1, 1-2, 3+ (h/d) | 1.00, 1.39, 2.08, 1.946 | + |
| 54 | Iversen et al[78] | M | 0, < 10, 10-19, 20-29, 30+ (year) | 1.00, 0.70, 1.20, 0.70, 1.10 | |
| F | 0, < 10, 10-19, 20-29, 30+ (year) | 1.00, 1.00, 1.40, 1.30, 1.60 | + | ||
Table 3 demonstrates clear evidence of a positive association, about three-quarters of the main analysis RR estimates exceeding 1. Seventeen are significantly (P < 0.05) increased, and none significantly decreased. Study 16 contributed 31% of the total weight, with studies 20, 27, 30 and 38 each contributing about 10%.
The main meta-analysis (Table 4) shows a clear positive association, with the random-effects RR estimate 1.18 (95%CI: 1.12-1.24) based on 75 individual estimates. The RR is little changed in sensitivity analyses preferring unadjusted to adjusted estimates (1.16, 1.09-1.24), or preferring current to ever exposure estimates (1.19, 1.13-1.26). It is somewhat increased if studies 15, 16 and 30 are excluded (1.23, 1.17-1.29).
There is clear (P < 0.001) heterogeneity between estimates for all these analyses. Analyses by subset (based on the main analysis) show highly significant (P < 0.001) variation by various factors:
Sex: Estimates are lower for males than for females or sexes combined.
Continent: Estimates are lower for North America than for Europe, Asia or elsewhere.
Publication period: Estimates are higher for the oldest (1984-1991) and newest (2010-2016) studies than for studies in intermediate periods.
Number of cases: Studies with fewer cases give higher estimates, consistent with the significant (P < 0.001) publication bias for the overall analysis.
Study type: Estimates are lower for prospective than for case-control or cross-sectional studies.
Spouse the index: Estimates are lower where the spouse is the index, and where the analysis is limited to married subjects.
Fatality: Estimates are lower when based on fatal cases.
Heart disease definition: Estimates are lower for IHD specifically than for other definitions.
Type of control: In case-control studies, estimates are lower where hospital/diseased controls rather than healthy controls, are used.
Source of diagnosis: Estimates are lower when diagnosis derives from death certificates or self-report than from medical data.
Definition of never smoker: Estimates are higher where the definition allowed “never smoking” subjects to smoke products other than cigarettes, or to have a limited smoking history.
Despite the heterogeneity, each RR estimate in Table 4 for each data subset exceeds 1.00, generally significantly so. Our analyses demonstrated 11 factors with highly significant (P < 0.001) heterogeneity by level, when considered one at a time. However, many were inter-correlated. To isolate the important factors, stepwise regression analysis was conducted (see Supplementary File 3). Only three of the 11 factors independently predicted heart disease risk at P < 0.05, with source of diagnosis introduced first into the model, then spouse the index, and then number of cases. While, for the factors remaining in the model, the direction of effect remained, the magnitude of variation between levels was slightly reduced from that shown in Table 4.
Table 3 also shows RRs for household exposure for five studies where separate results are available for both spousal and household exposure. Overall, there are 37 household exposure estimates from 22 studies, 10 showing a significant increase in risk, and none a significant decrease. The combined random-effects estimate is 1.19 (95%CI: 1.13-1.25). There is no marked heterogeneity between the estimates overall, and little indication of variation between males and females, continents, periods of publication or numbers of cases. Estimates do vary by study design (P < 0.01), being higher for case-control studies than other designs.
As shown in Table 5, 13 studies reported dose-response results for smoking by the spouse, 11 for smoking by household members, and one (study 47) for both. While only two studies providing dose-response data for spousal smoking reported a significant (P < 0.05) positive trend, nine did so for exposure to household members. These trend tests included the unexposed group. Had they excluded the unexposed group, they would have been significant for only one (study 26). There were no significant negative trends.
Table 6 presents results for ETS exposure at work, in childhood, a combined index of total exposure, and a biochemical index of exposure. For these four indices, results are available from, respectively, 14, 4, 24 and 8 studies. For some studies the estimates for total exposure are the same as those for the main exposure index. The RRs are supported by Figures 2-5, while Table 7 presents results of meta-analyses, and Table 8 the dose-response data. Again, fuller details of meta-analyses are given in Supplementary File 2. Supplementary File 2 also includes results for spousal smoking specifically.
| Study No. | Ref.1 | Sex | Exposure index2 | Relative risk (95%CI)3 | Exposure description |
| 3 | Lee et al[31] | M | Workplace | 0.66 (0.26-1.66) | |
| F | Workplace | 0.69 (0.26-1.87) | |||
| M | Total | 0.39 (0.17-0.90) | Home, work, travel, leisure | ||
| F | Total | 0.52 (0.24-1.09) | Home, work, travel, leisure | ||
| 5 | Svendsen et al[33] | M | Workplace | 1.40 (0.80-2.50) | |
| M | Total | 1.17 (0.62-1.19) | Spouse, work | ||
| 9 | Jackson et al[37] | M | Workplace | 1.80 (0.94-3.46) | |
| F | Workplace | 1.55 (0.48-5.03) | |||
| M | Total | 1.14 (0.76-1.70) | Home, work | ||
| F | Total | 1.56 (0.76-3.20) | Home, work | ||
| 12 | Dobson et al[40] | M | Workplace | 0.95 (0.51-1.78) | |
| F | Workplace | 0.66 (0.17-2.62) | |||
| M | Total | 1.09 (0.72-1.63) | Home, work | ||
| F | Total | 2.24 (1.28-3.91) | Home, work | ||
| 18 | Muscat et al[44] | M | Workplace | 1.20 (0.60-2.20) | |
| F | Workplace | 1.00 (0.40-2.50) | |||
| M | Childhood | 0.79 (0.39-1.63) | Mother, father, other relatives | ||
| F | Childhood | 0.72 (0.30-1.72) | Mother, father, other relatives | ||
| 19 | Tunstall-Pedoe et al[45] | M + F | Total | 1.34 (1.07-1.67) | Exposure to tobacco smoke from someone else in the previous three days |
| M + F | Biomarker | 1.13 (0.93-1.38) | Serum cotinine | ||
| 20 | Steenland et al[46] | M | Workplace | 1.03 (0.89-1.19) | |
| F | Workplace | 1.06 (0.84-1.34) | |||
| 22 | Kawachi et al[48] | F | Workplace | 1.68 (0.81-3.47) | |
| F | Total | 1.71 (1.03-2.84) | Home, work | ||
| 24 | McElduff et al[50] | M | Total | 0.82 (0.55-1.22) | Daily at home, work |
| F | Total | 2.15 (1.18-3.92) | Daily at home, work | ||
| 26 | He et al[52] | F | Workplace | 1.85 (0.86-4.00)4 | |
| F | Total | 2.87 (1.36-6.05) | Spouse, work | ||
| 27 | Iribarren et al[53] | M | Total | 1.07 (0.96-1.19) | Home, small spaces, large indoor areas |
| F | Total | 1.10 (1.01-1.20) | Home, small spaces, large indoor areas | ||
| 28 | Rosenlund et al[54] | M | Workplace | 1.14 (0.78-1.67) | |
| F | Workplace | 0.94 (0.59-1.50) | |||
| M + F | Total | 1.18 (0.87-1.60) | Spouse, work | ||
| 29 | Pitsavos et al[55] | M + F | Workplace | 1.97 (1.16-3.34) | |
| M | Total | 1.33 (0.94-1.88) | Home, work | ||
| F | Total | 1.39 (0.87-2.23) | Home, work | ||
| 31 | Chen et al[56] | M + F | Workplace | 1.70 (0.90-3.20) | |
| M + F | Total | 1.50 (1.03-2.20) | Other people’s tobacco smoke in the previous three days | ||
| M + F | Biomarker | 0.86 (0.64-1.16) | Serum cotinine | ||
| 32 | Nishtar et al[57] | M + F | Total | 2.87 (1.28-6.42) | Unspecified, but includes spouse and others |
| 33 | Whincup et al[58] | M | Biomarker | 1.67 (1.03-2.72) | Serum cotinine |
| 36 | Hedblad et al[61] | M | Biomarker | 2.22 (1.21-4.09) | Blood carboxyhaemoglobin |
| 37 | Stranges et al[62] | M | Workplace | 0.97 (0.64-1.48) | |
| F | Workplace | 0.96 (0.60-1.55) | |||
| M | Childhood | 1.04 (0.72-1.52) | Unspecified | ||
| F | Childhood | 0.93 (0.57-1.51) | Unspecified | ||
| M | Total | 1.11 (0.69-1.77) | Lifetime; home, work, public places; RR is compared to lower tertile of exposure | ||
| F | Total | 0.58 (0.33-1.03) | Lifetime; home, work, public places; RR is compared to lower tertile of exposure | ||
| 38 | Teo et al[63] | M + F | Total | 1.37 (1.27-1.48) | Family, friends, co-workers |
| 39 | Wen et al[64] | F | Workplace | 1.21 (0.74-2.01)5 | |
| F | Childhood | 1.49 (1.01-2.22)5 | In early life from family members | ||
| F | Total | 1.25 (0.69-2.25) 5 | Spouse, work, early life | ||
| 43 | He et al[67] | F | Total | 1.69 (1.31-2.18) | Home, work |
| 45 | Vozoris et al[69] | M + F | Total | 1.00 (0.80-1.20) | Exposed on most days in the previous month |
| 47 | Gallo et al[71] (EPIC) | M | Workplace | 0.93 (0.46-1.90)6 | |
| F | Workplace | 0.76 (0.47-1.24)6 | |||
| M | Childhood | 1.11 (0.72-1.69)6 | Parents | ||
| F | Childhood | 1.18 (0.88-1.57)6 | Parents | ||
| 48 | Hamer et al[72] | M | Biomarker | 1.50 (0.85-2.64) | Salivary cotinine |
| 49 | Jefferis et al[73] | M + F | Biomarker | 0.94 (0.59-1.51) | Serum cotinine |
| 50 | Peinemann et al[74] | M + F | Total | 1.27 (0.84-1.92) | Home, work, other |
| 51 | Chen[75] | M + F | Total | 1.16 (0.93-1.45)7 | Home, work, other |
| 52 | He et al[76] | M | Total | 2.24 (0.76-6.59) | Lifetime; home, work |
| F | Total | 2.10 (0.69-6.33) | Lifetime; home, work | ||
| 54 | Iversen et al[78] | M | Total | 0.97 (0.61-1.55) | Time spent in smoke-filled rooms |
| F | Total | 0.70 (0.44-1.12) | Time spent in smoke-filled rooms | ||
| 55 | Kastorini et al[79] | M + F | Total | 4.33 (1.52-12.38) | Partner, parents, children, roommates, colleagues; 30+ min/d |
| 56 | Rostron[80] | M + F | Biomarker | 1.02 (0.70-1.47) | Serum cotinine |
| 57 | Batty et al[81] | M | Biomarker | 0.49 (0.19-1.25) | Salivary cotinine |
| F | Biomarker | 1.26 (0.70-2.24) | Salivary cotinine | ||
| 58 | Shiue[82] | M + F | Total | 1.47 (0.96-2.24) | Home, work, other |
| Fixed-effect | Random-effects | Publication bias | Heterogeneity | ||||
| Index of exposure | n2 | Relative risk (95%CI) | Relative risk (95%CI) | P3 | χ2 | DF4 | P5 value |
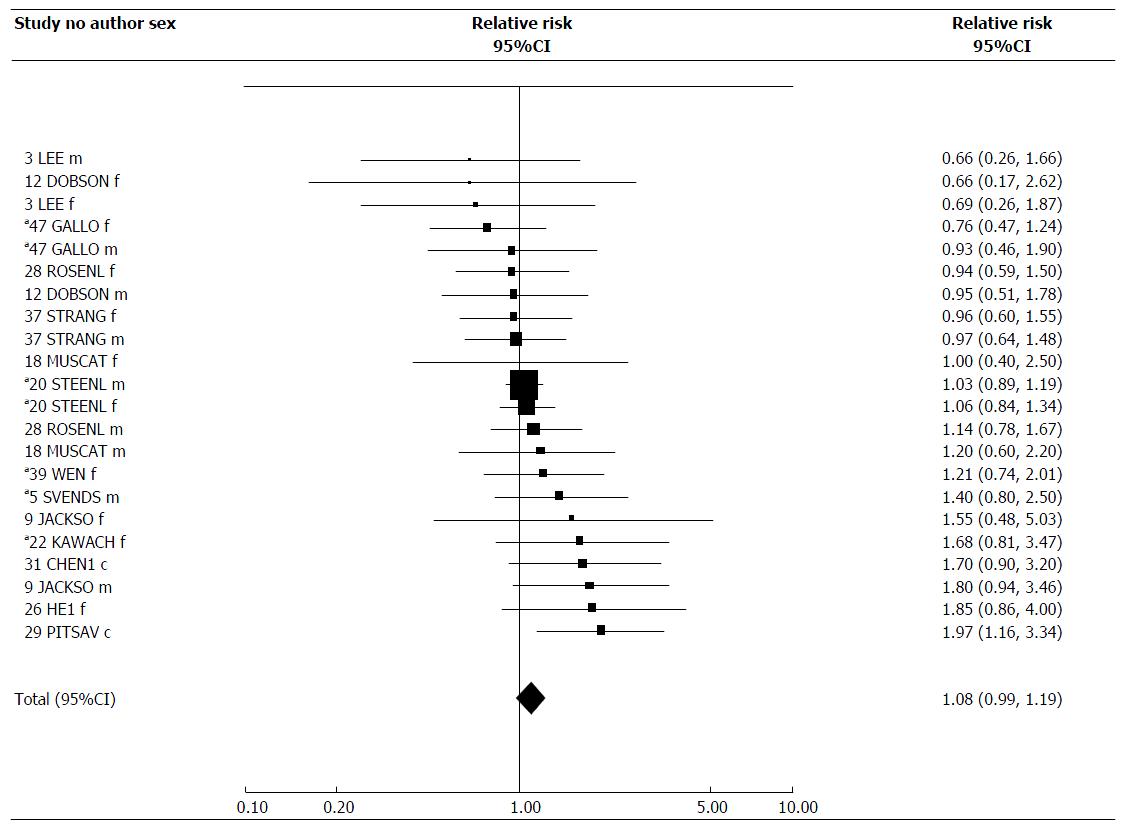
| Workplace | 22 | 1.08 (0.99-1.19) | 1.08 (0.99-1.19) | NS | 20.12 | 21 | NS |
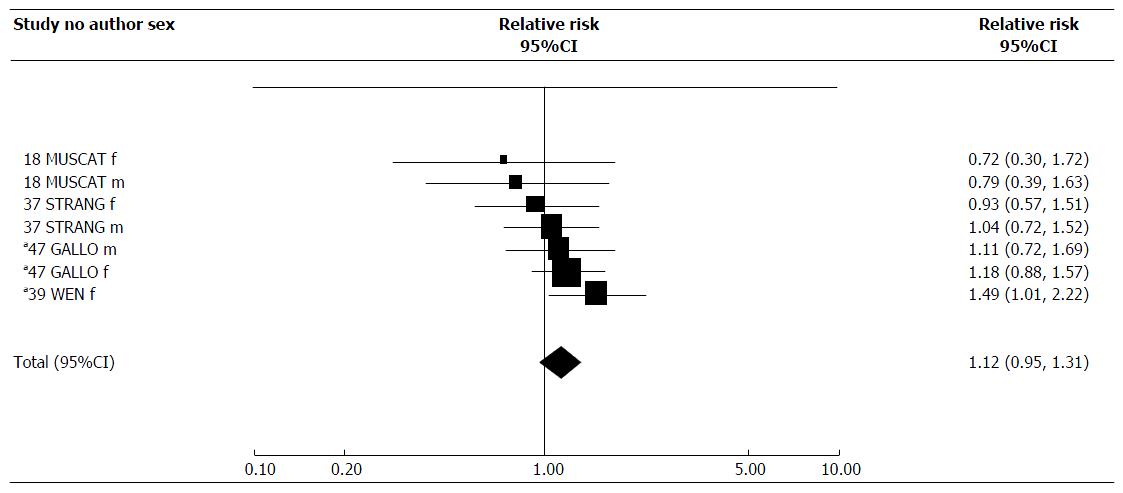
| Childhood | 7 | 1.12 (0.95-1.31) | 1.12 (0.95-1.31) | < 0.1 | 4.77 | 6 | NS |
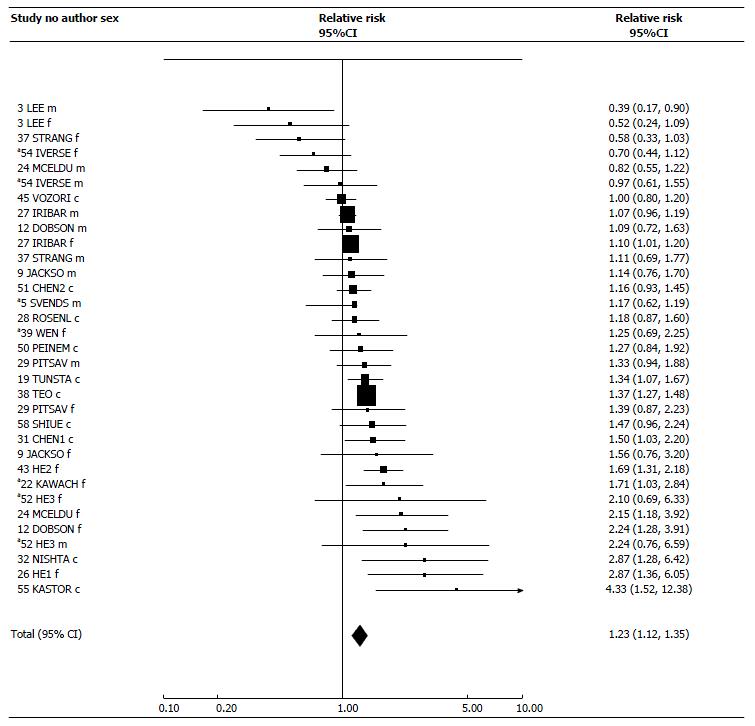
| Total | 33 | 1.21 (1.16-1.26) | 1.23 (1.12-1.35) | NS | 90.21 | 32 | P < 0.001 |
| Biomarker | 9 | 1.11 (0.98-1.26) | 1.15 (0.94-1.40) | NS | 15.40 | 8 | P < 0.1 |
| Study No. | Author1 | Sex | Exposure grouping | Relative risk by grouping (95%CI)2 | Significance3 |
| Workplace exposure | |||||
| 22 | Kawachi et al[48] | F | No, occasional, regular | 1.00, 1.49, 1.92 | |
| 26 | He et al[52] | F | 0-5, 6-10, 11-20, 21+ cigs/d | 1.00, 0.87, 2.95, 3.56 | + |
| F | 0-5, 6-15, 16+ year | 1.00, 3.08, 1.56 | |||
| F | 0, 1-2, 3, 4+ smokers | 1.00, 1.16, 5.06, 4.11 | + | ||
| F | 0, 1-2, 3-4, 5+ h/d | 1.00, 0.62, 4.03, 21.32 | + | ||
| F | 0, 1-2000, 2001-4000, 4000+, cigs/d × year × smokers × h | 1.00, 1.00, 2.05, 9.23 | + | ||
| 28 | Rosenlund et al[54] | M + F | 0, 1-31, 32+ yr | 1.00, 1.04, 1.30 | |
| M + F | 0, 1-68, 69+ h-year (= h/d × year) | 1.00, 0.99, 1.48 | |||
| 39 | Wen et al[64] | F | 0, < 10, 10-24, > 24 yr | 1.00, 0.86, 0.96, 0.934 | |
| 40 | Eisner et al[65] | M + F | Per 10 yr exposure | 1.04 | |
| Childhood exposure | |||||
| 18 | Muscat et al[44] | Exposure to mother, father, other relatives | |||
| M | None, 1-17, > 17 yr | 1.0, 0.9, 0.7 | |||
| F | None, 1-17, > 17 yr | 1.0, 0.6, 0.8 | |||
| 39 | Wen et al[64] | F | In early life from family members5 | ||
| 0, < 20, 20+, year | 1.00, 1.21, 1.364 | + | |||
| Total exposure | |||||
| 3 | Lee et al[31] | Home, work, travel, leisure combined index | |||
| M | Score: 0-1, 2-4, 5-12 | 1.00, 0.43, 0.43 | |||
| F | Score: 0-1, 2-4, 5-12 | 1.00, 0.59, 0.81 | |||
| 5 | Svendsen et al[33] | Spousal and/or workplace exposure | |||
| M | Neither, spouse, work, both | 1.0, 1.2, 1.0, 1.7 | |||
| 9 | Jackson[37] | Exposure at home and/or work6 | |||
| M | No, yes | 1.00, 1.14 (0.76-1.70) | |||
| F | No, yes | 1.00, 1.56 (0.76-3.20) | |||
| 12 | Dobson et al[40] | Exposure at home and/or work | |||
| M | No, yes | 1.00, 1.09 (0.72-1.63) | |||
| F | No, yes | 1.00, 2.24 (1.28-3.91) | + | ||
| 19 | Tunstall-Pedoe et al[45] | Exposure to tobacco smoke from someone else in the previous three days | |||
| M + F | None, little, some, a lot, (self-classified) | 1.00, 1.2, 1.5, 1.6 | + | ||
| 22 | Kawachi et al[48] | Exposure at home and/or work | |||
| F | None, occasional, regular | 1.00, 1.58, 1.91 | + | ||
| 26 | He et al[117] | ETS exposure from spouse and/or work | |||
| F | Neither, spouse, work, both | 1.00, 2.07, 2.53, 4.18 | + | ||
| 27 | Iribarren et al[53] | Exposure at home, in small spaces, in large indoor areas | |||
| M | 0, 1-9, 10-39, 40+ total h/wk | 1.00, 0.90, 1.08, 1.13 | + | ||
| F | 0, 1-9, 10-39, 40+ total h/wk | 1.00, 0.86, 1.07, 1.17 | + | ||
| 28 | Rosenlund et al[54] | Exposure from spouse and/or work | |||
| M + F | 0, > 16, 7-16, 1-6, < 1, year ago | 1.00, 0.92, 1.11, 1.30, 1.39 | |||
| M + F | 0, 1-12, 13-23, 24-34, 35+, year | 1.00, 0.72, 0.97, 1.54, 1.48 | + | ||
| M + F | 0, 1-17, 18-41, 42-89, 90+, h-year, (= year × h/d) | 1.00, 0.70, 1.22, 1.27, 1.55 | + | ||
| 29 | Pitsavos et al[55] | Exposure at home and/or work | |||
| M | None, occasional, regular | 1.00, 1.25, 1.47 | + | ||
| F | None, occasional, regular | 1.00, 1.29, 1.56 | + | ||
| 31 | Chen et al[56] | Exposure to tobacco smoke from someone else in the previous three days | |||
| M + F | None, a little, some, a lot | 1.00, 1.30, 1.50, 1.80 | + | ||
| Exposure to other people’s tobacco smoke | |||||
| M + F | 0, > 0-2, 3-5, ≥ 6 h/d | 1.00, 1.20, 1.60, 1.70 | |||
| 37 | Stranges et al[62] | Cumulative lifetime ETS exposure at home, work and in public settings | |||
| M | Tertile: 1, 2, 3 | 1.00, 0.93, 1.40 | |||
| F | Tertile: 1, 2, 3 | 1.00, 0.50, 0.67 | |||
| 38 | Teo et al[63] | Exposure from family, friends, co-workers | |||
| M + F | < 1, 1-7, 8-14, 15-21, 22+ h/wk | 1.00, 1.32, 1.52, 1.73, 1.49 | + | ||
| 43 | He et al[67] | Exposed at home and/or work | |||
| F | 0, 1-9, 10-19, 20+, cigs/d | 1.00, 1.41, 1.85, 1.77 | + | ||
| 0, 1-20, 21-40, 41+, min/d | 1.00, 1.46, 1.78, 1.86 | + | |||
| 52 | He et al[76] | Exposed at home and/or work7 | |||
| M + F | None Low Moderate High | 1.00, 1.74, 2.25, 3.79 | + | ||
| 54 | Iversen et al[78] | Time spent in a smoke-filled rooms | |||
| M | 0, 1-6, > 6, h/d | 1.00, 1.00, 0.80 | |||
| F | 0, 1-6, > 6, h/d | 1.00, 0.70, 0.70 | |||
| 58 | Shiue[82] | Exposed at home, work, other people’s home | |||
| M + F | 0, 1, 2+ of these places | 1.00, 1.37, 2.64 | + | ||
| Biomarker | |||||
| 19 | Tunstall-Pedoe et al[45] | Serum cotinine (ng/mL) | |||
| M + F | 0, > 0-1.05, 1.06-3.97, 3.98-17.49 | 1.00, 1.00, 1.30, 1.20 | |||
| 31 | Chen et al[56] | Serum cotinine (ng/mL) | |||
| M + F | 0, > 0-1.05, 1.06-3.97, 3.98-17.49 | 1.00, 0.70, 1.00, 1.10 | |||
| 33 | Whincup et al[58] | Serum cotinine (ng/mL) | |||
| M | ≤ 0.7, 0.8-1.4, 1.5-2.7, 2.8-14.0 | 1.00, 1.54, 1.89, 1.67 | + | ||
| 36 | Hedblad et al[61] | Blood carboxyhaemoglobin (%) | |||
| M | 0.13-0.49, 0.50-0.57, 0.58-0.66, 0.67-5.47 (quartiles) | 1.00, 1.26, 1.77, 3.71 | + | ||
| 48 | Hamer et al[72] | Salivary cotinine (ng/mL) | |||
| M + F | ≤ 0.05, 0.06-0.70, 0.71-14.99 | 1.00, 1.33, 2.00 | + | ||
| Per unit increase in log cotinine | 1.60 (1.11-2.31) | ||||
| 49 | Jefferis et al[73] | Serum cotinine (ng/mL) | |||
| M + F | ≤ 0.05, 0.06-0.19, 0.20-0.70, 0.71-15 | 1.00, 0.91, 0.99, 0.94 | |||
| Per doubling of cotinine | 1.00 (0.86-1.16) | ||||
| 56 | Rostron[80] | Serum cotinine (ng/mL) | |||
| M + F | < 0.1, 0.1- < 1, 1- < 15 | 1.00, 0.97, 1.41 | |||
| 57 | Batty et al[81] | Salivary cotinine (ng/mL) | |||
| M | ≤ 0.3, 0.4-1.2, 1.3-15.0 | 1.00, 0.41, 0.62 | |||
| F | ≤ 0.3, 0.4-1.2, 1.3-15.0 | 1.00, 0.99, 1.70 | |||
For workplace exposure, there were 22 estimates, with only one showing a significant increase, the combined estimate of 1.08 (95%CI: 0.99-1.19) being almost significantly raised. There was no evidence of heterogeneity, and little evidence of variation by any factor considered.
For childhood exposure, one of the seven estimates showed a significant increase in risk. However, the combined estimate of 1.12 (95%CI: 0.95-1.31) was not significant.
For total exposure, the 33 estimates showed clear heterogeneity (P < 0.001), 11 estimates showing a significant (P < 0.05) positive association, and one a significant negative association. However, there was a clear preponderance of positive associations, with the random-effects estimate 1.23 (95%CI: 1.12-1.35). Subgroup analyses showed higher estimates for Asia; for case-control studies, and for females and sexes-combined.
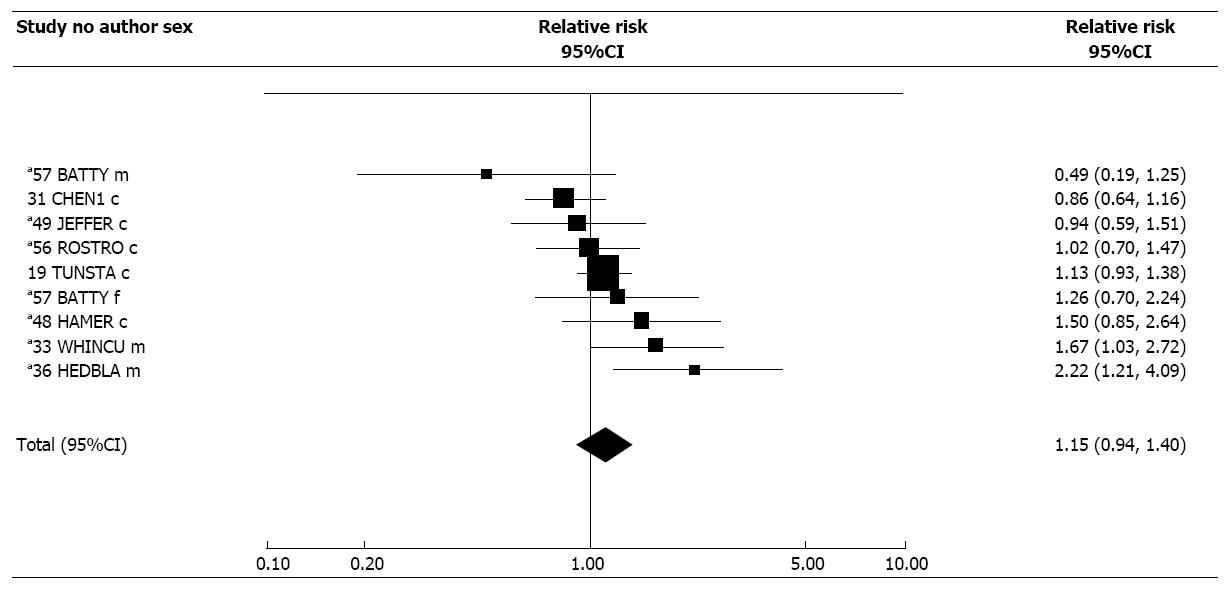
Of nine estimates for biomarker based exposure indices, all were cotinine-based apart from one based on COHb. There was some indication of heterogeneity (P < 0.1), the random-effects estimate of 1.15 (95%CI: 0.94-1.40) showing no clear association.
Table 8 presents dose-response data for these exposure indices. For studies reporting dose-response results, significant positive trends were seen (for at least one index) in 12 of 17 studies for total exposure, 3 of 8 studies for biomarker-based exposure, 1 of 5 studies for workplace exposure, and 1 of 2 studies for childhood exposure. No significant negative trends were seen.
Twelve studies presented RR estimates and/or dose-response results for one or more other exposure indices (Supplementary File 4). These results relate to many different indices, and are somewhat variable, with clear evidence of an increase being seen for studies 29 and 32, but a number of other studies showing no relationship with the indices studied.
Based on 58 studies, we present meta-analyses relating ETS exposure to heart disease risk in never smokers. Using an exposure index as equivalent as possible to having a spouse who ever smoked, a random-effects meta-analysis gave a significantly increased RR of 1.18 (95%CI: 1.12-1.24) based on 75 RR estimates. Positive associations, not all significant at P < 0.05, were also noted with spousal exposure specifically (1.10, 1.04-1.17, n = 34), household exposure (1.19, 1.13-1.25, n = 37), workplace exposure (1.08, 0.99-1.19, n = 22), childhood exposure (1.12, 0.95-1.31, n = 22), and total exposure (1.23, 1.12-1.35, n = 33). The overall estimate was also elevated for a biomarker-based index (1.15, 0.94-1.40, n = 9). There was also evidence of dose-response.
While the relationship of smoking with heart disease[83] suggests some effect may be evident for ETS, exposure to smoke constituents from ETS is much less than from active smoking. For example, studies of cotinine indicate relative exposure of ETS compared to smoking of 0.6% to 0.4%[84-86], while studies of particulate matter suggest a lower factor, < 0.02%[87-95]. In interpreting our meta-analyses, one must note the clear heterogeneity between the RR estimates. Thus, for the main exposure index, estimates were higher for females, United States studies, and small studies, and smaller for prospective studies and for fatal cases, and varied by definition of exposure and source of diagnosis. Although these factors are not independent, and the variations may reflect characteristics of studies with a large weight, they do add to the difficulties in interpreting the overall estimate.
Below, we comment on various aspects of the findings and discuss potential sources of bias.
For the main exposure index, there was clear publication bias (P < 0.001), RRs from smaller studies (more likely not to be published if finding no association) being much greater than from larger studies. Thus, for studies of > 1000 cases of heart disease, the RR was 1.08 (95%CI: 1.02-1.15, n = 18) while for studies of < 100 cases it was 1.66 (1.30-2.11, n = 13). This variation by study size explains why the random-effects estimate (1.18, 1.12-1.24) was higher than the fixed-effect estimate (1.10, 1.08-1.13), as small studies contribute relatively more to random-effects analyses. The random-effects estimate may be an overestimate, due to publication bias.
Some studies clarified that never smoking related to never smoking any product, and others that never smoking related only to cigarettes. However, many studies merely stated the subjects were never smokers. The distinction is more important in countries where smoking of other products is more common. Some studies also made it clear that the definition allowed inclusion of those with a limited history of smoking, and a few rejected individuals with cotinine levels typical of current smokers. However, the estimated RR for the main index varied little depending on the definition.
No study attempted to determine whether self-reported never smokers had in fact smoked previously. However, as noted above and in Table 2, a few studies excluded those with cotinine levels indicative of current smoking In our recent review of ETS and lung cancer[96], we presented analyses demonstrating that correction for misclassification bias substantially reduced the estimated RR for husband’s smoking. We did not attempted such correction here, partly because the extent of bias depends on the magnitude of the active smoking RR, which is much lower for heart disease than for lung cancer. However, we are aware of a study[97] which reported particularly high heart disease mortality among smokers who deny smoking, which, if confirmed, suggests misclassification bias might be of some relevance.
While random errors in determining ETS exposure will tend to underestimate any association with heart disease, errors may not be random. Thus, studies of case-control or cross-sectional design, are subject to recall bias if subjects with heart disease tend to overestimate their exposure relative to those without heart disease. Only two studies[45,56] used biomarker data to try to avoid recall bias. Some support for the existence of recall bias arises from the RRs for the main index being higher for case-control and cross-sectional studies than for prospective studies.
While prospective studies avoid recall bias, they may underestimate any true association if ETS exposure is determined only at baseline, and not updated. This was the case for the great majority of such studies. Thus, RRs for the index “spouse current smoker” may be underestimated by inclusion of some spouses who give up after baseline. However, the similarity of the RR estimates preferring current to ever spousal exposure and preferring ever to current spousal exposure suggests this is not a major issue.
In some case-control studies using population controls, the control group may not have been fully representative of the population from which the cases derived, while some hospital studies merely ensured that the controls were not suffering from heart disease, and may have included patients with other diseases associated with ETS exposure.
Ten of the 58 studies considered were of cross-sectional design. Apart from the possibility of recall bias, this design does not exclude the theoretical possibility that disease onset might have occurred before ETS exposure.
A major determinant of heterogeneity for the main index related to source of diagnosis, with RRs substantially lower for estimates based only on death certificates (1.06, 95%CI: 1.02-1.11), than when based on medical data (1.34, 1.23-1.46), the few estimates based on self-report giving intermediate results (1.17, 1.07-1.27). Note, however, that this classification correlates considerably with that for study type. Thus, all the estimates based on self-report are from cross-sectional studies, nearly all those based only on death certificates are from prospective studies, with case-control studies contributing largely to estimates based on medical data.
The actual disease for which results are available varies by study, with some studies presenting results for multiple definitions. Higher RRs were seen for the main index where the definition was based on MI (1.29, 95%CI: 1.14-1.46) rather than on IHD (1.12, 1.05-1.19) or other/mixed definitions (1.20, 1.10-1.30). However, again there is a correlation with study type, there being few prospective studies using a definition of MI.
There are manifold risk factors for heart disease, a study published in 1986[98] mentioning over 300. As several studies[53,99-103] showed differences in many lifestyle factors between smoking and non-smoking households, a potential for confounding is certainly present. Though difficult to assess precisely, partly because of the numerous risk factors involved, and partly because studies rarely present results showing the effect of adjustment for individual factors, some insight can be gained by comparing RR estimates across studies according to the number of risk factors adjusted for. Though the number of risk factors may be correlated with other aspects of the study, the results did not suggest the association was due to confounding, RRs being somewhat higher where more confounders were accounted for.
Three meta-analyses published in the late 1990s[2-4] deliberately excluded results reported by Layard[25], based on the National Mortality Followback Survey (NMFS), and by LeVois and Layard[26], based on the American Cancer Society (ACS) Cancer Prevention Studies I (CPS I) and II (CPS II). The results from these studies showed no evidence of a relationship of spousal smoking to heart disease mortality. Though we have not used the cited CPS II results, more detailed results being reported later by the ACS[46], we included the results from NMFS[25] and CPS I[26]. Apart from wishing to consider all the evidence, and particularly not omit data from the very large CPS I, we found the reasons for excluding these studies to be unconvincing.
One reason given[2] was that their results were inconsistent with other data, and reported by tobacco industry consultants. As regards inconsistency, it seems better to include all data, and investigate reasons for inconsistency, than to reject results not fitting in with preconceptions. As regards tobacco industry support, the test is whether the analyses presented were sound. We note no attempt was made by any critic to check the results from the publicly available NMFS, or by the ACS to check results from their CPS I. The ACS did conduct their own analyses of CPS II[46] using somewhat different methodology, their findings failing to indicate errors in the results of LeVois and Layard[26].
Another reason[4] given was that results were only presented for ever spousal exposure, rather than current spousal exposure. Apart from not noting that results for current spousal exposure were readily available from the CPSI data presented[26], the results being included in our analysis, Thun et al[4] also did not mention that their own analyses included results from other studies (studies 1, 2 and 8) based on ever spousal exposure! In fact, as we show, the overall RRs as can be seen in our main analysis, are very similar whether preferring ever to current spousal exposure (1.18, 95%CI: 1.12-1.24), or preferring current to ever spousal exposure (1.19, 1.13-1.26).
We have also included results reported by Enstrom and Kabat[27] in our analysis (Study 30), despite publication of the paper in the BMJ being subject to a large number of critical responses. As the authors noted in a final rapid response in the BMJ, none of the responses identified “any impropriety, bias, or omission in the review process” with “only about 3%” referring to “actual data in the paper”. “No one has identified a single error in the paper, not even Thun, who is in a position to check our findings”. We agree with Enstrom and Kabat that “the unethical tactics used by the ACS and others, including ad hominem attacks and condemnation of legitimate research based solely on the source of funding, have no place in scientific discourse”. The authors noted that “Our current research funding comes from Philip Morris USA and three other sources not connected with the tobacco industry”. As shown in Table 4, exclusion from our meta-analysis of the three studies in question (studies 15, 16 and 30) slightly increased the RR estimate for our main index, from 1.18 (95%CI: 1.12-1.24) to 1.23 (95%CI: 1.17-1.29), but did not affect the conclusion that there was a clear association of ETS exposure with heart disease risk.
Since the first study in 2004[104], which reported a 40% reduction in hospital admissions from AMI following introducing a local law banning smoking in public places and workplaces, numerous further studies have investigated ban effects at national, regional and local level. In a recent review[105], based on 45 studies, we used a consistent approach to adjust for time trends and seasonal effects. We estimated the post-ban risk reduction as 4.2% (95%CI: 1.8%-6.5%) initially, which reduced to 2.6% (1.1%-4.0%) after excluding regional studies where national estimates were available, and also studies where adjustment for the underlying trend in the heart disease rate was not possible. Although these estimates are much less than those from some earlier reviews[106-108] which used less precise techniques, they do suggest a small true ban effect. However, the effect cannot be directly attributed to reductions in risk arising from reduced ETS exposure. Some of the estimated effect might be because smokers reduced their daily cigarette consumption due to the more limited number of places where they are allowed to smoke.
The Institute of Medicine (IOM) report[7] discussed “pathophysiologic experiments that have investigated the cardiovascular effects of mainstream and sidestream tobacco smoke in cells, in animals and in humans”, noting that cigarette smoke could produce CVD by various “interrelated modes of action, including oxidative stress, hemodynamic and autonomic effects, endothelial dysfunction, thrombosis, inflammation, hyperlipidemia or other effects”. While beyond the scope of this paper to consider such evidence, we note that the report states most of the observed changes “have not been formally validated as clinical tests and there is not a consensus within the scientific community that they are predictive of actual clinical disease.” While the IOM Committee considered that these effects can “contribute to the biological plausibility that decreasing second-hand smoke could lead to a decrease in acute myocardial infarction”, they did not consider that the results, on their own, demonstrated a causal relationship of ETS exposure to heart disease.
In the introduction we referred to various other, conflicting, reviews of ETS and heart disease. Though it is beyond our scope to consider all these in detail, it is worth referring to a recently published systematic review[109] which concluded that ETS exposure “significantly increased the risk for …CVD”. This review was limited to prospective and case-control studies, but included studies of stroke, which we have reviewed separately[110]. While the authors’ combined RR estimate for cardiovascular disease of 1.23 (95%CI: 1.16-1.31) was similar to our main analysis estimate of 1.18 (1.12-1.24), we note they excluded a number of prospective and case-control studies we included. While some omissions were because they excluded abstracts and theses, and biomarker studies using COHb, we noted eight studies (13, 16, 21, 25, 32, 38, 46 and 55) where there seemed no good reason for the omission. Also, they did not separate results by source of ETS exposure or present any dose-response results.
In recent years, our group has carried out systematic reviews and meta-analyses of the relationship of ETS with various diseases in never smoking adults. These include lung cancer[111], breast cancer[112], other cancers[113], stroke[110] and COPD (submitted for publication). It is of interest to note that spousal smoking is associated with about 20% increased risk in never smokers, not only for heart disease, as we report here, but most studied diseases - stroke, COPD, lung cancer and breast cancer. Estimates are more limited for other cancers, many sites not showing any evidence of an effect, though significant increases were noted for cervix, nasosinus and kidney cancer. Whether evidence of an association for other diseases adds support to the argument that ETS exposure causes heart disease is unclear, as many of the problems of bias noted to affect the association with heart disease may also affect the association with other diseases.
Some, but not all, of the biases may be removed by limiting attention to prospective studies of ETS and total mortality. However, at this point in time, we have not carried out a review of the evidence, though we note that about half the prospective studies cited in Table 1 do give results for total mortality.
Do the results show that ETS exposure increases risk of heart disease? Here one can usefully cite the classic paper by Hill[114] which specified nine criteria to be considered when attempting to conclude causation. We consider these in turn below.
Strength: The observed association is clearly weak, with our main analyses estimating only an 18% increase in risk associated with ETS exposure.
Consistency: While some studies report no increased risk and a number do not report a statistically significant increased risk, this may reflect the difficulty in demonstrating a weak association, particularly with limited data. Even though there is clear heterogeneity for our main index of exposure, the meta-analysis estimates by level of a range of factors are all increased, and nearly always significantly increased. Thus, for example, significant increases are seen in each sex, in four continents, in prospective, case-control and cross-sectional studies, and in smaller and larger studies. There is certainly an element of consistency.
Specificity: ETS exposure is certainly not a necessary or sufficient cause of heart disease. While it is much easier to demonstrate causation where an agent is such a cause, this criterion is not really relevant here.
Temporality: While theoretically possible in the cross-sectional studies that some cases of heart disease might have preceded exposure to ETS, this could not be so for most cases in the 58 studies we considered.
Biological gradient: Though not all the studies demonstrate a dose-response relationship, many do. However, the significant trends observed are generally calculated including the unexposed group, and evidence of a dose-response within ETS exposed subjects is less clear.
Plausibility: There is clearly plausibility, given smoking causes heart disease and given the experimental evidence referred to above. However, the dose of smoke constituents from ETS is very much less than that from smoking, and it is unclear whether the short-term effects of ETS observed experimentally are actually predictive of heart disease.
Coherence: A cause-and-effect interpretation of the data does not, as far as we are aware, seriously conflict with other generally known facts concerning the history and biology of heart disease.
Experiment: The epidemiological evidence considered lacks any useful material to determine how the risk of heart disease varies following cessation of ETS exposure. However, the evidence from studies of smoking bans suggests that the introduction of smoking bans in public places has caused a modest reduction in risk of heart disease though, as noted, such studies, generally do not separate out effects of reduced ETS exposure in never smokers and of reduced opportunities to smoke in smokers.
Analogy: Whether effects of smoking and of ETS can be regarded as analogous is doubtful, given the substantial differences in extent of exposure and the somewhat different distribution of chemicals for the two types of exposure.
Considering all these points, there seems some inconclusive support for ETS exposure causing heart disease. An important issue not specifically considered in the Bradford Hill criteria, much more relevant for weak than strong associations, is whether the association might be explained by confounding or bias. As regards confounding, the observation that many studies adjusted for numerous risk factors for heart disease, and that RR estimates if anything, increase as more factors are adjusted for, suggests that confounding could not explain the relationship. Nor does it seem likely that the relationship could be fully explained by publication bias or recall bias, though the smaller estimates for large studies and for prospective studies suggest that these biases might have led to some overestimation of the association. Nor is it probable that misclassification of smoking status, or the inclusion of some smokers of products other than cigarettes or occasional or ex-smokers could explain the observed association. While we feel there may well be a true effect of ETS on heart disease risk, it is clear that it is difficult to come to a definitive conclusion, and even more difficult to estimate any true effect precisely.
In conclusion, Taken together with the known relationship of heart disease with smoking, the significantly increased risk for various indices of ETS exposure which can be seen in many study subsets, the evidence of a dose-response relationship, and the lack of any source of bias or confounding that can clearly explain the relationship, the evidence suggests that ETS exposure may cause some increase in the risk of heart disease. That said, the weakness of the overall relationship, the evidence of heterogeneity, the limitations of some of the studies, and the various possibilities of bias, certainly mean that any true effect of ETS exposure is very difficult to quantify precisely.
We thank Japan Tobacco International S.A. for supporting publication of this paper. The opinions and conclusions of the authors are their own, and do not necessarily reflect the position of Japan Tobacco International S.A. We also thank the United Kingdom Tobacco Manufacturers Association, Imperial Tobacco Ltd, British-American Tobacco Limited, and Philip Morris Products S.A. for earlier support in developing the databases used. Finally we thank Pauline Wassell, Diana Morris and Yvonne Cooper for assistance in typing various drafts of the paper and obtaining relevant literature, and all the researchers who published the reports which formed the basis of our work.
The authors consider evidence that environmental tobacco smoke (ETS) exposure might cause heart disease by presenting an up-to-date meta-analysis of the available evidence.
Based on 58 studies providing relevant data, the authors demonstrate an increase in heart disease risk in never smokers associated with ETS exposure by the spouse (or nearest equivalent), with an overall RR estimate of 1.18 (1.12-1.24). While increases were observed in all data subsets considered, there was evidence of heterogeneity, with risk estimates lower for North American studies, larger studies, prospective studies, and when based on fatal cases or death certificate data. Positive associations, not all significant at P < 0.05, were also seen with spousal exposure specifically (1.10, 1.04-1.17), workplace exposure (1.08, 0.99-1.19), childhood exposure (1.12, 0.95-1.31), total exposure (1.23, 1.12-1.35) and biomarker-based exposure (1.15, 0.94-1.40) and there was evidence of a dose-response relationship. Although the evidence has various limitations, it is suggestive of a causal relationship. However, the various possibilities of bias mean that any true effect of ETS exposure is very difficult to quantify precisely.
The new feature of the paper is the extent of the evidence considered, and the detail of the analyses conducted.
The authors analyses emphasise the difficulties in drawing inferences from weak associations seen in non-randomized epidemiological studies, where various biases may exist.
This is a meta-analysis of 58 studies that address the issue of environmental tobacco smoke and the development of heart disease. Overall, the authors found an association between exposure and heart disease risk.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: A den Uil C, Biondi-Zoccai G, Skobel E, Sabate M S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Wells AJ. Passive smoking as a cause of heart disease. J Am Coll Cardiol. 1994;24:546-554. [PubMed] [Cited in This Article: ] |
| 2. | Law MR, Morris JK, Wald NJ. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. BMJ. 1997;315:973-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 349] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 3. | He J, Vupputuri S, Allen K, Prerost MR, Hughes J, Whelton PK. Passive smoking and the risk of coronary heart disease--a meta-analysis of epidemiologic studies. N Engl J Med. 1999;340:920-926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 370] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Thun M, Henley J, Apicella L. Epidemiologic studies of fatal and nonfatal cardiovascular disease and ETS exposure from spousal smoking. Environ Health Perspect. 1999;107 Suppl 6:841-846. [PubMed] [Cited in This Article: ] |
| 5. | US Surgeon General. The health consequences of involuntary exposure to tobacco smoke. A report of the Surgeon General. Vol Atlanta, Georgia: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health 2006; 727 Available from: http://www.surgeongeneral.gov/library/reports/index.html. [Cited in This Article: ] |
| 6. | International Agency for Research on Cancer. Tobacco smoke and involuntary smoking. Vol 83. IARC Monogr Eval Carcinog Risks Hum Lyon, France: IARC 2004; 1452 Available from: http://monographs.iarc.fr/ENG/Monographs/vol83/index.php. [Cited in This Article: ] |
| 7. | Institute of Medicine. Secondhand smoke exposure and cardiovascular effects: making sense of the evidence. Vol Washington, D.C. : The National Academies Press 2010; 241. [Cited in This Article: ] |
| 8. | California Environmental Protection Agency. Proposed identification of environmental tobacco smoke as a toxic air contaminant - as approved by the Scientific Review Panel on June 24, 2005. Vol. 2005; Available from: https://www.arb.ca.gov/regact/ets2006/ets2006.htm. [Cited in This Article: ] |
| 9. | Zhang J, Watson RR. Environmental tobacco smoke and cardiovascular disease. Boca Raton, Florida: CRC Press LLC 2000; 309-318. [Cited in This Article: ] |
| 10. | Kaur S, Cohen A, Dolor R, Coffman CJ, Bastian LA. The impact of environmental tobacco smoke on women’s risk of dying from heart disease: a meta-analysis. J Womens Health (Larchmt). 2004;13:888-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Leone A, Giannini D, Bellotto C, Balbarini A. Passive smoking and coronary heart disease. Curr Vasc Pharmacol. 2004;2:175-182. [PubMed] [Cited in This Article: ] |
| 12. | Dunbar A, Gotsis W, Frishman W. Second-hand tobacco smoke and cardiovascular disease risk: an epidemiological review. Cardiol Rev. 2013;21:94-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Fischer F, Kraemer A. Meta-analysis of the association between second-hand smoke exposure and ischaemic heart diseases, COPD and stroke. BMC Public Health. 2015;15:1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Bailar JC. Passive smoking, coronary heart disease, and meta-analysis. N Engl J Med. 1999;340:958-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Lee PN, Roe FJC. Environmental tobacco smoke exposure and heart disease: a critique of the claims of Glantz and Parmley. Hum Ecol Risk Ass. 1999;5:171-218. [Cited in This Article: ] |
| 16. | Denson KWE. Environmental tobacco smoke and ischaemic heart disease: a critical assessment of recent meta-analyses and reviews. Med Sci Res. 1999;27:75-82. [Cited in This Article: ] |
| 17. | Mengersen KL, Merrilees MJ, Tweedie RL. Environmental tobacco smoke and ischaemic heart disease: a case study in applying causal criteria. Int Arch Occup Environ Health. 1999;72 Suppl:R1-40. [PubMed] [Cited in This Article: ] |
| 18. | Enstrom JE, Kabat GC. Environmental tobacco smoke and coronary heart disease mortality in the United States--a meta-analysis and critique. Inhal Toxicol. 2006;18:199-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Morris JA, Gardner MJ. Calculating confidence intervals for relative risks (odds ratios) and standardised ratios and rates. Br Med J (Clin Res Ed). 1988;296:1313-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 463] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 20. | Hamling J, Lee P, Weitkunat R, Ambühl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27:954-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 495] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 21. | Fleiss JL, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol. 1991;44:127-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 296] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 22. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39087] [Cited by in F6Publishing: 41759] [Article Influence: 1988.5] [Reference Citation Analysis (1)] |
| 23. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34245] [Cited by in F6Publishing: 36095] [Article Influence: 1336.9] [Reference Citation Analysis (1)] |
| 24. | Lee PN. Uses and abuses of cotinine as a marker of tobacco smoke exposure. Gorrod JW and Jacob P, III: Analytical determination of nicotine and related compounds and their metabolites. Amsterdam: Elsevier 1999; 669-719. [Cited in This Article: ] |
| 25. | Layard MW. Ischemic heart disease and spousal smoking in the National Mortality Followback Survey. Regul Toxicol Pharmacol. 1995;21:180-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | LeVois ME, Layard MW. Publication bias in the environmental tobacco smoke/coronary heart disease epidemiologic literature. Regul Toxicol Pharmacol. 1995;21:184-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Enstrom JE, Kabat GC. Environmental tobacco smoke and tobacco related mortality in a prospective study of Californians, 1960-98. BMJ. 2003;326:1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Draper NR, Smith H. Applied Regression Analysis. Vol 3rd edition. Wiley Series in Probability and Statistics New York: Wiley Interscience 1998; . [Cited in This Article: ] |
| 29. | Hirayama T. Lung cancer in Japan: effects of nutrition and passive smoking. In: Mizell M and Correa P, editors. Lung cancer: causes and prevention. Proceedings of the International Lung Cancer Update Conference; March 3-5, 1983; New Orleans, Louisiana. Deerfield Beach, Florida: Verlag Chemie International, Inc, 1984: 175-195. . [Cited in This Article: ] |
| 30. | Garland C, Barrett-Connor E, Suarez L, Criqui MH, Wingard DL. Effects of passive smoking on ischemic heart disease mortality of nonsmokers. A prospective study. Am J Epidemiol. 1985;121:645-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 83] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Lee PN, Chamberlain J, Alderson MR. Relationship of passive smoking to risk of lung cancer and other smoking-associated diseases. Br J Cancer. 1986;54:97-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 102] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Martin MJ, Hunt SC, Williams RR. Increased incidence of heart attacks in nonsmoking women married to smokers [Abstract]. Proceedings of the 114th Annual Meeting of American Public Health Association; October 1. 1986;1. [Cited in This Article: ] |
| 33. | Svendsen KH, Kuller LH, Martin MJ, Ockene JK. Effects of passive smoking in the Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1987;126:783-795. [PubMed] [Cited in This Article: ] |
| 34. | Butler TL. The relationship of passive smoking to various health outcomes among Seventh day Adventists in California [Thesis]. Los Angeles: University of California 1988; 275. [Cited in This Article: ] |
| 35. | Palmer JR, Rosenberg L, Shapiro S. Passive smoking and myocardial infarction in women [Abstract]. CVD Newsletter. 1988;43:29. [Cited in This Article: ] |
| 36. | Hole DJ, Gillis CR, Chopra C, Hawthorne VM. Passive smoking and cardiorespiratory health in a general population in the west of Scotland. BMJ. 1989;299:423-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 89] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Jackson RT. The Auckland Heart Survey [Thesis]. Auckland, New Zealand: University of Auckland 1989; . [Cited in This Article: ] |
| 38. | Sandler DP, Comstock GW, Helsing KJ, Shore DL. Deaths from all causes in non-smokers who lived with smokers. Am J Public Health. 1989;79:163-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 70] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Humble C, Croft J, Gerber A, Casper M, Hames CG, Tyroler HA. Passive smoking and 20-year cardiovascular disease mortality among nonsmoking wives, Evans County, Georgia. Am J Public Health. 1990;80:599-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Dobson AJ, Alexander HM, Heller RF, Lloyd DM. Passive smoking and the risk of heart attack or coronary death. Med J Aust. 1991;154:793-797. [PubMed] [Cited in This Article: ] |
| 41. | Gardiner AJS, Forey BA, Lee PN. The relationship between avian exposure and bronchogenic carcinoma. In: Lester JN, Perry R and Reynolds GL, editors. Proceedings of the Quality of the Indoor Environment; April 1992; Selper Ltd, 1992: 691-703. [Cited in This Article: ] |
| 42. | La Vecchia C, D’Avanzo B, Franzosi MG, Tognoni G. Passive smoking and the risk of acute myocardial infarction GISSI-EFRIM investigations. Lancet. 1993;341:505-506. [PubMed] [Cited in This Article: ] |
| 43. | Mannino DM, Siegel M, Rose D, Etzel R. Health effects of environmental tobacco smoke exposure in US adults: data from the 1991 National Health Interview Survey. Epidemiology. 1995;6:56S. [Cited in This Article: ] |
| 44. | Muscat JE, Wynder EL. Exposure to environmental tobacco smoke and the risk of heart attack. Int J Epidemiol. 1995;24:715-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Tunstall-Pedoe H, Brown CA, Woodward M, Tavendale R. Passive smoking by self report and serum cotinine and the prevalence of respiratory and coronary heart disease in the Scottish heart health study. J Epidemiol Community Health. 1995;49:139-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Steenland K, Thun M, Lally C, Heath C. Environmental tobacco smoke and coronary heart disease in the American Cancer Society CPS-II cohort. Circulation. 1996;94:622-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Janghorbani M, Sadeghi-Golmakani N. Passive smoking and the risk of coronary heart disease among married non-smoking women. Med J Islam Repub Iran. 1997;11:203-208. [Cited in This Article: ] |
| 48. | Kawachi I, Colditz GA, Speizer FE, Manson JE, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of passive smoking and coronary heart disease. Circulation. 1997;95:2374-2379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Ciruzzi M, Pramparo P, Esteban O, Rozlosnik J, Tartaglione J, Abecasis B, César J, De Rosa J, Paterno C, Schargrodsky H. Case-control study of passive smoking at home and risk of acute myocardial infarction. Argentine FRICAS Investigators. Factores de Riesgo Coronario en América del Sur. J Am Coll Cardiol. 1998;31:797-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | McElduff P, Dobson AJ, Jackson R, Beaglehole R, Heller RF, Lay-Yee R. Coronary events and exposure to environmental tobacco smoke: a case-control study from Australia and New Zealand. Tob Control. 1998;7:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Spencer CA, Jamrozik K, Lambert L. Do simple prudent health behaviours protect men from myocardial infarction? Int J Epidemiol. 1999;28:846-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | He Y, Lam TH, Li LS, Li LS, Du RY, Jia GL, Huang JY, Shi QL, Zheng JS. Passive smoking from husbands as a risk factor for coronary heart disease in women in Xi’an, China, who have never smoked. Lu R, Mackay J, Niu S and Peto R, editors. 1997;Beijing, China. [Cited in This Article: ] |
| 53. | Iribarren C, Friedman GD, Klatsky AL, Eisner MD. Exposure to environmental tobacco smoke: association with personal characteristics and self reported health conditions. J Epidemiol Community Health. 2001;55:721-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Rosenlund M, Berglind N, Gustavsson A, Reuterwall C, Hallqvist J, Nyberg F, Pershagen G. Environmental tobacco smoke and myocardial infarction among never-smokers in the Stockholm Heart Epidemiology Program (SHEEP). Epidemiology. 2001;12:558-564. [PubMed] [Cited in This Article: ] |
| 55. | Pitsavos C, Panagiotakos DB, Chrysohoou C, Skoumas J, Tzioumis K, Stefanadis C, Toutouzas P. Association between exposure to environmental tobacco smoke and the development of acute coronary syndromes: the CARDIO2000 case-control study. Tob Control. 2002;11:220-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Chen R, Tavendale R, Tunstall-Pedoe H. Environmental tobacco smoke and prevalent coronary heart disease among never smokers in the Scottish MONICA surveys. Occup Environ Med. 2004;61:790-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Nishtar S, Wierzbicki AS, Lumb PJ, Lambert-Hammill M, Turner CN, Crook MA, Mattu MA, Shahab S, Badar A, Ehsan A. Waist-hip ratio and low HDL predict the risk of coronary artery disease in Pakistanis. Curr Med Res Opin. 2004;20:55-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Whincup PH, Gilg JA, Emberson JR, Jarvis MJ, Feyerabend C, Bryant A, Walker M, Cook DG. Passive smoking and risk of coronary heart disease and stroke: prospective study with cotinine measurement. BMJ. 2004;329:200-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 59. | McGhee SM, Ho SY, Schooling M, Ho LM, Thomas GN, Hedley AJ, Mak KH, Peto R, Lam TH. Mortality associated with passive smoking in Hong Kong. BMJ. 2005;330:287-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Qureshi AI, Suri MF, Kirmani JF, Divani AA. Cigarette smoking among spouses: another risk factor for stroke in women. Stroke. 2005;36:e74-e76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Hedblad B, Engström G, Janzon E, Berglund G and Janzon L. COHb% as a marker of cardiovascular risk in never smokers: results from a population-based cohort study. Scand J Public Health. 2006;34:609-615. [Cited in This Article: ] |
| 62. | Stranges S, Bonner MR, Fucci F, Cummings KM, Freudenheim JL, Dorn JM, Muti P, Giovino GA, Hyland A, Trevisan M. Lifetime cumulative exposure to secondhand smoke and risk of myocardial infarction in never smokers: results from the Western New York health study, 1995-2001. Arch Intern Med. 2006;166:1961-1967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Teo KK, Ounpuu S, Hawken S, Pandey MR, Valentin V, Hunt D, Diaz R, Rashed W, Freeman R, Jiang L. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368:647-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 626] [Cited by in F6Publishing: 588] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 64. | Wen W, Shu XO, Gao YT, Yang G, Li Q, Li H, Zheng W. Environmental tobacco smoke and mortality in Chinese women who have never smoked: prospective cohort study. BMJ. 2006;333:376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Eisner MD, Wang Y, Haight TJ, Balmes J, Hammond SK, Tager IB. Secondhand smoke exposure, pulmonary function, and cardiovascular mortality. Ann Epidemiol. 2007;17:364-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 66. | Hill SE, Blakely T, Kawachi I, Woodward A. Mortality among lifelong nonsmokers exposed to secondhand smoke at home: cohort data and sensitivity analyses. Am J Epidemiol. 2007;165:530-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | He Y, Lam TH, Jiang B, Wang J, Sai X, Fan L, Li X, Qin Y, Hu FB. Passive smoking and risk of peripheral arterial disease and ischemic stroke in Chinese women who never smoked. Circulation. 2008;118:1535-1540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 68. | Sulo G, Burazeri G, Dehghan A, Kark JD. Partner’s smoking status and acute coronary syndrome: population-based case-control study in Tirana, Albania. Croat Med J. 2008;49:751-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 69. | Vozoris N, Lougheed MD. Second-hand smoke exposure in Canada: prevalence, risk factors, and association with respiratory and cardiovascular diseases. Can Respir J. 2008;15:263-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Ding D, Wing-Hong Fung J, Zhang Q, Wai-Kwok Yip G, Chan CK, Yu CM. Effect of household passive smoking exposure on the risk of ischaemic heart disease in never-smoke female patients in Hong Kong. Tob Control. 2009;18:354-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Gallo V, Neasham D, Airoldi L, Ferrari P, Jenab M, Boffetta P, Overvad K, Tjønneland A, Clavel-Chapelon F, Boeing H. Second-hand smoke, cotinine levels, and risk of circulatory mortality in a large cohort study of never-smokers. Epidemiology. 2010;21:207-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Hamer M, Stamatakis E, Kivimaki M, Lowe GD, Batty GD. Objectively measured secondhand smoke exposure and risk of cardiovascular disease: what is the mediating role of inflammatory and hemostatic factors? J Am Coll Cardiol. 2010;56:18-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 73. | Jefferis BJ, Lawlor DA, Ebrahim S, Wannamethee SG, Feyerabend C, Doig M, McMeekin L, Cook DG, Whincup PH. Cotinine-assessed second-hand smoke exposure and risk of cardiovascular disease in older adults. Heart. 2010;96:854-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | Peinemann F, Moebus S, Dragano N, Möhlenkamp S, Lehmann N, Zeeb H, Erbel R, Jöckel KH, Hoffmann B. Secondhand smoke exposure and coronary artery calcification among nonsmoking participants of a population-based cohort. Environ Health Perspect. 2011;119:1556-1561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Chen R. Association of environmental tobacco smoke with dementia and Alzheimer’s disease among never smokers. Alzheimers Dement. 2012;8:590-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | He Y, Jiang B, Li LS, Li LS, Ko L, Wu L, Sun DL, He SF, Liang BQ, Hu FB. Secondhand smoke exposure predicted COPD and other tobacco-related mortality in a 17-year cohort study in China. Chest. 2012;142:909-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 77. | Clark ML, Butler LM, Koh WP, Wang R, Yuan JM. Dietary fiber intake modifies the association between secondhand smoke exposure and coronary heart disease mortality among Chinese non-smokers in Singapore. Nutrition. 2013;29:1304-1309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Iversen B, Jacobsen BK, Løchen ML. Active and passive smoking and the risk of myocardial infarction in 24,968 men and women during 11 year of follow-up: the Tromsø Study. Eur J Epidemiol. 2013;28:659-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 79. | Kastorini CM, Georgousopoulou E, Vemmos KN, Nikolaou V, Kantas D, Milionis HJ, Goudevenos JA, Panagiotakos DB. Comparative analysis of cardiovascular disease risk factors influencing nonfatal acute coronary syndrome and ischemic stroke. Am J Cardiol. 2013;112:349-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 80. | Rostron B. Mortality risks associated with environmental tobacco smoke exposure in the United States. Nicotine Tob Res. 2013;15:1722-1728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Batty GD, Gale CR, Jefferis B, Kvaavik E. Passive smoking assessed by salivary cotinine and self-report in relation to cause-specific mortality: 17-year follow-up of study participants in the UK Health and Lifestyle Survey. J Epidemiol Community Health. 2014;68:1200-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Shiue I. Modeling the effects of indoor passive smoking at home, work, or other households on adult cardiovascular and mental health: the Scottish Health Survey, 2008-2011. Int J Environ Res Public Health. 2014;11:3096-3107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | US Surgeon General. The health consequences of smoking - 50 years of progress: a report of the Surgeon General. Vol Atlanta, Georgia: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health 2014; 944 Available from: http://www.surgeongeneral.gov/library/reports/index.html. [Cited in This Article: ] |
| 84. | Office of Population Censuses and Surveys. Health survey for England 1994. Volume I: Findings. Volume II: Survey methodology and documentation. Series HS no. 4. Colhoun H and Prescott-Clarke P, editors. Vol London: HMSO 1996; 607. [Cited in This Article: ] |
| 85. | Ziegler RG, Mason TJ, Stemhagen A, Hoover R, Schoenberg JB, Gridley G, Virgo PW, Altman R, Fraumeni JF. Dietary carotene and vitamin A and risk of lung cancer among white men in New Jersey. J Natl Cancer Inst. 1984;73:1429-1435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 86. | Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA. 1996;275:1233-1240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 87. | Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169:236-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 466] [Cited by in F6Publishing: 500] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 88. | Phillips K, Bentley MC, Howard DA, Alván G. Assessment of air quality in Stockholm by personal monitoring of nonsmokers for respirable suspended particles and environmental tobacco smoke. Scand J Work Environ Health. 1996;22 Suppl 1:1-24. [PubMed] [Cited in This Article: ] |
| 89. | Phillips K, Bentley MC, Howard DA, Alván G, Huici A. Assessment of air quality in Barcelona by personal monitoring of nonsmokers for respirable suspended particles and environmental tobacco smoke. Environ Int. 1997;23:173-196. [DOI] [Cited in This Article: ] |
| 90. | Phillips K, Howard DA, Bentley MC, Alván G. Assessment of air quality in Kuala Lumpur by personal monitoring of nonsmokers at home and in the workplace by reference to respirable suspended particles (RSP) and environmental tobacco smoke (ETS). In: Gee IL and Leslie GB, editors. Indoor and built environment problems in Asia. Proceedings of the Conference; 4th & 5th September 1997; Kuala Lumpur, Malaysia. Rothenfluh, Switzerland: The International Society of the Built Environment, 1997: 151-159. . [Cited in This Article: ] |
| 91. | Phillips K, Howard DA, Bentley MC, Alván G. Assessment by personal monitoring of respirable suspended particles and environmental tobacco smoke exposure for non-smokers in Sydney, Australia. Indoor Built Environ. 1998;7:188-203. [DOI] [Cited in This Article: ] |
| 92. | Phillips K, Howard DA and Bentley MC. Assessment of environmental tobacco smoke and respirable suspended particle exposures for nonsmokers in Lisbon by personal monitoring. Environ Int. 1998;24:301-324. [DOI] [Cited in This Article: ] |
| 93. | Phillips K, Howard DA, Bentley MC, Alván G. Measured exposures by personal monitoring for respirable suspended particles and environmental tobacco smoke of housewives and office workers resident in Bremen, Germany. Int Arch Occup Environ Health. 1998;71:201-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Phillips K, Bentley MC, Howard DA, Alván G. Assessment of environmental tobacco smoke and respirable suspended particle exposures for nonsmokers in Prague using personal monitoring. Int Arch Occup Environ Health. 1998;71:379-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Phillips K, Howard DA, Bentley MC, Alván G. Environmental tobacco smoke and respirable suspended particle exposures for non-smokers in Beijing. Indoor Built Environ. 1998;7:254-269. [DOI] [Cited in This Article: ] |
| 96. | Lee PN, Fry JS, Forey B, Hamling JS and Thornton AJ. Environmental tobacco smoke exposure and lung cancer: a systematic review. World J Metaanal. 2016;4:10-43. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Suadicani P, Hein HO, Gyntelberg F. Mortality and morbidity of potentially misclassified smokers. Int J Epidemiol. 1997;26:321-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Hopkins PN, Williams RR. Identification and relative weight of cardiovascular risk factors. Cardiol Clin. 1986;4:3-31. [PubMed] [Cited in This Article: ] |
| 99. | Thompson DH, Warburton DM. Lifestyle differences between smokers, ex-smokers and non-smokers, and implications for their health. Psychol Health. 1992;7:311-321. [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 100. | Thornton A, Lee P, Fry J. Differences between smokers, ex-smokers, passive smokers and non-smokers. J Clin Epidemiol. 1994;47:1143-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 90] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 101. | Matanoski G, Kanchanaraksa S, Lantry D, Chang Y. Characteristics of nonsmoking women in NHANES I and NHANES I epidemiologic follow-up study with exposure to spouses who smoke. Am J Epidemiol. 1995;142:149-157. [PubMed] [Cited in This Article: ] |
| 102. | Dallongeville J, Marécaux N, Fruchart JC, Amouyel P. Cigarette smoking is associated with unhealthy patterns of nutrient intake: a meta-analysis. J Nutr. 1998;128:1450-1457. [PubMed] [Cited in This Article: ] |
| 103. | Forastiere F, Mallone S, Lo Presti E, Baldacci S, Pistelli F, Simoni M, Scalera A, Pedreschi M, Pistelli R, Corbo G. Characteristics of nonsmoking women exposed to spouses who smoke: epidemiologic study on environment and health in women from four Italian areas. Environ Health Perspect. 2000;108:1171-1177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 104. | Sargent RP, Shepard RM, Glantz SA. Reduced incidence of admissions for myocardial infarction associated with public smoking ban: before and after study. BMJ. 2004;328:977-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 373] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 105. | Lee PN, Fry JS, Forey BA. A review of the evidence on smoking bans and incidence of heart disease. Regul Toxicol Pharmacol. 2014;70:7-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 106. | Glantz SA. Meta-analysis of the effects of smokefree laws on acute myocardial infarction: an update. Prev Med. 2008;47:452-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 107. | Lightwood JM, Glantz SA. Declines in acute myocardial infarction after smoke-free laws and individual risk attributable to secondhand smoke. Circulation. 2009;120:1373-1379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 108. | Meyers DG, Neuberger JS, He J. Cardiovascular effect of bans on smoking in public places: a systematic review and meta-analysis. J Am Coll Cardiol. 2009;54:1249-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 224] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 109. | Lv J, Qi L, Yu C, Yang L, Guo Y, Chen Y, Bian Z, Sun D, Du J, Ge P. Consumption of spicy foods and total and cause specific mortality: population based cohort study. BMJ. 2015;351:h3942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 110. | Lee PN, Thornton AJ, Forey BA, Hamling JS. Environmental Tobacco Smoke Exposure and Risk of Stroke in Never Smokers: An Updated Review with Meta-Analysis. J Stroke Cerebrovasc Dis. 2017;26:204-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 111. | Lee PN, Forey BA, Hamling JS and Thornton AJ. ETS and heart disease meta-analyses. Vol Sutton, Surrey: PN Lee Statistics and Computing Ltd 2015; 4. [accessed 2015] Available from: http://www.pnlee.co.uk/Reports.htm. [Cited in This Article: ] |
| 112. | Lee PN, Hamling JS. Environmental tobacco smoke exposure and risk of breast cancer in nonsmoking women. An updated review and meta-analysis. Inhal Toxicol. 2016;28:431-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 113. | Lee PN, Thornton AJ, Hamling JS. Epidemiological evidence on environmental tobacco smoke and cancers other than lung or breast. Regul Toxicol Pharmacol. 2016;80:134-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295-300. [PubMed] [Cited in This Article: ] |
| 115. | Hirayama T. Passive smoking [Letter]. N Z Med J. 1990;103:54. [PubMed] [Cited in This Article: ] |
| 116. | Wells AJ. Heart disease from passive smoking in the workplace. J Am Coll Cardiol. 1998;31:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |