Published online Aug 26, 2015. doi: 10.13105/wjma.v3.i4.193
Peer-review started: October 28, 2014
First decision: November 28, 2014
Revised: June 30, 2015
Accepted: July 11, 2015
Article in press: July 13, 2015
Published online: August 26, 2015
Processing time: 321 Days and 20.5 Hours
AIM: To evaluate existing evidence for the association between different type of brassiere exposures and the risk of breast cancer.
METHODS: Ovid Medline, CINAHL, Cochrane Data Base of Systematic Reviews, Pubmed, Scopus, Proquest, Sciencedirect, Wiley Online Library, WanFang Data, Hong Kong Index to Chinese Periodicals, China Journal Net, Chinese Medical Current Contents, Chinese Biomedical Literature Database, China Academic Journals Full-Text database, Taiwan Electronic Periodical Services and HyRead; reference lists of published studies; original research studies published in English or Chinese examining the association between type and duration of brassiere-wearing and breast cancer risk. Data were abstracted by a first reviewer and verified by a second. Study quality was rated according to predefined criteria. “Fair” or “good” quality studies were included. Results were summarised by meta-analysis whenever adequate material was available.
RESULTS: Twelve case-control studies were included in the review. Meta-analysis showed brassiere wearing during sleep was associated with a two times of increased odds.
CONCLUSION: The present review demonstrates insufficient evidence to establish a positive association between the duration and type of brassiere wearing and breast cancer. Further research is essential; specifically, a large-scale epidemiological study of a better design is needed to examine the association between various forms of brassiere exposure in detail and breast cancer risk, with adequate control of confounding variables.
Core tip: This systematic review and meta-analysis aimed to evaluate the association between 8 areas of brassiere-wearing practices and the risk of breast cancer. Twelve case-control studies met inclusion criteria for review. Although the meta-analysis shows statistically significant findings to support the association between brassiere wearing during sleep and breast cancer risk, evidence was insufficient to establish a positive association between brassiere wearing (duration and type) and breast cancer risk. A large-scale epidemiological study is needed to examine the relationship between various forms of brassiere exposure and breast cancer risk.
- Citation: So WK, Chan DN, Lou Y, Choi KC, Chan CW, Shin K, Kwong A, Lee DT. Brassiere wearing and breast cancer risk: A systematic review and meta-analysis. World J Meta-Anal 2015; 3(4): 193-205
- URL: https://www.wjgnet.com/2308-3840/full/v3/i4/193.htm
- DOI: https://dx.doi.org/10.13105/wjma.v3.i4.193
Breast cancer is the most prevalent invasive cancer in women, comprising 22.9% of all such cancers in women worldwide and causing 458503 deaths in 2008. The incidence rate of the disease varies across countries, ranging from 18 to 90 per 100000, with the lowest rate found in developing countries and the highest in the developed world[1]. A number of factors have been examined for their association with the risk of breast cancer: demographic factors (gender and age); heredity and disease history (history of benign breast conditions); reproductive and hormonal factors (menstrual periods and diethylstilbestrol exposure); lifestyle and environmental factors (handedness, smoking and alcohol consumption, physical activity, previous chest radiation, body weight and night work)[2-5].
Among these, lifestyle-related factors are modifiable and thus are the most common areas to be targeted in breast cancer prevention. Apart from those listed above, brassiere wearing seems to be a subtle lifestyle-related factor that constantly arouses discussion among researchers. The brassiere is designed to support and uplift the breasts by utilising the tension of elastic materials, and this has now become a widespread habit where a brassiere is considered as a kind of fashion item with a protective function[6,7]. It is commonly thought that brassieres help to give women a better body shape, and that the underwired type provides better support for the breasts and prevents them from sagging[6,7]. Women usually wear a brassiere during normal daily activity for the purpose of breast support, and it has become an indispensable part of everyday attire. Furthermore, brassiere wearing seems to conform to a social norm whereby women cover their breasts to avoid any embarrassment and to improve their self-confidence[6].
Given the increasing incidence of breast cancer, attention has been paid to investigating this increasingly common habit. Studies have shown that wearing an underwired brassiere, sleeping with a brassiere, wearing one for more than 12 h a day, and incorrect brassiere wearing are potential risk factors[6-14]. However, such studies have failed to control risk factors such as body weight and epidemiological data, thereby diminishing the validity of their results. Findings from a multicentre study, though inconsistent, showed a non-significant association between daily use of a brassiere and breast cancer in premenopausal women[3,8-16].
In view of emerging concerns about breast cancer in relation to brassiere wearing and about the inconsistent results available from previous research, the purpose of this systematic review and meta-analysis is to evaluate the existing evidence for the association between different brassiere-wearing practices and breast cancer risk, to provide a clearer picture of the evidence and in that way to inform breast cancer prevention planning.
Two investigators made a comprehensive literature search in January 2015 of relevant articles published in English or Chinese, using Ovid Medline (since 1946), Cumulative Index to Nursing and Allied Health Literature (CINAHL; since 1937), Cochrane Data Base of Systematic Reviews (since 1995), PubMed, Scopus (since 1823), Proquest (since 1923), Sciencedirect, Wiley Online Library, WanFang Data (since 1993), Hong Kong Index to Chinese Periodicals, China Journal Net (since 1915), Chinese Medical Current Contents (CMCC; since 1994), Chinese Biomedical Literature Database (since 1980), China Academic Journals Full-Text database (since 1994), Taiwan Electronic Periodical Services and HyRead (since 1974). Relevant keywords and search terms used were “breast cancer, breast neoplasm, breast carcinoma, bra, brassiere, constrictive clothing, underwear, undergarment, risk factor”. A secondary search for studies not identified through databases was conducted by manually reviewing reference lists of the twelve studies finally chosen.
The authors developed inclusion and exclusion criteria for abstracts and articles based on the target population, risk factor and outcome measure. Only original research studies in either English or Chinese were included. The target population (case) consisted of women with breast cancer. Measurements of exposure to brassiere wearing are included in the data collection section. The outcome measure covered risk factors of breast cancer, and is reported in the results section, either in the text or in a table. We included original research studies with the full text available, and excluded abstracts, unpublished studies and articles written in languages other than English or Chinese. When a study met the inclusion criteria but there was insufficient data in the paper itself, the corresponding author was contacted for further information.
A reviewer abstracted data from the studies identified and transferred it into a structured form, which included the following information: year and country of study, study design, sample size, participant characteristics (including age, sources and residential status), diagnostic method, information on brassiere exposure, adjusted covariates, outcome results and study quality. A second reviewer confirmed the accuracy of the data.
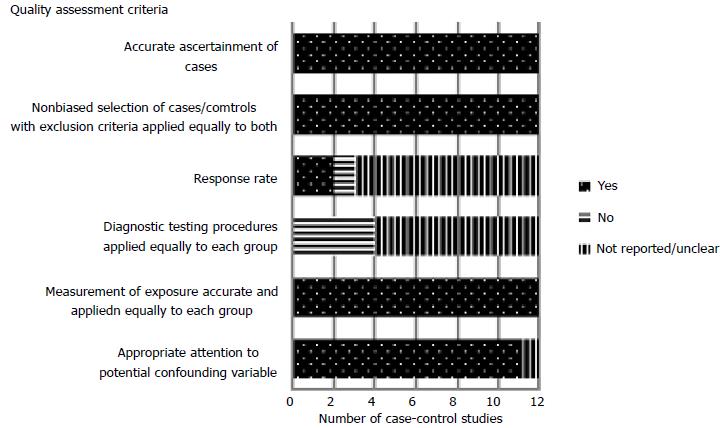
Two reviewers assessed the quality of the studies according to predefined criteria developed by the US Preventive Services Task Force[17], which specifically grades the internal validity of case-control studies. Six aspects were evaluated: accurate ascertainment of cases, non-biased selection of cases/controls with exclusion criteria applied equally to both, response rate, diagnostic testing procedures applied equally to each group, accurate measurement of exposure applied equally to each group, and appropriate attention to potential confounding variable A “good” study meets all these criteria, while one of “fair” quality does not meet all criteria but is without a fatal flaw invalidating its results. Two reviewers independently rated the quality of each study as “good”, “fair” or “poor” (Table 1) and resolved discrepancies by consensus in the presence of a third reviewer. Only studies of fair or good quality were included in the review.
| Criteria |
| Accurate ascertainment of cases |
| Nonbiased selection of cases/controls with exclusion criteria applied equally to both |
| Response rate |
| Diagnostic testing procedures applied equally to each group |
| Measurement of exposure accurate and applied equally to each group |
| Appropriate attention to potential confounding variable |
| Definition of ratings based on above criteria |
| Good: Appropriate ascertainment of cases and nonbiased selection of case and control participants; exclusion criteria applied equally to cases and controls; response rate equal to or greater than 80%; diagnostic procedures and measurements accurate and applied equally to cases and controls; and appropriate attention to confounding variables |
| Fair: Recent, relevant, without major apparent selection or diagnostic work-up bias but with response rate less than 80% or attention to some but not all important confounding variables |
| Poor: Major selection or diagnostic work-up biases, response rates less than 50%, or inattention to confounding variables |
Results of the studies were synthesised in a narrative way in an evidence table corresponding to the topic of the review. We assessed the heterogeneity of the studies qualitatively by their study designs, participant characteristics, data collection and analytic methods. Specific quantitative results were synthesised narratively. To define the suitability of the risk factors to be included in the meta-analysis, the homogeneity of study method, participant background and statistical methods were considered. Specifically, meta-analysis was conducted for the effect on the risk of breast cancer entailed by wearing a brassiere during sleep. This was the only factor assessed in seven studies, with the results shown in six of them. Another factor, tightness of the brassiere was assessed in three studies, but not considered further, as multivariable adjusted analysis produced insignificant results. The combined estimate of risk effects, together with its 95%CI, was calculated by using the estimates in individual studies, producing the best control over other potential risk factors and confounders. In particular, the odds ratio of each study, with as many other risk factors and/or confounders as possible adjusted, was chosen for meta-analysis. A total of six studies were judged appropriate for analysis, with five having adjusted odds ratios. Heterogeneity among the studies was assessed by Cochrane Q-test and I2 statistics[18]. As neither the Q test (P < 0.05) nor I2 > 40% were significant[18], the fixed-effects (weighted inverse variance) method was applied to the meta-analysis. Review Manager (RevMan5.3, Cochrane Collaboration, Oxford, England) was used for the meta-analysis.
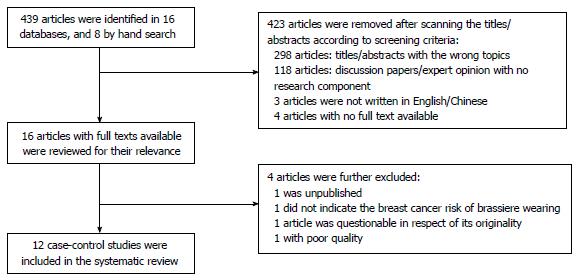
A total of 439 studies were identified through 16 databases. Of these, 423 were removed, after scanning of abstracts and titles, because they were concerned with an irrelevant topic, discussion paper or expert opinion, or were not written in English or Chinese, or the full text was not accessible (Figure 1). This left sixteen articles with full texts, which were further reviewed for relevance. After a detailed examination, four further articles were excluded, one of which, with the same statistical results as another article published in 2006[9], raised concerns about its originality. The twelve remaining studies that met all inclusion criteria were further assessed for quality. Two studies of good and ten of fair quality examining brassiere exposure and breast cancer risk were finally included in the systematic review and meta-analysis. Characteristics, results and quality assessment of the twelve studies are illustrated in Tables 2 and 3 and Figure 2, respectively.
| Ref. | Design | Country | No. of participants | Source of participants | Age of participants | Years of residence of participants | Residential status of participants | Age-match between participants | Ratio of cases to controls | Diagnosis of breast cancer status | ||||
| Cases | Controls | Cases | Controls | Cases | Controls | Casescontc | Controls | |||||||
| Hsieh et al[3] | Case-control | Greece, United States, Wales, Brazil, Yugoslavia, Taiwan, Japan | 2325 | 7008 | Hospital | Same hospital as cases | Over 35 years old | Over 35 years old | Not reported | Yes | ± 2 yr | 1:3 | Diagnosed in hospital | Not reported |
| Feng et al[20] | Case-control | China | 262 | 262 | Hospital | Same hospital as cases, relative/neighbor of index case | Not reported | Not reported | Not reported | Yes | ± 5 yr | 1:1 | Diagnosed in hospital | Not reported |
| Lee et al[10] | Case-control | Taiwan | 250 | 219 | Out-patient clinic or cancer center | Same as cases | Mean: 47.2; | Mean: 46.4 | Not reported | Yes | ± 5 yr | 1:1 | Pathological examination | Mammogram |
| Zhu et al[9] | Case-control | China | 246 | 246 | Hospital | Same hospital as cases; relative/neighbor of index case | Mean: 50.4 | Mean: 50.0 | Not reported | Not reported | ± 5 yr | 1:1 | Diagnosed in hospital | Not reported |
| Zhang et al[11] | Case-control | China | 284 | 669 | Hospital | Health check program | Over 35 years old | Over 35 years old | Not reported | Yes | Not reported | 1:3 | Pathological examination | Not reported |
| Chen[21] | Case-control | China | 167 | 334 | Hospital | Neighbor | Not reported | Not reported | Over 10 years | Yes | ± 2 yr | 1:2 | Diagnosed in hospital | Not reported |
| Hu and Lin[16] | Case-control | China | 95 | 95 | Hospital | Same hospital as cases | Median: 48 | Median: 48 | Over 10 years | Yes | Not reported | 1:1 | Pathological examination | Not reported |
| Liu et al[15] | Case-control | China | 365 | 365 | Not reported | Randomly obtained from population | Mean: 46.4 | Mean: 45.6 | Over 10 years | Yes | ± 3 yr | 1:1 | Pathological examination | Not reported |
| Yao et al[13] | Case-control | China | 200 | 200 | Hospital | Same hospital | Mean: 47.30 | Mean: 45.88 | Over 10 years | Yes | ± 2 yr | 1:1 | Pathological examination | Not reported |
| Chen et al[19] | Case-control | United States | Ducta: 454 Lobular: 590 | 469 | Cancer Surveillance System | Randomly obtained from population | Between 55-74 | Between 55-74 | Not reported | Yes | ± 5 yr | 1:1:1 | Pathological examination and tumor tissue specimens | Not reported |
| Liu et al[14] | Case-control | China | 208 | 416 | Hospital | Coworker/neighbor | Mean: 50.1 | Mean: 49.2 | Over 5 years | Yes | ± 5 yr | 1:2 | Pathological examination | Not reported |
| Shen et al[12] | Case-control | China | 275 | 275 | Hospital | Not reported | Mean: 45.6 | Mean: 48.5 | Not reported | Yes | Not reported | 1:1 | Pathological examination | Physical examination |
| Ref. | Study years | Area of brassiere exposure | Effect size: OR (95%CI) | Adjusted covariates | Study quality | ||||||||
| Duration of Brassiere wearing per day | Brassiere users | Sleeping with brassiere | Tightness of brassiere wearing | Wear underwired brassiere | Age began brassiere wearing | Brassiere cup size 1 yr before reference data | Appropriateness of brassiere wearing | Modifiable | Non-modifiable | ||||
| Hsieh et al[3] | Not reported | Yes | (Premenopausal) non-brassiere-users vs brassiere-users: 0.44 (0.17-1.15) | Age at first birth Parity Obesity | Age at interview Study center | Fair | |||||||
| Feng et al[20] | 1 yr | Yes | Yes | Sleep without brassiere vs with brassiere: 0.26 (0.09-0.77) Tightness of brassiere wearing: Not significant | Occupational contact of chemicals Emotional adjustment Psychological distress History of abortion Diet (high fat, rich in beans, take breakfast, drink tea) Night work Personality | History of benign breast disease Family history of breast cancer Menarche history | Fair | ||||||
| Lee et al[10] | 3 yr | Yes | Yes | Wear brassiere (≥ 12 h/d) vs (< 12 h/d) (> age 40): 3.0 (1.6-5.7) Wear brassiere (≥ 13 h/d) vs ( ≤ 13 h/d) (all ages): 2.1 (1.3-3.3) Sleep with brassiere: Not significant | Physical activity Education High fat food Supplement and allium use Total calories | Age | Good | ||||||
| Zhu et al[9] | 1 yr | Yes | Yes | Sleep with brassiere vs without brassiere: 2.32 (1.32-4.10) Tightness of brassiere wearing: Not significant | Parity Diet (high fat, rich in beans, drink tea) Psychological distress Personality Occupational contact of chemicals | Menarche history Family history of breast cancer History of benign breast disease | Fair | ||||||
| Zhang et al[11] | 10 mo | Yes | (Premenopausal) sleep without brassiere vs with brassiere: 0.401 (0.250-0.644) Post-menopausal: not significant | Oral contraceptive use Physical activity Education Emotional problem/adjustment | Family history of breast cancer History of benign breast disease or breast biopsy Breast pain during menstruation | Fair | |||||||
| Chen[21] | 2 yr | Yes | Yes | Both are not significant | Body mass index Lactation Oral contraceptive use Education Psychological distress Smoking history Night work Diet Occupational contact of chemicals or radiation | Age Family history of breast cancer History of benign breast disease Age at menarche and menopause | Fair | ||||||
| Hu and Lin[16] | 3 yr | Yes | Wear brassiere with an underwire (Yes vs No): 6.729 (2.001-22.635) | Lactation Oral contraceptive use Sexual life Passive smoking Psychological distress Diet (fried and oily food) Sleeping hours History of abortion | History of benign breast disease, cervical disease, ovarian cancer, hepatitis - | Fair | |||||||
| Liu et al[15] | 3 yr | Yes | Sleep with brassiere vs without brassiere: 2.313 (1.323-4.121) | Lactation Parity History of abortion Age at first birth Occupational contact of chemicals Passive smoking Diet (high fat, spicy/salty food, preserved food, seafood, drink water) Psychosocial problem (trauma, family problem, distress, anger) | Age at menarche Family history of breast cancer - | Fair | |||||||
| Yao et al[13] | 15 mo | Yes | Yes | Sleep with brassiere vs without brassiere: 1.902 (1.177-3.072) Wear brassiere with an underwire (Yes vs No): Not significant | Large scale renovation Non-environmental friendly decoration materials Interval between renovations Nature of occupation Lactation Number of birth labour Job-related life events Fruit intake High fat intake Salted food intake Workplaces condition Sleeping hours and quality - | Family history of other tumors Family history of breast cancer History of cancer in first degree relatives Mammary hyperplasia The death of a loved one | Fair | ||||||
| Chen et al[19] | 4 yr 3 mo | Yes | Yes | Yes | Yes | All are not significant | Education Annual household income BMI Bra band size Age at first full-term pregnancy Mammogram screening Parity Use of hormonal therapy | Race/ethnicity Family history of breast cancer Types of menopause | Good | ||||
| Liu et al[14] | 2 yr 7 mo | Yes | Wear brassiere (≥ 12 h/d) vs (< 12 h/d): 1.064 (1.001-1.132) | Income Occupation Oral contraceptive use Passive smoking Use of royal jelly Use of other supplement Work-related stress Personality Emotional adjustment Interpersonal relationship Contact of chemicals Psychological distress Adverse life events Sleeping hours | History of benign breast disease | Fair | |||||||
| Shen et al[12] | 4 yr | Yes | Appropriately wearing vs inappropriately wearing: 2.313 (1.112-5.43) | History of abortion Lactation Work intensity Long working hours Poor sleep quality Family discord Satisfaction towards life Fatigue strength Smoking | Family history of other tumors Family history of breast cancer Mammary hyperplasia | Fair | |||||||
All twelve studies were published between 1991 and 2014, and all were of a case-control design, where people with breast cancer (case participants) were compared with others free of the disease (control participants), with respect to the risk of breast cancer from wearing a brassiere (risk factor). One multicentre study was conducted across Europe, Asia and North and South America[3], one in the metropolitan areas in the United States[19] and ten in Asia[9-16,20,21]. The ratio of case to control was 1:1 (n = 8)[9,10,12,13,15,16,19,20], 1:2 (n = 2)[14,21] or 1:3 (n = 2)[3,11]. The number of participants varied across studies, from 190 to 9333. Seven reported the mean age of participants, varying from 45.6 to 50.4[9,10,12-16]. Two studies only stated that they included participants of 35 or more[3,11], one reported participants aged between 55-74[19], while two did not report the age[20,21]. The control participants were age-matched with the case participants within 2-5 years. Two studies reported the response rate (83%-87%)[10,19]. Data on participants’ demographic data and information on brassiere exposure were collected by means of either questionnaire or interview. Eleven studies indicated that their participants (both case and control) were residents in the study area[3,10-16,19-21], one stated over 5 years of residence[14], and four further stated that participants had lived in the area for more than 10 years[13,15,16,21]. Nearly all case participants were recruited from hospital, outpatient clinic or cancer centre, with control participants recruited from the same hospital[3,9,13,16,20], outpatient clinic or cancer centre[10], health-check programme[11], among neighbours/relatives of the case participants[9,14,20,21], randomly obtained from the local population[15,19], or from the same residential area[12]. All case participants had their cancer diagnosis confirmed in hospital and eight studies specifically reported that the diagnosis was confirmed by pathological examination[10-16,19]. Nevertheless, eleven studies did not clearly state what diagnostic tests had been carried out on participants in the control group[3,9,11-16,19-21], reporting only that they were found to be healthy and were not currently suffering from breast cancer. One study had conducted mammography screening[10] and one had underwent physical examination[12] to exclude the presence of breast cancer among control participants.
Eight aspects of brassiere exposure have been examined previously and included in the present review: brassiere users and non-users[3], duration of brassiere wearing per day[10,14,19], sleeping with or without a brassiere[9-11,13,15,20,21], tightness of the brassiere[9,20,21], wearing an underwired brassiere[13,16,19], age began brassiere wearing[19], brassiere cup size 1 year before reference data[19], and appropriateness of brassiere wearing[12].
Univariate and multivariable logistic regression analysis of the data was used to explore 8 areas of brassiere wearing practice and breast cancer risk. All studies used multivariable logistic regression analysis, with the results shown in the Results section, in the text and/or in table form. Of the twelve studies, nine presented the univariate results in tabular form[9-16,19]. Two reported that they had derived significant results from the univariate analysis. However, no detailed figures were given in either text or tabular form[20,21]. Ten studies reported that significant variables detected in the univariate analysis were further included in the multivariable logistic regression model[9-16,20,21].
To identify any significant relationship between brassiere wearing and breast cancer risk, all the studies paid appropriate attention to the covariates which also provided reliable and comparable data considered as confounding variables during statistical analysis, although not all known non-modifiable and modifiable factors were included.
Eight known non-modifiable risk factors were considered in the analysis of the twelve studies. They were age[3,10,21], history of benign breast disease[9,11,14,16,20,21], family history of breast cancer[9,11-13,15,19-21], family history of other tumors[12,13], age at menarche and menarche history[9,15,20,21], age at menopause[21], mammary hyperplasia[12,13], death of a loved one[13]. Factors that had an uncertain effect on breast cancer risk were also reported, such as breast pain during menstruation[11]. One study considered the study centre to be one of the covariates, since it was conducted in seven countries[3]. Chen et al[19] also evaluated race/ethnicity and types of menopause as covariates as potential confounders.
Several known modifiable risk factors were considered in the analysis. These factors are grouped under 5 broad categories, namely lifestyle-related, reproductive, nutritional, psychological and emotional, and others modifiable factors with uncertain effects. Lifestyle-related factors such as obesity[3], body mass index[19,21], physical activity[10,11], oral contraceptive use[11,14,16,21] and lactation[12,13,15,16,21], and reproductive factors such as parity[3,9,15,19] and age at first birth[3,15,19]. Additionally, dietary habits, being uncertain factors of breast cancer risk, were commonly referred to in the studies, including diets high in fat[9,10,13,15,20], beans[9,20], dried or oily food[16], spicy, salty, preserved seafood or other foods[13,15], breakfast[20], tea or water[9,15,20], supplement and allium use[10,14], and total calorie intake[10]. Psychological and emotional factors such as emotional adjustment[11,14,20], psychological distress[9,14-16,20,21], and psychological problems (trauma, family problems, anger, fatigue, and stress)[12,14,15] constituted another uncertain category commonly found in the studies. Some factors with uncertain effects on breast cancer risk were also considered, such as occupational contact with chemicals or radiation[9,14,15,20,21], night work[20,21], personality[9,14,20], smoking history or passive smoking exposure[12,14-16,21], sex life[16], sleeping hours[13,14,16], education[10,11,19,21], and any history of abortion[12,15,16,20].
Univariate analyses were conducted to identify candidate variables for further multivariable logistic regression analysis. Among eight areas of brassiere exposures that has been studied, brassiere wearing more than twelve hours[10,14], sleeping with a brassiere on[9-11,13,15,20,21], tightness of the brassiere[9,20,21], wearing an underwired type[13,16], and incorrect brassiere wearing[12] were found to have a significant association with breast cancer. Two studies reported in their Results sections that sleeping with a brassiere on and tightness of the brassiere were significantly associated with the occurrence of breast cancer, but no data or figures were provided either in the text or in tabular form[20,21].
After univariate analyses, multivariable analysis was carried out to investigate further brassiere exposure and the risk of breast cancer. One study found that pre-menopausal women who were not brassiere users were less than half as likely to contract the disease as those who did wear one (OR = 0.44; 95%CI: 0.17-1.15)[3]. Hu and Lin[16] studied the effect of wearing an underwired brassiere on the incidence of breast cancer, and claimed that women who wore that type were 6.7 times more likely to have breast cancer than those who did not (OR = 6.7; 95%CI: 2.0-22.6)[16]. However, insignificant association was found in other 2 studies[13,19].
Inconsistent results were noted in the case of brassiere wearing during sleep. Two studies found no significant difference in the breast cancer risk between sleeping with or without a brassiere on[10,21]. However, five others found that sleeping with a brassiere on was a significant risk factor[9,11,13,15,20], interpreting the results in different ways. Feng et al[20] reported that sleeping without a brassiere was much less risky than sleeping with one (OR= 0.26; 95%CI: 0.09-0.77)[20]. Zhang et al[11] investigated this by classifying the participants into pre- and post-menopausal groups. They found that the former, sleeping without a brassiere, were less than half as likely to contract breast cancer than those who did wear one (OR = 0.40; 95%CI: 0.25-0.64)[11]. Three studies showed that women who slept with a brassiere on were 1.9-2.3 times more likely to have breast cancer than those who did not[9,13,15]. In three studies, the tightness of the brassiere was found to have an association but was not a significant risk factor[9,20,21].
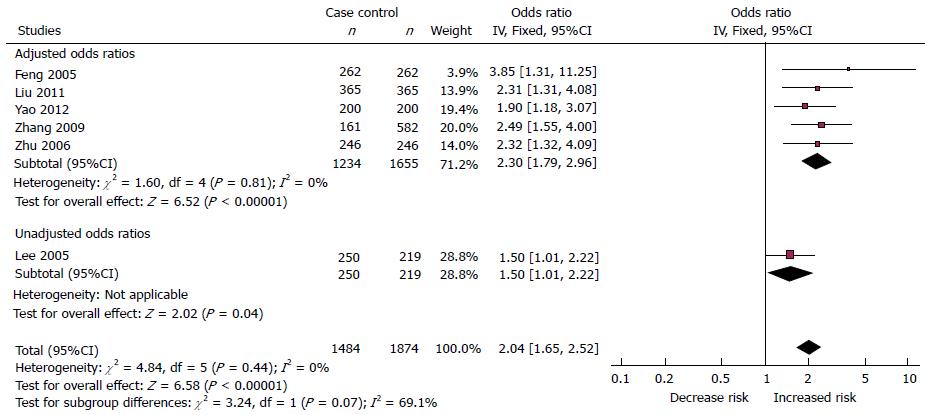
The results between duration of daily brassiere use and breast cancer risk are in disagreement. Chen et al[19] found insignificant association between duration of brassiere wearing per day and the risk the breast cancer, but Liu et al[14] and Lee et al[10] reported more than 12 h or daily brassiere wear increased the risk of breast cancer. Meta-analysis was conducted to evaluate the findings on brassiere wearing during sleep. Although a total of seven studies reported findings related to brassiere wearing during sleep[9-11,13,15,20,21], only six provided numerical results[9-11,13,15,20], and only data from these six are therefore included in the meta-analysis (Figure 3). The total case-to-control ratio was 1607:1961. The meta-analysis of the five studies with adjusted odds ratios and the other with an unadjusted odds ratio did not show significant heterogeneity. The fixed-effects method was applied to the meta-analysis, showing a significant association between brassiere wearing during sleep and breast cancer (pooled OR=2.04; 95% CI: 1.65-2.52).
According to the American Cancer Society, a number of modifiable and non-modifiable factors that may contribute to the incidence of breast cancer have been identified[22]. However, there are still many uncertain factors awaiting clarification and confirmation in respect of breast cancer risk. The growing popularity of brassiere wearing and public awareness of breast cancer prevention have led researchers to investigate the possible association between brassiere wearing and breast cancer risk. An initial examination of 439 published studies investigating that association was conducted, and as a result a total of twelve “fair” to “good” quality case-control studies involving over 10000 participants were included in the present systematic review.
This review shows that 8 areas of brassiere exposure[12,13,19,20] have been studies in relation to breast cancer risk. There are inconsistent results in respect of the association between brassiere wearing during sleep, duration of daily brassiere use and breast cancer. Meta-analyses, when used to evaluate these associations further, show a positive relation between brassiere wearing during sleep and breast cancer with an OR of 2.04 (95% CI 1.65-2.52). These may be simply the result of prolonged wearing of a brassiere - a finding consistent with what Singer and Grismaijer concluded in 1995[8]. The assumption of both Singer and Grismaijer[8] and Kumar[23] was that brassiere wearing might inhibit the temperature regulatory system of the breast and engender breast neoplasm. However, the idea of changes in surface temperature over the breast area is challenged by King[24], who suggests that the temperature may correlate with the amount of clothing worn, but it is hard to determine how much breast glandular tissue has been exposed to abnormal temperature as the result of clothing. To be more accurate, the temperature should be measured at the breast tissue itself rather than the surface[24].
In addition to the duration of brassiere wearing, one study included in this review also showed that wearing an underwired brassiere had a 6.7 times increased risk of breast cancer[16] although 2 studies reported no association between the two[13,19]. This raises further consideration of the influence of different brassiere designs. Underwired types have thin rigid material sewn into the underside of the cup of the bra. The material may be metal, plastic or resin. Compared to other types, underwired brassieres provide better lifting and shaping effects and are thus preferred by many women. However, the underwired type is more uncomfortable in comparison with its wire-free counterpart, as it puts added pressure on the breasts, potentially clogging the milk duct and lymphatic system. Obstructing the latter over the breast area may cause fluid, toxin and carcinogen to accumulate, and cause breast cancer as a result[8,23]. Nevertheless, various studies have opposed the suggestion of a blockage in the lymphatic drainage system, on the grounds that lymphatic fluid drains upward to the armpit but not down to the bottom of brassiere, which is considered to be the constricted region, and in fact it has been shown that lymphatic drainage is not affected even if a tight-fitting or underwired brassiere is worn[25-28]. This may be confirmed by the results of three other studies included in this review, which found a non-significant association between the tightness of the brassiere and breast cancer risk[9,20,21]. Additionally, the one study that gives a certain risk factor connected with underwired brassieres does not specifically report any history of using such types, which generates further concern about its results. Interestingly, an investigation into awareness and knowledge of breast cancer risk among Malaysian women revealed that more than a third of participants linked the wearing of underwire brassieres with breast cancer[29], indicating that women paid attention to brassiere wearing in the prevention of breast cancer even without robust evidence of its risks.
Apart from the association between the kind of brassiere, the duration of wearing one and breast cancer, Hsieh and Trichopoulos’s study reported an association between brassiere use and breast cancer among pre-menopausal women (OR = 0.44; 95%CI: 0.17-1.15; P about 0.09)[3]. However, the authors went on to speculate that this might have been related to obesity, as the brassiere users in the study were substantially heavier than non-users.
Though not conclusive, findings in this review raise concerns about brassiere wearing in general - not only about the duration, but also the nature of the garment itself (e.g., construction method and materials) and how and when it is worn. Brassiere-wearing practice is undoubtedly a serious issue whose relation to breast cancer risk needs to be explored. However, the evidence accumulated in previous studies, as discussed in the current review, is not strong enough to draw reliable conclusions. To explore in greater depth the effects of brassiere wearing on the risk of breast cancer, further research is recommended to examine the physiological responses of the breast to brassiere exposure in association with cancer risks, with proper adjustment for confounding variables during the analysis.
There are several limitations to the present review that may affect the validity and generalisability of its findings. The eight studies included were all of the case-control design. Although such design is commonly used to identify risk factors contributing to a medical condition, its potential bias may influence the reliability of results. This includes selection, information and confounding biases.
First, there was selection bias in respect of the source of participants. Of the current twelve studies, two are the population-based case-control type[15,19], another seven recruited case and control participants from the same hospitals[3,9-11,13,16,20], two matched control participants from the neighbourhood[14,21], and the remaining one with unclear source of sample[12]. This could lead to admission rate bias. The control participants from hospital are not as representative as those from the community. For example, people in areas remote from hospital and people unable to afford medical care may not be included in the study. Furthermore, only two studies in this review recruited incident cases[3,9], while the others might have prevalent-incidence bias, whereby breast cancer patients reduce the use of brassiere after diagnosis.
Second, information bias might dilute the results of the review. Case-control studies usually suffer from measurement bias due to participants’ failure to recall past events[30]. Only two studies in this review reported the response rate[10,19], and this limited our ability to evaluate the bias caused by response rates in different studies. Additionally, of the twelve studies, only two indicated the use of mammography or physical examination and pathological examination to confirm breast cancer status in both case and control groups[10,12]. It would certainly be difficult and might be unethical to ask control participants to undergo pathological examination or mammography, but misclassification bias might otherwise be introduced, as it is possible that some control participants are potential breast cancer patients. Furthermore, the search for studies in this review was limited to those written in English or Chinese, and those in other languages were not considered, potentially limiting the number included and thus affecting the generalisability of the findings to different populations. It was also possible that certain related studies could not be identified, despite the use of multiple databases.
Third, confounding bias might influence the association detected between brassiere-wearing practices and breast cancer risk. Studies varied in the degree to which confounding variables were adjusted during analysis, and none was able to control all known non-modifiable and modifiable factors, leading to potential bias in the estimates of odds ratios. Obesity was identified as a confounding factor that related to the use of a brassiere and the occurrence of breast cancer. However, only three studies controlled the factor of obesity or BMI during the analysis stage[3,19,21]. Also, the use of a brassiere and a preference for its particular design are related to socio-economic status, which itself is associated with breast cancer risk. Three studies in the review adjusted for the education factor[11,19,21], and eight considered the effect of diet[9,10,13-16,20,21]. Further studies should take annual income into account to eliminate the confounding influence of socio-economic status.
In conclusion, the present review demonstrates insufficient evidence to establish a positive association between brassiere wearing (duration and type) and breast cancer risk, although the meta-analysis shows statistically significant findings which support the association between brassiere wearing during sleep and breast cancer risk. Twelve studies reviewed suffered from selection bias, information bias or improper adjustment of confounding variables, which all affect the validity and generalisability of the findings to different populations. Further research is essential - specifically, a large-scale epidemiological study of a better design is needed to examine the association between various forms of brassiere exposure in detail and breast cancer risk, with adequate control of confounding variables. In this way, women may be granted the informed view of brassiere usage that they need and deserve. Since wearing brassieres has already become common among women in developed countries, education on the proper use of such garments can help maintain better breast health.
Given an increasing incidence of breast cancer, attention has been paid to investigating common modifiable lifestyle-related factors including brassiere wearing practice for breast cancer prevention. However, inconsistent results have been reported in the association between use of a brassiere and risk of breast cancer.
The authors performed the first systematic review and meta-analysis to investigate the association between different type of brassiere exposures and the risk of breast cancer.
Eight aspects of brassiere exposure have been examined by 12 Case-control studies were included in the present review: brassiere users and non-users, duration of brassiere wearing per day, sleeping with or without a brassiere, tightness of the brassiere, wearing an underwired brassiere, age began brassiere wearing, brassiere cup size one year before reference data, and appropriateness of brassiere wearing. Meta-analysis was conducted to evaluate the findings on brassiere wearing during sleep. The results showed that brassiere wearing during sleep was associated with a two times of increased odds.
The meta-analysis shows statistically significant findings to support the association between brassiere wearing during sleep and breast cancer risk, however, all the twelve studies reviewed suffered from selection bias, information bias or improper adjustment of confounding variables, which all affect the validity and generalisability of the findings to different populations. A large-scale epidemiological study is needed to examine the relationship between various forms of brassiere exposure and breast cancer risk, with adequate control of confounding variables.
Brassiere is designed to support and uplift the breasts by utilising the tension of elastic materials, and this has now become a widespread habit where a brassiere is considered as a kind of fashion item with a protective function.
This paper addresses the breast cancer risk associated with wearing brassiere. The paper is very well written and well structured. The article also has the potential to add to what the we know about risk factors of breast cancer.
P- Reviewer: Khajehei M, Yao HR S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | International Agency for Research on Center. World Cancer Report. World Health Organisation. 2008; Available from: http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/wcr_2008.pdf. |
| 2. | Chen X, Kong Z. Chinese Cancer Registry Annual Report 2009. Beijing: Military Medical Science Press 2010; . |
| 3. | Hsieh CC, Trichopoulos D. Breast size, handedness and breast cancer risk. Eur J Cancer. 1991;27:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | American Cancer Society. Breast cancer overview, 2012. Available from: http://www.cancer.org/cancer/breastcancer/overviewguide/index. |
| 5. | Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2005;41:2023-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 274] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Risius D, Thelwell R, Wagstaff C, Scurr J. Influential factors of bra purchasing in older women. J Fash Market Manag. 2012;16:366-380. [DOI] [Full Text] |
| 7. | Liao CS, Lee CW. The application of codesign in new bra product innovations. Int J Cloth Sci Technol. 2010;22:211-227. [DOI] [Full Text] |
| 8. | Singer S, Grismaijer S. Dressed to kill: The link between breast cancer and bras. New York: Avery Publishing Group 1995; . |
| 9. | Zhu LH, Li SF. A case-control study on risk factors of female breast cancer in Zhengzhou City. J Chin Prim Med Pharm. 2006;13:679-680. |
| 10. | Lee MM, Chang IY, Horng CF, Chang JS, Cheng SH, Huang A. Breast cancer and dietary factors in Taiwanese women. Cancer Causes Control. 2005;16:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Zhang AQ, Xia JH, Wang Q, Li WP, Xu J, Chen ZY, Yang JM. Risk factors of breast cancer in women in Guangdong and the countermeasures. Nanfang Yike Daxue Xuebao. 2009;29:1451-1453. [PubMed] |
| 12. | Shen M, Gu JF, Dong JY. Risk factors of breast cancer incidence and nursing strategies. Chin J Gen Pract. 2014;12:782-785. |
| 13. | Yao XY, Ni SS, Zhou J, Hu HY, Li LL, Wan F, Wang YK, Chen YD. [A case-control study on risk factors of female breast cancer in Zhejiang province]. Zhejiang Daxue Xuebao Yixueban. 2012;41:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Liu L, Ding H, Jia ZX, Qiu LP, Tao MF, Yan M. A case-control on risk factors of female breast cancer in Beijing. Matern Child Health Care China. 2014;21:3407-3408 (in Chinese). [DOI] [Full Text] |
| 15. | Liu SP, Qiu YX, Li YL. A study on risk factors of breast cancer in the Zhoushan island. J Hebei Med Univ. 2011;32:1336-1338. |
| 16. | Hu PX, Lin FC. A case-control study on risk factors of female breast cancer in the city of Shenzhen: A report of 95 cases. New Med. 2011;42:291-294. |
| 17. | Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, Atkins D. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20:21-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1142] [Article Influence: 47.6] [Reference Citation Analysis (1)] |
| 18. | Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. New York: Avery Publishing Group 2011; Available from: http://handbook.cochrane.org/. |
| 19. | Chen L, Malone KE, Li CI. Bra wearing not associated with breast cancer risk: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2014;23:2181-2185. [PubMed] |
| 20. | Feng Y, Wu JG, Shi LY. A case-control study with multivariate analysis for 262 cases of female breast cancer. Chin J Epidemiol. 2005;26:925. |
| 21. | Chen HM. A case-control study on risk factors of breast cancer for women in Shantou in recent years. Med Inf. 2011;24:1607-1608. |
| 22. | American Cancer Society. Breast cancer, 2012. Available from: www.cancer.org/acs/groups/cid/documents/webcontent/003090-pdf.pdf. |
| 23. | Kumar A. Burn the bra! (and men’s tight underpants too): compromised ‘chaotic’ cooling by constrictive clothing in the causation of testicular and breast cancers. Med Hypotheses. 2009;73:1079-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | King CR. Bras, breast carcinoma, and cryptorchid testis. Lancet. 1979;1:45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Swenson KK, Nissen MJ, Leach JW, Post-White J. Case-control study to evaluate predictors of lymphedema after breast cancer surgery. Oncol Nurs Forum. 2009;36:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Al-Dubai SA, Qureshi AM, Saif-Ali R, Ganasegeran K, Alwan MR, Hadi JI. Awareness and knowledge of breast cancer and mammography among a group of Malaysian women in Shah Alam. Asian Pac J Cancer Prev. 2011;12:2531-2538. [PubMed] |
| 30. | Pagano M, Gauvreau K. Principles of Biostatistics. 2nd ed. Pacific Grove, California: Duxbury 2000; . |