Published online Jun 26, 2017. doi: 10.5662/wjm.v7.i2.33
Peer-review started: February 10, 2017
First decision: March 28, 2017
Revised: April 10, 2017
Accepted: May 3, 2017
Article in press: May 5, 2017
Published online: June 26, 2017
Processing time: 143 Days and 9.7 Hours
Integration of the cancer registry and clinical research departments can have a significant impact on the accreditation process of a Commission on Cancer (CoC) Program. Here in we demonstrate that the integration of both departments will benefit as there is increased knowledge, manpower and crossover in job responsibilities in our CoC-accredited Academic Comprehensive Cancer Center. In our model this integration has led to a more successful cooperative interaction among departments, which has in turn created an enhanced combined effect on overall output and productivity. More manpower for the cancer registry has led to increased caseloads, decreased time from date of first contact to abstraction, quality of data submissions, and timely follow-up of all patients from our reference date for accurate survival analysis along with completeness of data. In 2016, our Annual Facility report showed an additional 163 cases over prediction by the state of Maryland Cancer Registry and a 39% increase in case completeness. As proof of the synergetic effectiveness of our model within one year of its implementation, the cancer center was able to apply for, and was awarded membership from Alliance for Clinical Trials in Oncology, Central IRB, and in turn led to increased clinical trial accrual from 2.8% in 2014 compared to 13.2% currently. Our cancer registry in year one submitted over 150 more cases than predicted, improved quality outcome measures displayed by our Cancer Program Practice Profile reports and had more timely and complete data submissions to national and state registries. This synergetic integration has led to a better understanding, utilization and analysis of data by an integrated team with Clinical Research expertise.
Core tip: In the current era, the evolution of healthcare management has focused on limiting resources while increasing the value of healthcare delivery. As hospitals and health care organizations operate under tighter budgets year after year, the executive teams must prioritize and utilize the resources available in the optimal way to produce the best patient care with limited funding. Integrating the cancer registry and clinical research departments can have a significant impact on outcomes. Both departments benefit as there is increased knowledge, manpower and crossover in job responsibilities. This leads to increased caseloads, decreased time from date of first contact to abstraction, and quality and completeness of data.
- Citation: Bedra M, Vyskocil T, Emel J, Edwards C, Boutros C. Synergetic role of integrating the departments of cancer registry and clinical research at an academic comprehensive cancer center. World J Methodol 2017; 7(2): 33-36
- URL: https://www.wjgnet.com/2222-0682/full/v7/i2/33.htm
- DOI: https://dx.doi.org/10.5662/wjm.v7.i2.33
Health organizations all over the world are required to set priorities and allocate resources within the constraint of limited funding[1]. The Commission on Cancer (CoC) is a program of the American College of Surgeons (ACoS) that was established in 1922. CoC membership is composed of 110 individuals who are either surgeons representing the ACoS or who are representatives from one of the 56 national professional organizations or member organizations affiliated with the CoC[2]. Patients who obtain care at a CoC-accredited cancer program receive many benefits and they are directly related to the quality of their cancer care. They have the opportunity to receive surgical treatment in innovative ways including equipment such as robotic, laparoscopic and other minimally invasive approaches to cancer treatment. Accredited programs participate in multidisciplinary cancer conferences for each specialty where all key physicians are present to decide the best patient-centered care for each individual. In addition to cancer treatment, CoC-accredited programs also offer a vast range of support services for patients who receive treatment at their facilities. Some examples of support services include social work, dietitians, genetics counselors, nurse navigators, nurse practitioners specializing in survivorship which includes life after cancer treatment is complete, clinical research opportunities and a cancer registry that collects data on cancer type, stage, treatment result, and offers lifelong patient follow-up. Being part of a CoC-accredited program raises the bar by ensuring all programs adhere to the ACoS CoC program standards on an annual basis.
Clinical Research and Cancer Registry departments play an integral role in achieving the standards set forth by the CoC for accredited programs. There is currently one standard for clinical research. CoC Standard 1.9 states, “as appropriate to the cancer program category, the required percentages of patients are accrued to cancer-related clinical research studies each calendar year. The Clinical Research Coordinator documents and reports clinical research study enrollment information to the cancer committee annually[3]”. It is required that each accredited cancer program accrue the minimum percentage of patients based on the program’s CoC designated category, and the number of reportable cancer cases on an annual basis. For the cancer registry there are two standards that outline the minimum percentage of follow-up of cancer patient’s year around. CoC Standard 5.3 states, “for all eligible analytic cases, an 80% follow-up rate is maintained from the cancer registry reference date”. CoC Standard 5.4 states, “a 90 percent follow-up rate is maintained for all eligible analytic cases diagnosed within the last five years or from the cancer registry reference date, whichever is shorter[3]”. These two standards are applicable to both departments as ensuring a high percentage of patients in the cancer registry are followed from the registries reference date forward in turn leads to accurate survival analysis and opportunities for retrospective research.
Each CoC-accredited program is required to report data to the National Cancer Data Base (NCDB) during the annual Call for Data which falls during the beginning of each calendar year. Reporting of data falls under two standards. CoC standard 5.5 states, “each year, complete data for all requested analytic cases are submitted to the NCBD in accordance with the annual Call for Data[3]”. CoC Standard 5.6 states, “annually, cases submitted to the NCDB that were diagnose on January 1, 2003, or later meet the established quality criteria and resubmission deadline specified in the annual Call for Data[3]”. Reporting of this data is mandatory for every CoC-accredited program regardless of the program category on an annual basis. By reporting based on the standards above, it helps measure performance of the program and its cancer care quality. The data is used to monitor treatment patterns and outcomes and to also enhance cancer control and clinical surveillance activities. Utilization of this data helps in the development of effective educational interventions and clinical research to improve cancer prevention, early detection, cancer care delivery and outcomes in health care settings[3].
Synergetic integration of the cancer registry and clinical research departments can have a significant impact on outcomes of a CoC accredited Academic Comprehensive Cancer Program (ACAD). As the standards of the CoC continue to develop and set the bar higher for accredited programs, individual cancer programs need to meet or exceed these standards. In the current state of healthcare, there is a major question about the priority setting and the dilemma of resource scarcity. This process should be evidenced based and encompass a wide range of challenges[1]. Today, there is a significant increase in the workload which is needed to comply with CoC accreditation and deliver quality care to patients. As health organizations all over the world are required to set priorities and allocate resources within the constraint of limited funding, this has led to an increase in workload within the cancer registry and clinical research departments[1]. These departments already have limited resources which has led us to the development of our model to still deliver quality care with the current scarce resources. This project was started as a vision by our facility to combine two departments which have one common theme, data. The idea was put into place in August of 2015 as there was a need to utilize the vast amount of data available in the cancer registry for research. The two teams were merged and the data was utilized for both departments in many ways.
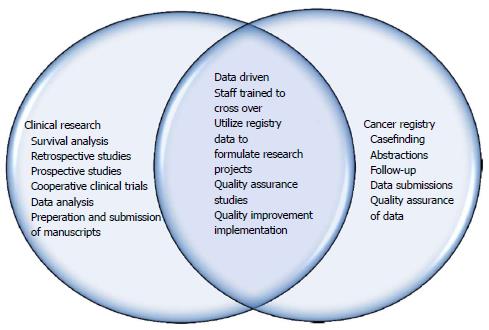
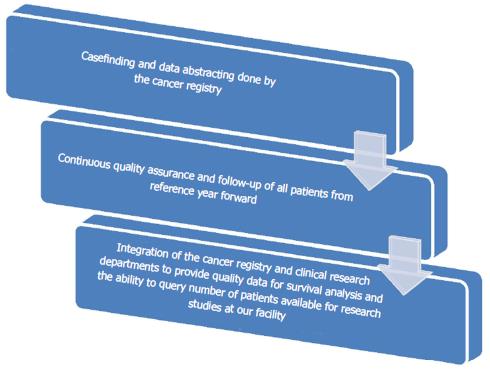
Results have shown that both departments have benefited as there is increased knowledge, manpower and crossover in job responsibilities. This integration has led to a more successful cooperative interaction among departments, which has in turn created an enhanced combined effect on overall output and productivity. More manpower for the Cancer Registry has led to increased caseloads, decreased time from date of first contact to abstraction, quality of data submissions, and timely follow-up of all patients from our reference date for accurate survival analysis along with completeness of data. In 2016, our Annual Facility report showed an additional 163 cases over prediction by the state of Maryland Cancer Registry and a 39% increase in case completeness. Figure 1 below shows the roles and responsibilities of the two departments along with how the integration has led to a combined effort and crossover within the departments. Figure 2 below represents the synergistic integration and the flow of effects it has had on our success as an ACAD with less resources and more productivity.
Since becoming a part of Alliance for Clinical Trials in Oncology and Central IRB, our model has led to increased clinical trial accrual from 2.8% in 2014 compared to 13.2% currently. This synergetic integration has led to a better understanding, utilization and analysis of data by an integrated team with Clinical Research expertise.
Based on our experience, we advocate for synergetic integration and implementation of our model in a CoC-accredited program. Our model will assure the ability to continuously meet standards of accreditation and add value to healthcare delivery while limiting cancer program resources.
Manuscript source: Invited manuscript
Specialty type: Medical Laboratory Technology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Neri V, Ni Y, Sonoda K S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Mitton C, Donaldson C. Health care priority setting: principles, practice and challenges. Cost Eff Resour Alloc. 2004;2:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Commission on Cancer. American College of Surgeons Website. 1996. [updated. 2016;Jan 1, accessed 2017 Jan] Available from: https://www.facs.org/quality-programs/cancer/coc. |
| 3. | American College of Surgeons. Cancer program standards: ensuring patient-centered care (2016 ed). Chicago, IL: American College of Surgeons 2015; 1-82. |