Published online Dec 14, 2018. doi: 10.5662/wjm.v8.i4.51
Peer-review started: September 28, 2018
First decision: October 29, 2018
Revised: November 6, 2018
Accepted: November 16, 2018
Article in press: November 16, 2018
Published online: December 14, 2018
Processing time: 77 Days and 0.1 Hours
Radiotherapy has long been used as an adjunct to neurosurgery for the treatment of malignant and benign intracranial tumors and other intracranial lesions. Intracranial tumors can be irradiated in three different ways: I) fractional radiotherapy, II) stereotactic radiotherapy and III) stereotactic radiosurgery. The third is most often by means of a gamma knife or a specially designed linear accelerator. Additionally, radiosurgery is increasingly used in combination with systemic therapy to treat metastases.
Core tip: Intracranial tumors can be irradiated in three different ways: with fractional radiotherapy, stereotactic radiotherapy, and stereotactic radiosurgery. Additionally, radiosurgery is increasingly used in combination with systemic therapy to treat metastases.
- Citation: Velnar T, Bosnjak R. Radiosurgical techniques for the treatment of brain neoplasms: A short review. World J Methodol 2018; 8(4): 51-58
- URL: https://www.wjgnet.com/2222-0682/full/v8/i4/51.htm
- DOI: https://dx.doi.org/10.5662/wjm.v8.i4.51
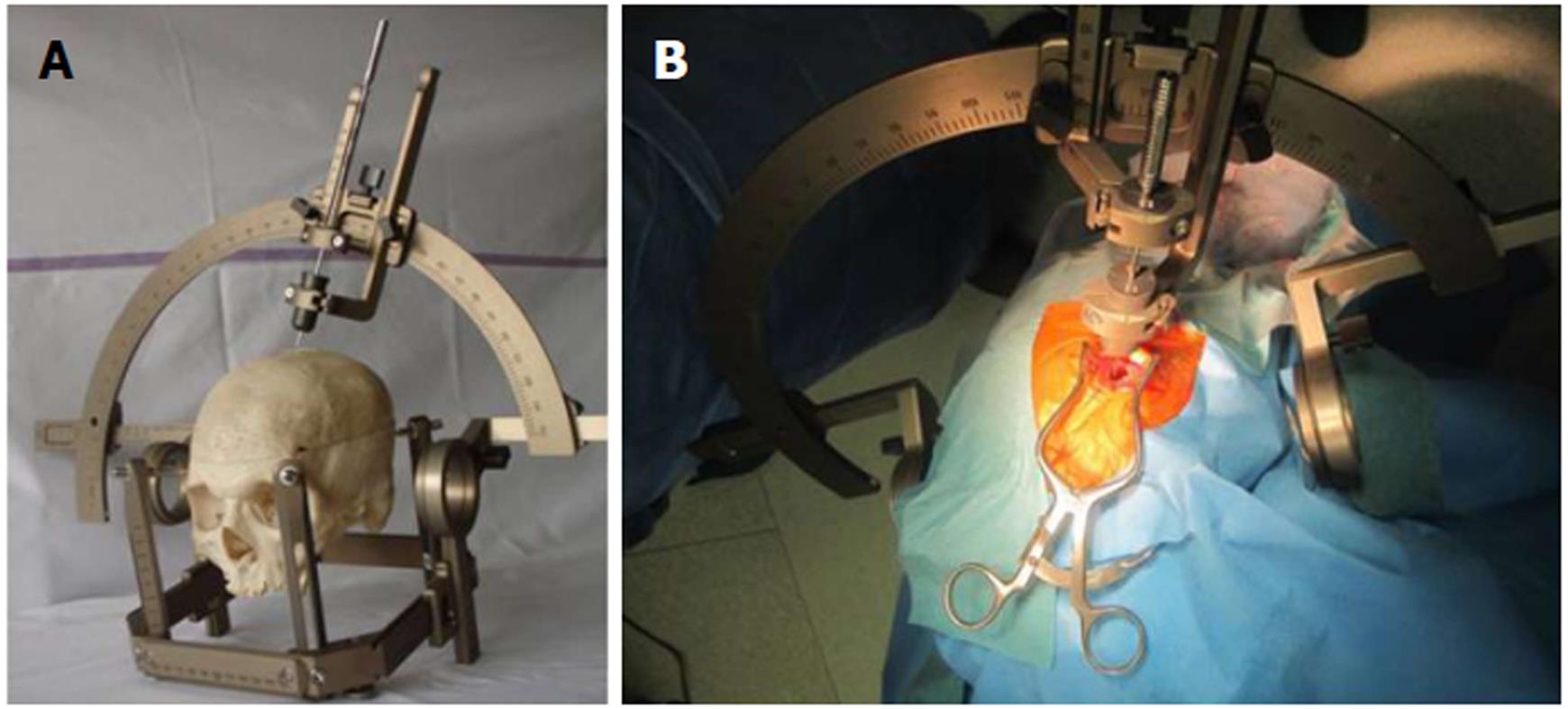
Radiotherapy has long been used to treat malignant and benign intracranial neoplasms. Its use permits greater control of tumor growth and leads to increased patient survival[1-3]. Recently, a number of new neurosurgical therapies have been developed, such as stereotactic surgery for deep-seated lesions, neuronavigation, minimally invasive endoport surgery, and navigated endoscopic surgery of small parenchymal lesions[2,4-6]. These allow a more effective and targeted treatment of lesions (removal or biopsy) whilst causing less damage to the surrounding tissue (Figure 1). In modern neuro-oncological radiotherapy, the same working principle was transferred from stereotactic neurosurgery into radiotherapy, with the aim being the treatment of surgically inaccessible lesions with a beam from a radioactive source or with the aid of electron acceleration[2]. A variety of radiosurgical techniques have recently emerged.
At the start of the 20th century, neurosurgeon Harvey Cushing carried out over 2000 brain tumor operations; amongst these, there were 800 glioma procedures. During some operations, he placed radioactive radium needles or wires, which he called radium “bombs”. These were designed to irradiate the tumor and hence improve patient survival[7]. The treatment, known as brachytherapy, is a form of radiotherapy where a radioactive source is placed directly into a tumor by means of a neurosurgical operation[8].
In some centers, this technique is still being used for treating brain metastases and primary brain tumors such as glioblastomas, oligodendrogliomas, astrocytomas, meningiomas, and craniopharyngiomas[9-12]. Although the results initially seemed positive, the use of brachytherapy did not ultimately increase survival times. The most harmful complication reported in connection to brachytherapy was the radiation-induced late tissue necrosis[8-13].
With the development of radiotherapy and surgical techniques, the tendency has been to use radiotherapy either as a primary or a secondary method to treating brain tumors. For the successful treatment of these tumors, a sufficient dose of ionizing radiation, which is aimed at the target volume, ensures the tumor tissue is destroyed, while conserving healthy tissue. This method of radiotherapy is possible with high-dose targeted radiation[14]. Three methods exist for the irradiation of intracranial tumors: (1) fractional radiotherapy, (2) stereotactic radiotherapy, and (3) stereotactic radiosurgery.
In fractional radiotherapy, many fractions of radiation are given to treat a lesion. Stereotaxy is the precise three-dimensional localization of a target within a space. In stereotactic radiotherapy, the radiation beams are stereotactically (very precisely) targeted onto a tumor in a single dose or fractionally, relying on detailed imaging and computerized three-dimensional treatment planning. Stereotactic radiosurgery is a highly accurate type of radiation therapy, which uses high-powered X-rays that are stereotactically focused onto a lesion. In contrast to other forms of radiation therapy, which are more likely to affect the nearby healthy tissue, stereotactic radiosurgery more specifically targets the abnormal area. Stereotactic radiosurgery is related to stereotactic neurosurgery and radiotherapy. All three methods are stereotactic methods, as they use the exact positional localization of a target and patient positioning to consistently and accurately direct radiation to the same area[14,15]. They differ by the number of fractions applied to form the total dose received by the patient, as well as the method of application[15]. In fractional and stereotactic radiotherapy, a number of fractions are given to reach the total dose, with stereotactic radiotherapy differing by stereotactically using a beam aimed at the target. In stereotactic radiosurgery, the whole dose is stereotactically applied to the target in one fraction[14].
The type of treatment will depend upon the size, location and number of lesions, the radiation dose needed to destroy the tumor mass, the volume of healthy tissue that will receive the dose, important surrounding structures, and the tolerance of the healthy tissue to the dose of radiation, and hence, its ability to regenerate. Dividing radiation into fractions allows healthy cells to repair any damage to the genetic material between the individual fractional treatments of low dose radiation at a faster rate than cancerous cells[14-17].
Swedish neurosurgeon Lars Leksell, the pioneer of stereotactic radiosurgery, who collaborated in the development of the gamma knife, defined radiosurgery as a method that directs a single high-dose ionizing radiation onto an intracranial target, precisely decided by computed tomography (CT) or magnetic resonance imaging (MRI). A stereotactic frame or a polyester mesh mask, to fix and reference the head, is used to stereotactically direct multiple beams to a target and apply a therapeutic dose without incision[14-17].
Imaging has an important role in radiotherapy treatment. Firstly, proper imaging is very important during the entirety of radiosurgery treatment, not only at the beginning for pre-treatment planning but then again during the treatment itself and during the follow-up[8,13]. Before deciding the treatment options with the patients, modern management of brain tumors includes comprehensive staging and surveillance with both CT and MRI to identify the intracranial metastases as early as possible[17,18]. Thereafter, prompt management with radiosurgery may commence, with the purpose to prevent the need for surgical resection, development of neurologic symptoms, and worsening of the clinical condition. Both CT and MRI are often needed for planning the stereotactic treatment. Additionally, pre-treatment position imaging verification is strongly advised to avoid possible mistreatments during the course of stereotactic radiosurgery, although the stereotactic system itself is extremely accurate[13,17,19]. In the follow-up period, imaging is important to monitor the effects of the stereotactic treatment. As brain metastases may at first increase in size after the stereotactic radiosurgery, it is very difficult to distinguish post-radiation changes from a possible tumor relapse. In these situations, imaging with multiparametric MRI techniques is very useful. In the initial identification of radiation-related changes, it enables assessment of metabolic and physiological characteristics of the tissue and possible tumor recurrence, and thus, may be useful for monitoring treatment changes in intracranial neoplasms[17-19].
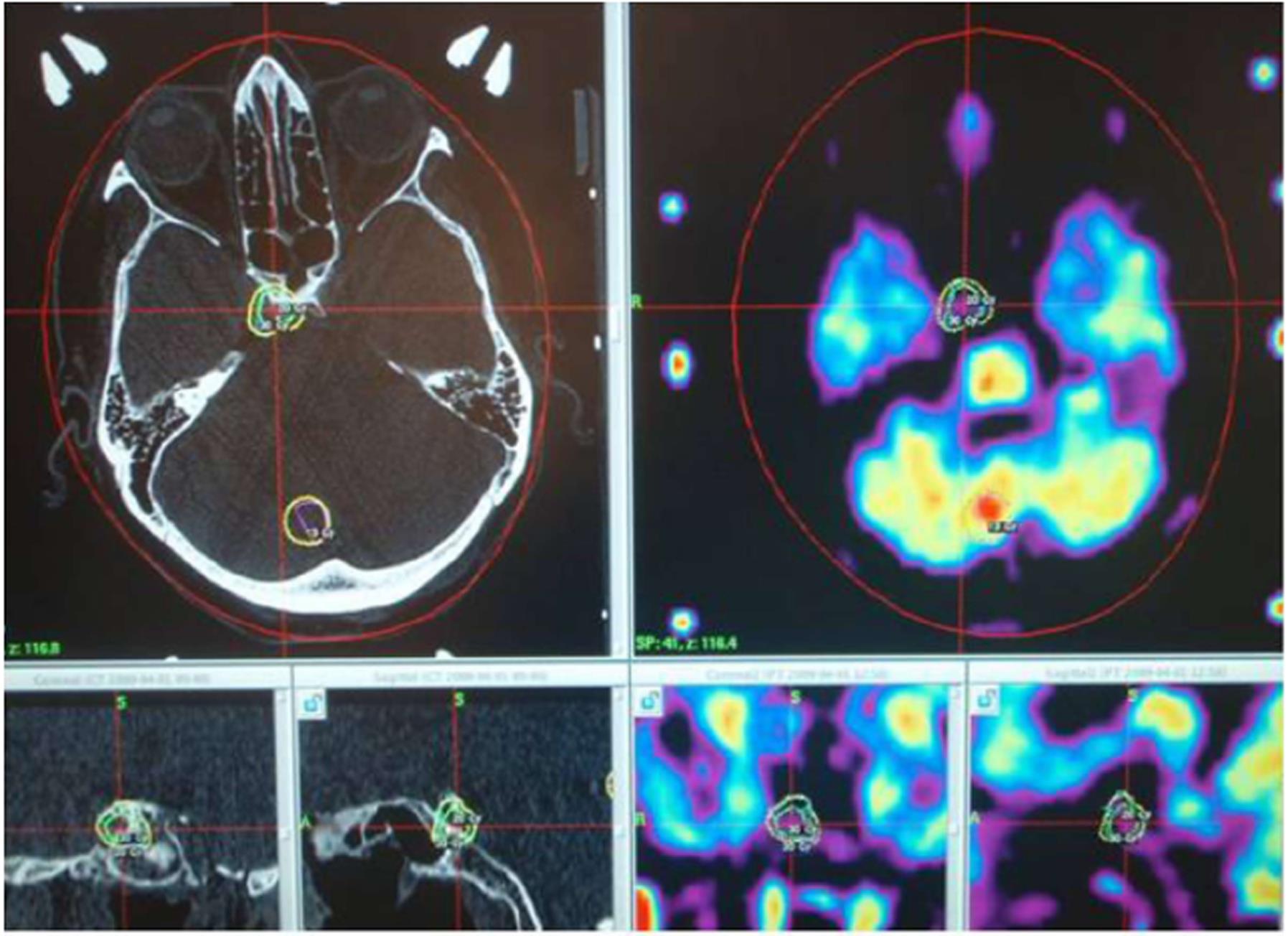
Secondly, proper radiotherapy treatment cannot be planned without accurate CT and MR images. The planning itself is performed on a computer, which combines and develops CT or MR images. At this point, an oncologist and a medical physicist or a radiographer select the target to be irradiated and calculate the dose that the tissue will receive. The target is defined by means of a coordinate system so that each target is defined with coordinates relating to the X-, Y-, and Z-axes. Before therapeutic irradiation, a computer performs a simulation during which the apparatus moves into the position for target irradiation[14-16]. The stereotactic frame is used while CT images are taken to plan the coordinates for the target, therefore playing a role as an external reference for the coordinate system. This method of irradiation allows the target to receive the maximal dose, with the dose received by neighboring tissue dropping significantly. The healthy tissue receives a negligible dose of radiation, allowing for decreased morbidity and better quality of life. With the maximum limit of the diameter of the target being around 3 cm, this method is particularly appropriate for the use in small brain metastases and primary intracranial lesions[15,16].
The two most used units in radiosurgery are the linear accelerator and gamma knife. The characteristics of both are: (1) application of a single high dose of radiation; (2) stereotactic localization of the lesion with CT imaging; (3) the use of computerized dose calculation, which can precisely define the amount of radiation that the central nervous system receives; (4) a steep dose-gradient, with the target receiving the highest dose and the dose steeply falling in the area surrounding it, so that these tissues receive a negligible or manageable dose; and (5) an accurate application of radiation, both spatially and in terms of dose. Technical development has allowed more accurate planning and irradiation of intracranial lesions[1,18,19].
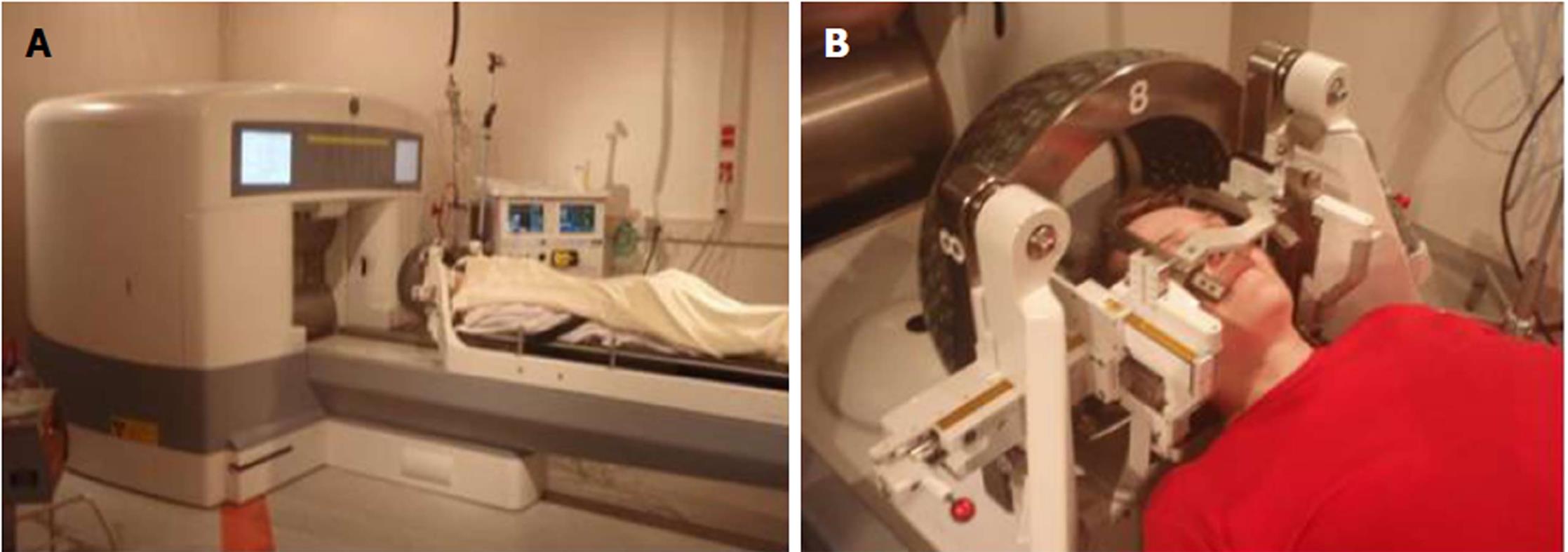
The gamma knife was first developed in 1968. The modern gamma knife is equipped with 201 sources of the radioactive isotope Cobalt-60 built into a special “hood” (Figure 2). The gamma knife allows for the application of a narrow beam of rays, accurately directed at a predetermined region in the brain where the lesion is located (Figure 3). Thus, the dose-gradient on the periphery of the target volume is very steep so that the target receives a high dose of radiation with the surrounding tissue preserved[2,20]. The treatment is planned using a stereotactically determined image base with a computer program. The treatment is usually completed in 1 d and one session. The gamma knife is suitable for the treatment of deep and surgically inaccessible intracranial lesions with low mortality and with few side effects from radiation. It is also suitable for the management of lesions that are insensitive to conventional radiotherapy. The gamma knife is also used to treat functional disorders, pain syndromes (such as trigeminal neuralgia), arteriovenous malformations, benign and malignant brain tumors and their remnants after surgery. Effective treatment is predominantly in the area of arteriovenous malformations and tumors (meningiomas, acoustic neuromas, pituitary adenomas, and brain metastases)[1,2,20].
In comparison with micro-neurosurgery, the neurological deficits in patients treated with radiosurgical techniques tend to be smaller, although technical limits and the risk of radiation damage to the brain do exist. Even though the gamma knife is a minimally invasive method for the treatment of a multitude of intracranial diseases, it is not without risk. In some patients, complications such as syncopal episodes, anxiety, and acute vessel complications can develop[21,22]. Late complications include headaches, strong facial pain (e.g., post trigeminal neuralgia treatment), newly developed motor dysfunction (e.g., ataxia caused by edema, paresis of the facial and oculomotor nerves, which can also occur a long time after the procedure), cases of hydrocephalus, and late-onset epileptic seizures. The most common indications for gamma knife treatment are: metastases (33% of patients), vestibular Schwannomas (17%), meningiomas (16%), neuropathic pain from trigeminal neuralgia (14%), arteriovenous malformations (8%), gliomas (5%) and the post-operative remnants of pituitary adenomas (2% of patients)[22-24].
Radiosurgery is also indicated for the treatment of some cases of epilepsy and movement disorders, as damage to a precisely defined point in the deep brain nuclei from electromagnetic irradiation may improve a patient’s symptoms. however, this treatment has fallen out of favor more recently, as neuromodulatory techniques are being used more frequently. Here, the cerebral nuclei or cortex is stimulated or inhibited by implanting an electrode to improve neural pathway function. The benefit of this technique is that it is reversible and causes less tissue damage[21].
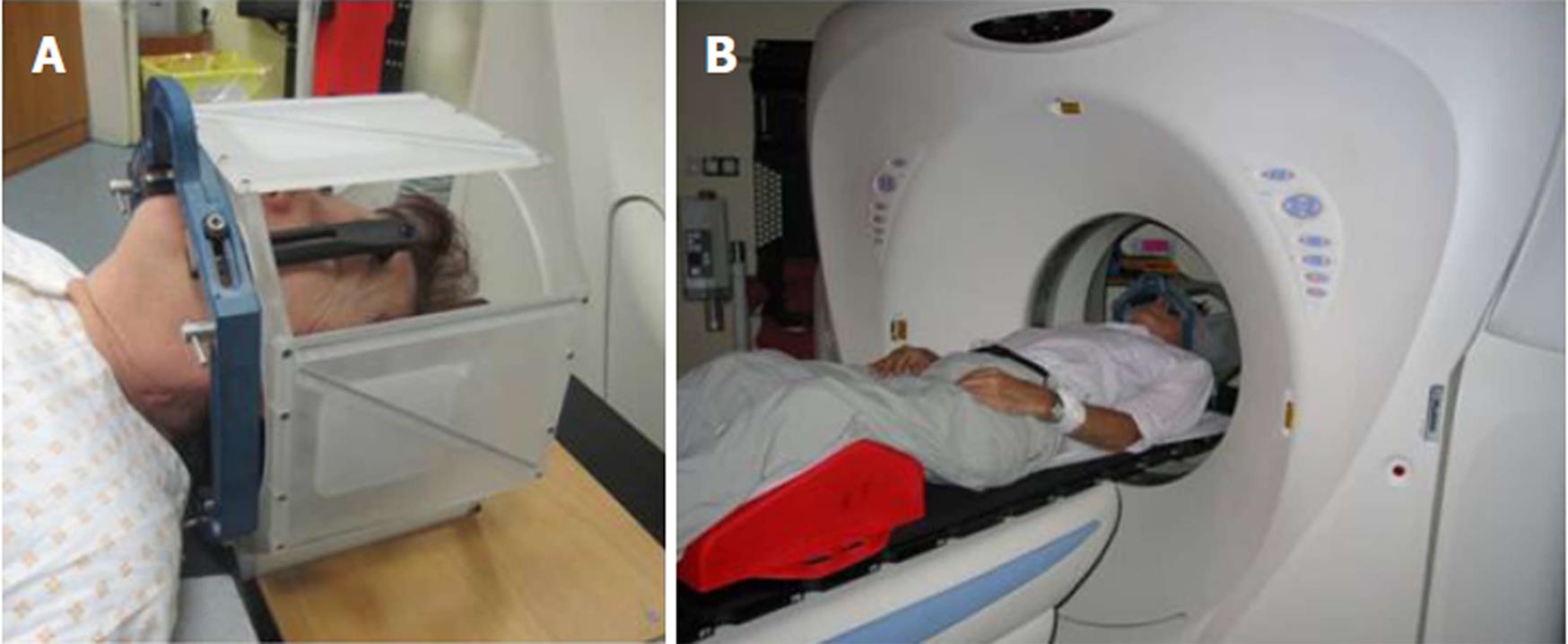
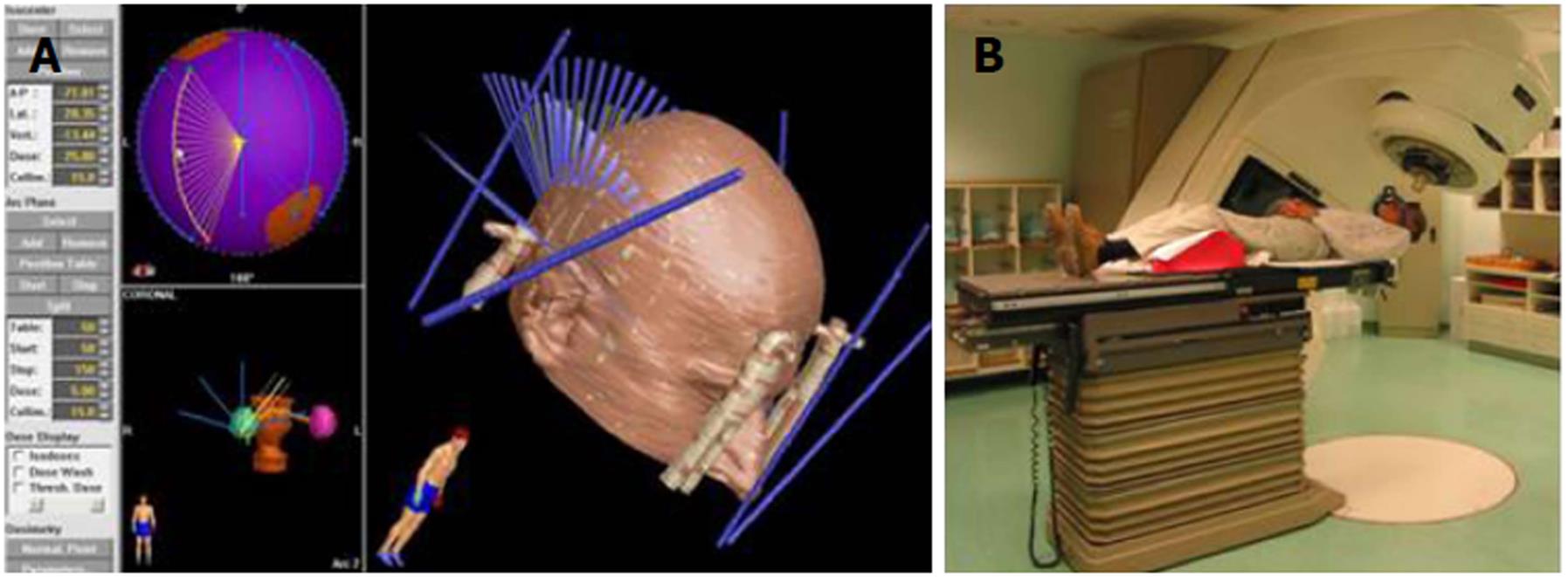
Linear accelerators were first used in the 1970s and are the most commonly used devices for conventional radiotherapy and radiosurgery today. They work by focusing a directed and shaped beam of X-rays onto a stereotactically determined target (Figure 4). The main direction of entry of the beam of X-rays from the linear accelerator (gantry) rotates around the patient to form a crisscross of beams, which all intersect at the target (Figure 5). The bed can rotate about the horizontal axis to allow irradiation from multiple trajectories to intersect at a certain point, the isocenter of the target[1,25]. As is also the case with the gamma knife, radiation beams that intersect on the target provide a large dose of ionizing radiation while minimizing the dose that the surrounding brain tissue receives. Linear accelerators use a special device called a collimator to shape and target the rays. In addition to primary and secondary collimators, rays can be further shaped with circular divergent tertiary collimators[25].
The modifications of linear accelerators include the gantry, which synchronously rotates the robotic table carrying the patient with the entry of the beam to the target, the system for stereotactic localization of the lesions, and improved collimators. With the wider use of linear accelerators, it is now possible to treat larger targets. Circular collimators were first developed for targets smaller than 3 cm in diameter and for irregularly shaped targets. In larger targets, the irradiated volume includes an unacceptably large volume of normal tissue. This is why collimators were developed. They allow radiosurgery with the dynamic form of the field of exposure, and hence, the irradiation of irregular and concave-shaped lesions. They are designed so that they are supplemented with a special multileaf collimator system, which allows exact coverage of the field of irradiation and comprises a multitude of micro-leaves that allow for irradiation of even bigger (diameter 3 cm to 4 cm) and geometrically more complex lesions. Recently, there has been a resurgence of interest in arc-based intensity modulated radiotherapy through the use of ‘conventional’ multileaf collimator systems that can treat large tumor volumes in a single or very few passes of the gantry[1,14,25,26].
The benefits of the linear accelerator over the gamma knife are the ability of more accurate shaping of the field of irradiation, a wider range of settings for the size of the collimator, a more homogeneous field of irradiation for large lesions, a higher energy of irradiation, the possibility to irradiate other parts of the body, the use of modern computer programs, and lower costs. Linear accelerators are also widely used for stereotactically focused fraction radiotherapy and for radiosurgical procedures. It is also possible to radiosurgically treat extracranial lesions[26].
The latest versions of linear accelerators are in the form of a robotic knife known as a Cyberknife[21]. The Cyberknife is a robotic radiosurgical system that is computer controlled with the help of CT or MRI. The system functions because of the interaction between the automatic guide, the imaging system, and the linear accelerator[21,22].
The guidance system includes a unit for X-ray imaging with the unit for the application of radiation being provided by a linear accelerator located on a robotic arm. There is a specific system for the guidance of the robotic arm during irradiation. Therefore, the robotic knife does not require a rigid attachment onto the stereotactic frame, as is the case with a linear accelerator[22]. Therefore, the position of the patient during the treatment is more comfortable in comparison to the gamma knife or linear accelerator. Treatment times typically range from 30 to 90 min, depending on the type of tumor. The treatment is usually concluded in one session. Occasionally, the patient can receive up to five sessions of radiotherapy. Besides intracranial tumors, the robotic knife can also be used to irradiate other organs, such as lungs, liver, prostate, kidneys, and pancreas. Another benefit of the Cyberknife is that tumors that move during respiration can also be treated[21-24].
Alongside neurosurgery, radiosurgery is a desirable secondary treatment option for many intracranial lesions. As a primary treatment, it is most suitable in cases of deep neurosurgically inaccessible or multiple lesions, where conventional forms of radiotherapy are not appropriate due to the greater risk of radiation tissue damage[25-29]. Examples include arteriovenous malformations, vestibular Schwannomas, meningiomas, certain primary brain tumors, and brain metastases. The number of metastases that can be treated with radiosurgery is up to five or a total diameter of 3.5 cm[30-32]. The uses of radiosurgery in treating epilepsy, functional motor, and behavioral disorders are limited to a small number of centers globally. The radiosurgical treatment of lesions requires a source of high energy rays and a method of accurately applying the radiation, conforming to the target volume and preserving the surrounding tissue[27,28].
Radiosurgical techniques can be used as an independent form of treatment, in case the volume of the lesion is not too large, or as a part of combined treatment with surgical removal or intravascular techniques. A combination of high-resolution diagnostic imaging, powerful computers, robotic systems, irradiation techniques, and new achievements in radio-biological research has enabled more successful treatment[30,31]. In addition to treating intracranial lesions, radiosurgery is effectively used in combination with systemic therapy in metastatic cancer treatment and as a local ablative therapy with stereotactic irradiation of metastases in the lungs, liver, and spine[30-34].
Manuscript source: Invited manuscript
Specialty type: Medical laboratory technology
Country of origin: Slovenia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Abdel Razek AAK, Neri V, Sotelo J S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Bian YN
| 1. | Rahman M, Murad GJ, Bova F, Friedman WA, Mocco J. Stereotactic radiosurgery and the linear accelerator: accelerating electrons in neurosurgery. Neurosurg Focus. 2009;27:E13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Noda SE, Lautenschlaeger T, Siedow MR, Patel DR, El-Jawahri A, Suzuki Y, Loeffler JS, Bussiere MR, Chakravarti A. Technological advances in radiation oncology for central nervous system tumors. Semin Radiat Oncol. 2009;19:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Eskelinen S. E-cadherin and β-catenin: dual roles in carcinogenesis. Acta Medico-biotechnica. 2010;3:9-14. |
| 4. | Air EL, Warnick RE, McPherson CM. Management strategies after nondiagnostic results with frameless stereotactic needle biopsy: Retrospective review of 28 patients. Surg Neurol Int. 2012;3:S315-S319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Nachbichler SB, Kreth FW. Brachytherapy of Intracranial Gliomas. Prog Neurol Surg. 2018;31:72-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Rakovec-Felser Z, Planinc S, Matvoz M, Vidovic L. Preparing patients to undergo surgery. Acta Medico-biotechnica. 2015;8:39-51. |
| 7. | Vitaz TW, Warnke PC, Tabar V, Gutin PH. Brachytherapy for brain tumors. J Neurooncol. 2005;73:71-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Fabrini MG, Perrone F, De Franco L, Pasqualetti F, Grespi S, Vannozzi R, Cionini L. Perioperative high-dose-rate brachytherapy in the treatment of recurrent malignant gliomas. Strahlenther Onkol. 2009;185:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Vanhauwaert D, Hallaert G, Baert E, Van Roost D, Okito JP, Caemaert J. Treatment of cystic craniopharyngioma by endocavitary instillation of yttrium90 radioisotope--still a valuable treatment option. J Neurol Surg A Cent Eur Neurosurg. 2013;74:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Kortmann RD, Seidel C, Müller K, Hirsch FW. Irradiation of Intracranial Gliomas in Children. Prog Neurol Surg. 2018;31:87-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Wernicke AG, Lazow SP, Taube S, Yondorf MZ, Kovanlikaya I, Nori D, Christos P, Boockvar JA, Pannullo S, Stieg PE. Surgical Technique and Clinically Relevant Resection Cavity Dynamics Following Implantation of Cesium-131 (Cs-131) Brachytherapy in Patients With Brain Metastases. Oper Neurosurg (Hagerstown). 2016;12:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Vrhovec L. Evidence-based medicine. Acta Medico-biotechnica. 2010;3:7-8. |
| 13. | Masucci GL. Hypofractionated Radiation Therapy for Large Brain Metastases. Front Oncol. 2018;8:379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Massager N. [Gamma knife radiosurgery]. Rev Med Brux. 2012;33:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Aiyama H, Yamamoto M, Kawabe T, Watanabe S, Koiso T, Sato Y, Higuchi Y, Ishikawa E, Yamamoto T, Matsumura A. Complications after stereotactic radiosurgery for brain metastases: Incidences, correlating factors, treatments and outcomes. Radiother Oncol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Niranjan A, Monaco EA III, Kano H, Flickinger JC, Lunsford LD. Stereotactic Radiosurgery in the Multimodality Management of Residual or Recurrent Glioblastoma Multiforme. Prog Neurol Surg. 2018;31:48-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Khuntia D, Tomé WA, Mehta MP. Radiation techniques in neuro-oncology. Neurotherapeutics. 2009;6:487-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Sahgal A, Ma L, Chang E, Shiu A, Larson DA, Laperriere N, Yin FF, Tsao M, Menard C, Basran P. Advances in technology for intracranial stereotactic radiosurgery. Technol Cancer Res Treat. 2009;8:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Yu C, Shepard D. Treatment planning for stereotactic radiosurgery with photon beams. Technol Cancer Res Treat. 2003;2:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Puataweepong P, Dhanachai M, Hansasuta A, Dangprasert S, Sitathanee C, Ruangkanchanasetr R, Yongvithisatid P. Clinical outcomes of perioptic tumors treated with hypofractionated stereotactic radiotherapy using CyberKnife® stereotactic radiosurgery. J Neurooncol. 2018;139:679-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Antypas C, Pantelis E. Performance evaluation of a CyberKnife G4 image-guided robotic stereotactic radiosurgery system. Phys Med Biol. 2008;53:4697-4718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Sakamoto GT, Borchers DJ 3rd, Xiao F, Yang HJ, Chang SD, Adler JR Jr. Cyberknife radiosurgery for trigeminal schwannomas. Neurosurgery. 2009;64:A14-A18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Nuyttens JJ, van de Pol M. The CyberKnife radiosurgery system for lung cancer. Expert Rev Med Devices. 2012;9:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Kondziolka D, Lunsford LD, Flickinger JC. The application of stereotactic radiosurgery to disorders of the brain. Neurosurgery. 2008;62 Suppl 2:707-19; discussion 719-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Friehs GM, Park MC, Goldman MA, Zerris VA, Norén G, Sampath P. Stereotactic radiosurgery for functional disorders. Neurosurg Focus. 2007;23:E3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Vachhrajani S, Fawaz C, Mathieu D, Ménard C, Cusimano MD, Gentili F, Hodaie M, Kenny B, Kulkarni AV, Laperriere N. Complications of Gamma Knife surgery: an early report from 2 Canadian centers. J Neurosurg. 2008;109 Suppl:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Vesper J, Bölke B, Wille C, Gerber PA, Matuschek C, Peiper M, Steiger HJ, Budach W, Lammering G. Current concepts in stereotactic radiosurgery - a neurosurgical and radiooncological point of view. Eur J Med Res. 2009;14:93-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Aizawa R, Uto M, Takehana K, Arakawa Y, Miyamoto S, Mizowaki T. Radiation-induced cystic brain necrosis developing 10 years after linac-based stereotactic radiosurgery for brain metastasis. Oxf Med Case Reports. 2018;2018:omy090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Song DY, Kavanagh BD, Benedict SH, Schefter T. Stereotactic body radiation therapy. Rationale, techniques, applications, and optimization. Oncology (Williston Park). 2004;18:1419-30; discussion 1430, 1432, 1435-1436. [PubMed] |
| 30. | Lo SS, Fakiris AJ, Teh BS, Cardenes HR, Henderson MA, Forquer JA, Papiez L, McGarry RC, Wang JZ, Li K. Stereotactic body radiation therapy for oligometastases. Expert Rev Anticancer Ther. 2009;9:621-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Rubin P, Brasacchio R, Katz A. Solitary metastases: illusion versus reality. Semin Radiat Oncol. 2006;16:120-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Vorsic M, Ravnik J, Bunc G. Pelvic expansion hidden by concomitant lumbar compression as a cause of sciatica- report of three cases. Acta Medico-biotechnica. 2010;3:45-50. |
| 33. | Alongi F, Arcangeli S, Filippi AR, Ricardi U, Scorsetti M. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist. 2012;17:1100-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 34. | Tran TA, Wu V, Malhotra H, Steinman JP, Prasad D, Podgorsak MB. Target and peripheral dose from radiation sector motions accompanying couch repositioning of patient coordinates with the Gamma Knife(®) Perfexion(™). Radiol Oncol. 2011;45:132-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |