Published online Mar 26, 2017. doi: 10.5662/wjm.v7.i1.25
Peer-review started: November 22, 2016
First decision: January 14, 2017
Revised: January 21, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: March 26, 2017
Processing time: 127 Days and 9.4 Hours
To evaluate the utility of patch test and cross-sensitivity patterns in patients with adverse cutaneous drug reactions (ACDR) from common anticonvulsants.
Twenty-four (M:F = 13:11) patients aged 18-75 years with ACDR from anticonvulsants were patch tested 3-27 mo after complete recovery using carbamazepine, phenytoin, phenobarbitone, lamotrigine, and sodium valproate in 10%, 20% and 30% conc. in pet. after informed consent. Positive reactions persisting on D3 and D4 were considered significant.
Clinical patterns were exanthematous drug rash with or without systemic involvement (DRESS) in 18 (75%), Stevens-Johnsons syndrome/toxic epidermal necrolysis (SJS/TEN) overlap and TEN in 2 (8.3%) patients each, SJS and lichenoid drug eruption in 1 (4.2%) patient each, respectively. The implicated drugs were phenytoin in 14 (58.3%), carbamazepine in 9 (37.5%), phenobarbitone in 2 (8.3%), and lamotrigine in 1 (4.7%) patients, respectively. Twelve (50%) patients elicited positive reactions to implicated drugs; carbamazepine in 6 (50%), phenytoin alone in 4 (33.3%), phenobarbitone alone in 1 (8.3%), and both phenytoin and phenobarbitone in 1 (8.33%) patients, respectively. Cross-reactions occurred in 11 (92%) patients. Six patients with carbamazepine positive patch test reaction showed cross sensitivity with phenobarbitone, sodium valproate and/or lamotrigine. Three (75%) patients among positive phenytoin patch test reactions had cross reactions with phenobarbitone, lamotrigine, and/or valproate.
Carbamazepine remains the commonest anticonvulsant causing ACDRs and cross-reactions with other anticonvulsants are possible. Drug patch testing appears useful in DRESS for drug imputability and cross-reactions established clinically.
Core tip: Anticonvulsants account for 20% of all adverse cutaneous drug reactions (ACDRs) while cross-reactions occur frequently among carbamazepine, phenytoin, phenobarbitone necessitating careful prescriptions. The clinical presentation alone is not diagnostic and identification of offending drug needs causality assessment that may be misleading in patients on multiple medications. Drug provocation, skin prick or intradermal tests have ethical issues for possibility of precipitating more severe reactions. Basophil degranulation/lymphocyte activation or drug specific IgE radioallergosorbent tests, histamine release and passive haemagglutination tests have limited use in clinical practice. Drug patch testing appears useful in anticonvulsant ACDRs, drug imputability and cross-reactions established clinically.
- Citation: Shiny TN, Mahajan VK, Mehta KS, Chauhan PS, Rawat R, Sharma R. Patch testing and cross sensitivity study of adverse cutaneous drug reactions due to anticonvulsants: A preliminary report. World J Methodol 2017; 7(1): 25-32
- URL: https://www.wjgnet.com/2222-0682/full/v7/i1/25.htm
- DOI: https://dx.doi.org/10.5662/wjm.v7.i1.25
Adverse cutaneous drug reaction (ACDR) is a frequent problem in clinical practice comprising 1%-2% of outdoor and 6%-30% of indoor patients in dermatology. ACDRs from anticonvulsants [carbamazepine, phenytoin, phenobarbitone (aromatic group), lamotrigine and sodium valproate] account for 20% of all drug rashes[1]. Lamotrigine itself is associated with high adverse cutaneous reactions in 10% or more cases and its combination with sodium valproate further enhances this risk. They cause transient maculopapular rash that may eventuate to more severe life threatening adverse cutaneous reactions like exanthematous drug hypersensitivity, drug rash with eosinophilia with or without systemic involvement (DRESS), Stevens-Johnsons syndrome/toxic epidermal necrolysis (SJS/TEN) collectively known as anticonvulsant hypersensitivity syndrome[2]. Cross-reactions especially aromatic anticonvulsants (carbamazepine, phenytoin, phenobarbitone), lamotrigine, and sodium valproate frequently makes selection of an alternative agent difficult[3]. The focus has shifted in recent years on the utility of drug patch test in cutaneous adverse drug reactions for ease and positive results can be useful to confirm drug imputability established on clinical grounds. Moreover, the risk with patch testing is considerably lower when compared to intracutaneous or oral provocation tests. Although the reliability of patch testing in identification of the culprit drug has been reported[4], the cross-reactions among anticonvulsants remain under studied. This study intended to evaluate the utility of patch test in patients with ACDRs from anticonvulsants and occurrence of cross-sensitivity patterns among these drugs.
Twenty four patients diagnosed and treated previously for ACDRs from anticonvulsants were patch tested after informed consent between April 2014 and March 2015 when they were off systemic treatments including corticosteroids for ≥ 4 wk. Pregnant and lactating women, children aged under 18 years, patients with recent acute reaction, suspected viral exanthem or autoimmune disorders, and who were using topical corticosteroids over the back within the last one week were excluded from the study. Clinical details of age, gender, onset, duration and progress of drug rash, the suspected offending anticonvulsant drug, all treatments taken before or after onset of rash, personal and type of ACDRs were recorded.
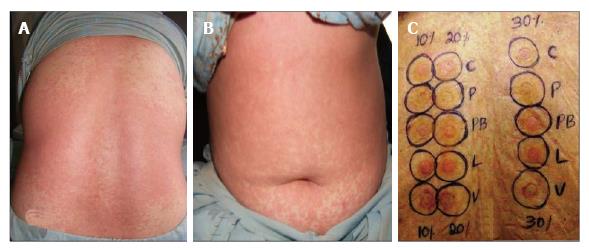
Since pure form of drugs could not be obtained, antigens for patch testing were prepared as suggested by Friedmann and Ardern-Jones[5] from pulverized prescribable tablets of carbamazepine, phenytoin, phenobarbitone, lamotrigine, and sodium valproate in petrolatum having active drug in 10%, 20%, 30% conc. The patch test was performed by Finn chamber (7 mm) method as described previously using 0.02 mL of test antigen[4]. The patch tests were applied on dry, non-hairy upper back after cleansing with ethanol. The patients returned for reading of results after 48 h (D2), 72 h (D3) and 96 h (D4) and results were graded as per International Contact Dermatitis Research Group criteria[6]. Reactions persisting on D3 or D4 were considered significant for final analysis. None of the test concentration elicited irritant/allergic reaction in ten healthy adult volunteers in prior testing. The relevance of positive patch test results was determined clinically. Any side effects from patch testing (adhesive tape reaction, itching/flare up, angry back phenomenon, or pigment alteration) were noted.
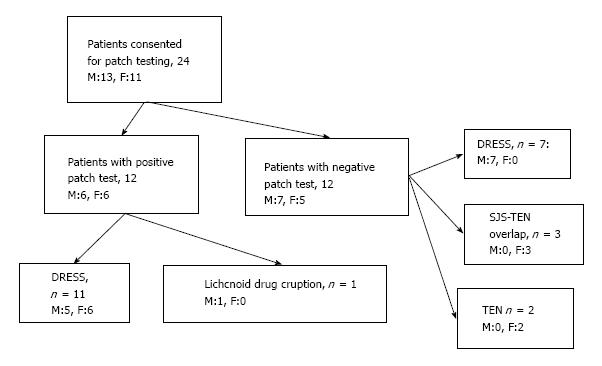
Tables 1 and 2 lists baseline characteristics of study patients, incubation period, common clinical patterns of ACDRs observed, individual implicated anticonvulsants, and time interval between complete recovery from ACDR and drug patch test. The majority, 14 (58.3%) patients were of DRESS and nine were from phenytoin (Figure 1). None of them had received any drug(s) other than anticonvulsant(s) before or after the onset of drug rash.
| Baseline characteristics | Number of patients underwent patch testing n = 24 (%) |
| Gender | |
| Male | 13 (54.2) |
| Female | 11 (45.8) |
| M:F | 1:1.8 |
| Age (yr) | |
| Range | 18-75 |
| Mean ± SD | 45.70 ± 16.29 |
| 18-30 | 4 (16.7) |
| 31-50 | 12 (50) |
| 51-70 | 6 (25) |
| > 70 | 2 (8.3) |
| Time interval (d) between drug intake and ACDRs | |
| Range | 7-45 |
| Mean ± SD | 22.54 ± 12.19 |
| Implicated drugs | |
| Phenytoin | 14 (58.3) |
| Carbamazepine | 9 (37.5) |
| Phenobarbitone | 2 (8.3) |
| Lamotrigine | 1 (4.7) |
| Phenytoin1 + Carbamazepine | 1 (4.7) |
| Phenytoin1 + Phenobarbitone | 1 (4.7) |
| Clinical spectrum of ACDRs | |
| DRESS | 18 (75) |
| SJS-TEN overlap | 2 (8.3) |
| TEN | 2 (8.3) |
| SJS | 1 (4.2) |
| Lichenoid drug eruption | 1 (4.2) |
| Time interval (mo) between complete recovery from ACDRs and Patch test | |
| Range | 1-24 |
| Mean ± SD | 9.62 ± 6.62 |
| Implicated drugs | Clinical patterns (n = 24 ) | ||||
| DRESS | SJS | SJS-TEN overlap | TEN | Lichenoid drug eruption | |
| Phenytoin | 9 | 1 | 1 | 1 | - |
| Carbamazepine | 6 | - | - | 1 | 1 |
| Phenytoin + Carbamazepine | - | - | 1 | - | - |
| Phenytoin + Phenobarbitone | 1 | - | - | - | - |
| Lamotrigine | - | - | 1 | - | - |
| Phenobarbitone | 1 | - | - | - | - |
| Sodium valproate + Lamotrigine | - | - | - | - | - |
Only 12 (50%) patients had positive patch test reactions from the primarily implicated drug and/or other anticonvulsants 4-9 mo after complete recovery from ACDR (Table 3). Carbamazepine elicited positive reactions in 6 of 8 patients with carbamazepine hypersensitivity and cross reactions from one or more drugs that included sodium valproate (3 patients), lamotrigine (4 patients), and phenobarbitone (2 patients), respectively (Figure 2). Similarly, phenytoin elicited positive reactions in 4 of 11 patients with phenytoin hypersensitivity. Cross-reactions were also observed in 3 patients from phenobarbitone (2 patients), and sodium valproate and lamotrigine in one patient. Phenobarbitone that had caused DRESS in one patient also elicited positive reaction in him along with cross sensitivity to carbamazepine, phenytoin, sodium valproate and lamotrigine. One patient with DRESS from combination of phenytoin and phenobarbitone showed positivity to both the drugs and cross sensitivity with lamotrigine. Lamotrigine in 7, carbamazepine, phenytoin in 6 patients each elicited more number of positive reactions with 30% concentration than their 20% and 10% concentrations. Patch test positivity from phenobarbitone (in 6 patients) or sodium valproate (in 4 patients) was more with 10% concentration than from their higher concentrations. Sodium valproate elicited positive reaction with all concentrations but more so with 10% and 30% (4 patients each) as compared to 20% eliciting positive reactions in two patients only (Table 4).
| Case No | Age (yr) and Sex | Clinical diagnosis | Implicated drug | Interval between drug rash and patch test (mo) | Patch test results (Grades) | Cross reactions (Grades) | Irritant reaction at D2 |
| 1 | 65 F | DRESS | Carbamazepine | 7 | Carbamazepine (2+) with 10%, 20%, 30% | Sodium valproate (1+) with 20%, 30% | - |
| 2 | 60 F | DRESS | Carbamazepine | 6 | Carbamazepine (3+) with 20%, 30% | Lamotrigine (3+) with 30% | Carbamazepine 10%, Lamotrigine 10%, Sodium valproate 10%, 30% Phenobarbitone 30% |
| 3 | 55 F | DRESS | Carbamazepine | 5 | Carbamazepine (1+) with 30% | Sodium valproate (2+) with 10%, 30% Lamotrigine (1+) with 10%, 20%, 30% | Carbamazepine 10% and 20%, Phenobarbitone 10%, 30% and Sodium valproate 20% |
| 4 | 52 M | Lichenoid drug eruptions | Carbamazepine | 6 | Carbamazepine (2+) with 10%, 20%, 30% | Phenobarbitone (2+) with 10%, 20%, 30% | Phenytoin 20%, Sodium valproate 20% and Lamotrigine 10% |
| 5 | 48 M | DRESS | Phenobarbitone | 9 | Phenobarbitone (3+) with 10%, 20%, 30% | Phenytoin (3+) with 10%, 20%, 30% Carbamazepine (2+) with 30% Sodium valproate (3+) with 10%, 30% Lamotrigine (1+) with 30% | - |
| 6 | 32F | DRESS | Carbamazepine | 6 | Carbamazepine (3+) with 10%, 20%, 30% | Sodium valproate (1+) with 10%, Lamotrigine (3+) with 10%, 30% Phenobarbitone (2+) with 10%, 30% | Phenytoin 10% |
| 7 | 31 M | DRESS | Phenytoin | 8 | Phenytoin (1+) with 30% | Lamotrigine (1+) with 30% | - |
| 8 | 26 M | DRESS | Phenytoin | 6 | Phenytoin (1+) with 30% | - | - |
| 9 | 63 M | DRESS | Carbamazepine | 4 | Carbamazepine (2+) with 10% | Lamotrigine (1+) with 30% | - |
| 10 | 31 M | DRESS | Phenytoin + Phenobarbitone | 8 | Phenytoin (3+) and Phenobarbitone (3+) with 10%, 20%, 30% | Lamotrigine (1+) with 30% | Sodium valproate 10% and 30%, |
| 11 | 43 F | DRESS | Phenytoin | 4 | Phenytoin (2+) with 10%, 30% | Phenobarbitone (2+) with 10%, 30% | Carbamazepine 30% |
| 12 | 75 F | DRESS | Phenytoin | 5 | Phenytoin (2+) with 20%, 30% | Phenobarbitone (2+) with 10%, 20%, Sodium valproate (2+) with 10%, 20%, 30% | Phenytoin 10% |
| 13 | 56 M | DRESS | Phenytoin | 1 | - | - | Sodium valproate 10%, 20%, 30% |
| 14 | 60 M | DRESS | Phenytoin | 1 | - | - | Sodium valproate 10%, 20%, 30% |
| Case No | Clinical diagnosis | Carbamazepine | Phenytoin | Phenobarbitone | Lamotrigine | Sodium valproate | ||||||||||
| 10% | 20% | 30% | 10% | 20% | 30% | 10% | 20% | 30% | 10% | 20% | 30% | 10% | 20% | 30% | ||
| 1 | DRESS | 2+ | 2+ | 2+ | - | - | - | - | - | - | - | - | - | - | 1+ | 1+ |
| 2 | DRESS | - | 3+ | 3+ | - | - | - | - | - | - | - | - | 3+ | - | - | - |
| 3 | DRESS | - | - | 1+ | - | - | - | - | - | - | 1+ | 1+ | 1+ | 2+ | - | 2+ |
| 4 | Lichenoid drug eruptions | 2+ | 2+ | 2+ | - | - | - | 2+ | 2+ | 2+ | - | - | - | - | - | - |
| 5 | DRESS | - | - | 2+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | - | - | 1+ | 3+ | - | 3+ |
| 6 | DRESS | 3+ | 3+ | 3+ | - | - | - | 2+ | - | 2+ | 3+ | - | 3+ | 1+ | - | - |
| 7 | DRESS | - | - | - | - | - | 1+ | - | - | - | - | - | 1+ | - | - | - |
| 8 | DRESS | - | - | - | - | - | 1+ | - | - | - | - | - | - | - | - | - |
| 9 | DRESS | 2+ | - | - | - | - | - | - | - | - | - | - | 1+ | - | - | - |
| 10 | DRESS | - | - | - | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | - | - | 1+ | - | -- | - |
| 11 | DRESS | - | - | - | 2+ | - | 2+ | 2+ | - | 2+ | - | - | - | - | - | - |
| 12 | DRESS | - | - | - | - | 2+ | 2+ | 2+ | 2+ | - | - | - | - | 2+ | 2+ | 2+ |
| 13 | DRESS | - | - | - | - | - | - | - | - | - | - | - | - | IR | IR | IR |
| 14 | DRESS | - | - | - | - | - | - | - | - | - | - | - | - | IR | IR | IR |
| Total | 4 | 4 | 6 | 3 | 3 | 6 | 6 | 4 | 5 | 2 | 1 | 7 | 4 | 2 | 4 | |
Overall, 24 irritant reactions were observed in nine patients (Table 3). These were from sodium valporate in 6 patients (4 reactions each from 10%, 20%, and 30%), carbamazepine (2 reactions from 10%, and one reaction each from 20% and 30%) and phenytoin in 3 patients each (2 reactions from 10% and one reaction from 20%). Phenobarbitone (one from 10%, and two reactions from 30%), and lamotrigine (two reactions from 10%) elicited irritant reactions in 2 patients each. The irritant reactions from sodium valproate in two patients were from all three concentrations lasting for > 72 h. No patient had patch test related side effects.
The patch testing is a preferred investigation in adverse ACDRs as well as it helps in studying cross-reactions and understanding the pathomechanisms of drug eruptions that is essentially same as that in patch testing for allergic contact dermatitis[4]. Briefly, it is type-4 (delayed type) hypersensitivity involving CD4 or CD8 T-lymphocytes producing different patterns of cytokines and/or cytotoxic factors. The antigen (drug molecule or the metabolite, the hapten, and protein complex) is presented to T-helper cells after processing by antigen presenting cells. The T-helper cells after getting activated proliferate and produce clones of specific immunogenic memory/effector T-cells having ability to activate immune effector mechanism (immunological memory) as well as help antibody (IgA, IgG, IgE) production from B cells during this sensitization phase lasting for 7-10 d. This is followed by elicitation phase when the offending drug will elicit similar clinical reaction on re-exposure and positive patch test reactions in individuals sensitized previously.
Carbamazepine, phenytoin, phenobarbitone and lamotrigine, alone or in combination, may induce ACDRs such as DRESS, SJS, SJS-TEN overlap, and TEN. Arene oxide metabolites from a shared metabolic pathway of carbamazepine, phenytoin and phenobarbitone, the commonest offending aromatic drugs, have been implicated in the pathogenesis of hypersensitivity reactions and cross reactivity among these anticonvulsants. Sodium valproate inhibits metabolism of lamotrigine and increases the risk of severe ACDRs. Frequency of positive drug patch tests varies between 7% and 87% in ACDRs from groups of drugs including anticonvulsants across studies[1,7-11]. The patch test positivity of 50% in the present study is comparable. The significance of drug patch test in SJS/TEN and exfoliative dermatitis due to anticonvulsants remains poorly elucidated since these patients usually elicit no or weak positive reactions. Contrarily, highest patch test positivity occurs in maculopapular/exanthematos drug rash such as DRESS[1,12] as was also observed in our 11 (92%) of 12 patients with DRESS. It is possibly due to the pathomechanism (Th2 cytokine response) involved in DRESS that differs from that in SJS/TEN (cytotoxic T-cell response). Carbamazepine has been the commonest drug eliciting positive patch test reactions in 24%-100% patients with DRESS followed by phenytoin and phenobarbitone in order of frequency[8,9,11]. The highest patch test positivity was with carbamazepine (50%) followed by phenytoin (33%), phenobarbitone (8.3%) and combination of phenytoin and phenobarbitone in one case and both eliciting positive patch test reactions in this study also corroborate.
Clinical cross reactivity among anticonvulsants occurs frequently from their structural homology. Cross sensitivity between carbamazepine and phenytoin was 18%-50% patients and was as high as 57% in two separate studies[2,13]. The cross sensitivity from one or more drugs was seen in 11 (92%) of 12 patients with positive patch tests in this study being common in 6 patients having positivity from carbamazepine. Common cross-reactions were with lamotrigine (4 patients) and sodium valproate (3 patients) and phenobarbitone (2 patients) in order of frequency. Similarly, 3 (75%) of 4 patients with positivity from phenytoin had cross sensitivity to one or more drugs that is phenobarbitone, lamotrigine, and sodium valproate. Another patient with DRESS from phenobarbitone showed positivity to carbamazepine, phenytoin, lamotrigine, and sodium valproate. Although sodium valproate does not cross react with these aromatic anticonvulsants, it was perhaps responsible for positive reaction per se in some of the patients in the current study. Nevertheless, multiple drug reactivity is not uncommon and reportedly occurs in 18% patients with DRESS from classes of drugs including anticonvulsants[14]. The phenomenon is considered to be from co-stimulatory signals provided by viral reactivation (herpes family virus reactivation in 76% patients) and/or first-drug sensitization acting as cofactors for enhanced immune response to another drug-protein conjugate. Increased sensitivity/irritability of skin after DRESS, especially when tested too early, is other plausible explanation. Since no patient in the study had received all the anticonvulsants concurrently or sequentially, the multiple positive patch test responses were considered cross-reactions.
It has been recommended to use between 1% and 10% (w/w) of pure drug or 30% (w/w) conc. of the powdered commercial tablet when pure drug form cannot be patch tested[5]. However, the conc. per se was not important in a series of patients with DRESS from carbamazepine for frequency or strength of positive patch test responses over varied drug concentrations from 1% to 20%[8]. According to Romano et al[11] anticonvulsants in 20% concentration are sufficient to induce positive patch test results. However, 20% drug concentration elicited only 14 (23%) of 61 positive reactions as compared to 28 (44.4%) positive reactions elicited by 30% drug concentration and 19 (31%) positive reactions from 10% drug concentration particularly in case of carbamazepine, phenytoin and lamotrigine in this study. Whereas, phenobarbitone positivity was more with 10% concentration than higher concentrations while sodium valproate showed equal positivity with both 10% and 30% concentration. Lin et al[2] also observed similar results with 30% carbamazepine concentration eliciting higher number and more intense positive reactions than 10% concentration. While positive patch test reactions with 5%, 10%, 15% and 20% concentrations of phenobarbitone and carbamazepine occurred in 60% patients, sodium valproate 15%, 30%, 45% and 60% concentrations elicited positivity in one (10%) patient only[11]. This variability of results is attributed to drugs’ capability to penetrate skin barrier more effectively in higher concentrations and ability to produce its metabolites in the skin in a manner that is dose and the drug type dependent[15]. Similarly, variability of our results also signifies the need for patch testing with several drug concentrations for accurate results especially when consensus for drug concentration for patch testing in patients with ACDRs remains elusive. It is also suggested to use prescribable drug for patch testing for its potential advantage of identifying drug hypersensitivity from excipients itself[16].
Irritant drug patch test reactions are not uncommon especially with sodium valproate and have been documented even in as low as 1% concentration[1]. Sodium valproate is highly irritant for being hygroscopic and getting converted rapidly to acidic form. Twenty-four reactions in 9 patients were considered irritant reactions in this study. While all three concentrations of sodium valproate elicited 12 (50%) irritant reactions in this study, carbamazepine, phenobarbitone, phenytoin and lamotrigine produced 4 (16.7%), 3 (12.5%), 3 (12.5%) and 2 (8.4%) irritant reactions, respectively. However, reasons of irritant reactions from drugs other than sodium valproate remain conjectural and might have been from multiple patch test applied concurrently, testing just 4 wk after DRESS (in few cases), or due to constituents of the excipient of prescribable drug that may cause irritant reaction from low pH or positive reactions in already sensitized individuals that may be non-relevant[17].
Unfortunately, there is little consensus for interval between recovery and time of patch test and interval of 6 wk to 6 mo has been considered appropriate by most workers[4,9]. The patients who were patch tested within 4-9 mo of recovery in this study had positive drug patch test reactions while longer interval of 10-24 mo elicited no reactions reflecting an important limitation of drug patch testing.
Small number of patients and use of commercial drugs for patch testing with possible excipient induced irritant reactions are the main limitations. Timing of one month or ≥ 6 mo after recovery for drug patch testing, patch test drug concentrations, or exposure time might have influenced some results. Late readings at D7 of patch test results were not performed.
In conclusion, drug patch testing appears useful tool to confirm drug imputability established on clinical grounds and cross-reactions in DRESS from anticonvulsants. Carbamazepine was the commonest drug causing positive patch test reactions. Cross-reactions are common among aromatic anticonvulsants and with structurally related lamotrigine while sodium valproate too has potential to cross-react increasing the risk of ACDRs necessitating prudent prescriptions.
Anticonvulsants, carbamazepine, phenytoin, phenobarbitone (aromatic group), lamotrigine and sodium valproate, are implicated in 20% of all adverse cutaneous reactions (ACDRs) and cross reactions among them are common. It is often difficult to identify the offending drug from temporal correlation/history alone since most patients will be on multiple medications and clinical picture is often not diagnostic. The re-challenge/provocation tests, intradermal tests or skin prick tests are time consuming and require expertise. Moreover, there are ethical concerns due to their ability to re-precipitate severe life-threatening adverse drug reaction such as SJS/TEN. Basophil degranulation/lymphocyte activation tests have limited availability, low sensitivity/specificity and may even be negative during acute stage. Radioallergosorbent test for drug specific IgE, histamine release test, and passive hemagglutination test with sensitivity/specificity nearly similar to skin tests have limited availability/applicability in routine clinical practice. The drug patch test, an in-vivo challenge test, in ACDRs is inexpensive, convenient and safe with reasonable certainty. This study evaluated utility of drug patch test for identification of culprit drug as well as cross reactions in patients with ACDRs from anticonvulsants.
Nearly 95% of adverse drug reactions are Type-A (augmented) reactions which are dose-dependent, predictable from primary and secondary drug pharmacology. Other, Type-B (Bizarre) reactions are idiosyncratic, unpredictable from known drug pharmacology, depend on patient-specific susceptibility factors and manifest varied clinical picture. These can be “non-immune mediated (drug intolerance)” due to inadequate or imperfect metabolic detoxification and present as hemolysis, bone marrow toxicity or neurotoxicity from toxic metabolites, or “pseudo-allergic” due to histamine, leukotrienes or other mediators released from direct basophil/mast cell de-granulation due to drugs like opiates, muscle relaxants or radio contrast media manifesting clinically as asthma, anaphylaxis, and urticaria/angioedema-like reactions. These are often indistinguishable from “true immunologically mediated” immediate (Type-I) hypersensitivity reactions. Depending upon immune effecter mechanisms involved the “true immunologically mediated” reactions has four main classes: (1) Type-I or IgE mediated (immediate or anaphylactic/urticaria type); (2) Type-II or complement mediated (cytotoxic); (3) Type-III or immune complex mediated (hypersensitivity vasculitis, serum sickness); and (4) Type-IV or T-cell (CD4 or CD8) mediated (tuberculin or contact dermatitis type) reactions. In Type-IV hypersensitivity reactions activated T-lymphocytes produce different patterns of cytokines and/or cytotoxic factors which are relevant for clinical patterns and drug patch testing: IFN-γ, TNF-α (Th1-Tc1cells) cause contact dermatitis/tuberculin reaction (type-IVa); IL-4, IL-13, IL-5, eosinophils (Th2 cells) cause maculopapular/exanthematous drug rash and eosinophilia with or without systemic involvement (DRESS) (type-IVb); perforin, granzyme-B, granulysin (cytotoxic T-cells) cause dermatitis, maculopapular drug rash, Stevens-Johnson syndrome (SJS), toxic epidermal necrosis (TEN) (type-IVc); and CXCL-8, GM-CSF, neutrophils (T-cells) cause acute generalized exanthematous pustulosis (type-IVd). Most positive drug patch test reactions will be elicited in these T-cells mediated ACDRs.
Many studies suggest that diagnosis of drug hypersensitivity by patch testing lacks clarity and standardized definitions of clinical and immunopathological processes. This has resulted in uncertainty that whether patch tests are used appropriately in T-cell-mediated ACDRs. Many studies have also used both skin prick and drug patch tests in different types of ACDRs without ascertaining the immune mechanism relevant to the clinical reaction or the tests used. Failure of carbamazepine to elicit positive patch test responses in some individuals despite T-cell mediated drug hypersensitivity confirmed by positive in-vitro T-cell responses also remains poorly understood. There seems no consensus for drug concentration for patch testing in patients with ACDRs. When a commercial form of the drug is used for patch testing it is usual to make up to a 30% by weight conc. of powdered tablet in white soft paraffin. However, there is also evidence that conc is not critical and in a series of patients with DRESS induced by carbamazepine there was no difference in the frequency or strength of positive patch test responses over a range of drug conc from 1%-20%. Thus, it may be better to use 10%, 20% and 30% conc. to avoid missing of true positive results. Usual recommendation is to test with 1%-10% of pure drug and 30% of commercial form. It is advisable to do pre test in healthy controls to avoid conc. high enough that may cause direct toxic, proinflammatory or irritant effects. Opinions also differ whether to use pure drug that is often difficult to procure or prescribable form for patch testing. The later have the advantage of easy availability and diagnosing “drug hypersensitivity” that is actually from the excipient only. There is also little consensus for interval between recovery from ACDRs and time of patch test. An interval of 6 wk to 6 mo has been considered appropriate by most workers. The choice of an appropriate vehicle for antigen preparation is also important. Similarly, late reading of patch test responses at day 7 may be required in some cases. Last but not the least, the role of genetic factors in drug metabolism, drug molecular weight and solubility, and skin barrier function and pathomechanism involved in each type of drug reaction and drug patch test also needs elucidation. More systematic studies and consensual approach in future studies will perhaps resolve some of these issues encouraging wider acceptance of this very safe and important diagnostic test in ACDRs.
The drug patch test works best for T-cell mediated ACDRs (exanthematous drug eruptions, acute generalized exanthematous pustulosis, DRESS, erythema multiforme major/SJS/TEN, fixed drug eruption and symmetrical drug-related intertriginous/flexural exanthem) particularly from aromatic anticonvulsants and some antibiotics but responses are inconsistent with many other drugs. Further, patch testing in SJS/TEN has low sensitivity. Testing with chemically/pharmacologically similar drugs may also help to identify cross-reactivity for these patients for prudent prescriptions.
Erythema multiforme major (EM-major), Stevens-Johnson syndrome (SJS), and Toxic epidermal necrolysis (TEN): This spectral drug hypersensitivity reaction is cytotoxic T-cell mediated and perforin, granzyme-B, granulysin are involved in its immunopathogenesis. Erythema multiforme major is characterized by well defined flat round target-like skin lesions with central necrotic macule/bulla with zone of pallor and outer erytematous rim usually accompanied by mucosal involvement, fever and prostration. It has tendency to become confluent, severe and extensive eventuating to SJS/TEN. SJS is characterized by macular erythema, blisters, and detachment of skin involving 10% body surface area and mucosal ulcerations. Atypical targetoid spots and bullae can occur beyond large sheets of necrotic skin. It may eventuate through SJS-TEN overlap (skin detachment between > 10% and 30% body surface area) to more severe TEN (skin detachment > 30% body surface area) with widespread skin/mucosal detachment and multi-organ involvement often ending fatally; Exanthemataous drug eruptions, Acute generalized exanthematous pustulosis (AGEP), and Drug rash with eosinophilia and systemic symptoms (DRESS): This spectral drug hypersensitivity reaction is T-cell mediated and Th2 cell cytokines (IL-4/-13, IL-5) and eosinophils are involved in its immunopathogenesis. Generalized exanthematous drug eruptions is characterized by moderate fever, facial and peri-orbital edema, and prominently pruritic maculopapular rash that occur during first 2 wk of drug intake (may appear even 10-14 d after stopping it). This can eventuate to AGEP (characterized by non-follicular pustular lesions over face and trunk) or progress to DRESS if multi-organ involvement, lymphadenopathy and eosinophilia develop.
This is an interesting study regarding patch testing and cross sensitivity of adverse cutaneous drug reactions due to anticonvulsants. In general, the methodology of the study is appropriate, the results are significant, and the findings are clinically relevant and scientifically interesting.
Manuscript source: Invited manuscript
Specialty type: Clinical medicine
Country of origin: India
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hu SCS, Kaliyadan F, Ni Y, Vasconcellos C S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Vatve M, Sharma VK, Sawhney I, Kumar B. Evaluation of patch test in identification of causative agent in drug rashes due to antiepileptics. Indian J Dermatol Venereol Leprol. 2000;66:132-135. [PubMed] |
| 2. | Lin YT, Chang YC, Hui RC, Yang CH, Ho HC, Hung SI, Chung WH. A patch testing and cross-sensitivity study of carbamazepine-induced severe cutaneous adverse drug reactions. J Eur Acad Dermatol Venereol. 2013;27:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Romano A, Viola M, Gaeta F, Rumi G, Maggioletti M. Patch testing in non-immediate drug eruptions. Allergy Asthma Clin Immunol. 2008;4:66-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Mahajan VK, Handa S. Patch testing in cutaneous adverse drug reactions: methodology, interpretation, and clinical relevance. Indian J Dermatol Venereol Leprol. 2013;79:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Friedmann PS, Ardern-Jones M. Patch testing in drug allergy. Curr Opin Allergy Clin Immunol. 2010;10:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Wilkinson DS, Fregert S, Magnusson B, Bandmann HJ, Calnan CD, Cronin E, Hjorth N, Maibach HJ, Malalten KE, Meneghini CL. Terminology of contact dermatitis. Acta Derm Venereol. 1970;50:287-292. [PubMed] |
| 7. | Barbaud A. Drug patch testing in systemic cutaneous drug allergy. Toxicology. 2005;209:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Santiago F, Gonçalo M, Vieira R, Coelho S, Figueiredo A. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Dermatitis. 2010;62:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Barbaud A, Gonçalo M, Bruynzeel D, Bircher A; European Society of Contact Dermatitis. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001;45:321-328. [PubMed] |
| 10. | Barbaud A. [Drug patch tests in the investigation of cutaneous adverse drug reactions]. Ann Dermatol Venereol. 2009;136:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Romano A, Pettinato R, Andriolo M, Viola M, Guéant-Rodriguez RM, Valluzzi RL, Romano C, Elia M, Ventura MT, Guéant JL. Hypersensitivity to aromatic anticonvulsants: in vivo and in vitro cross-reactivity studies. Curr Pharm Des. 2006;12:3373-3381. [PubMed] |
| 12. | Balachandran C, Shenoi SD, Sarkar D, Ravikumar BC. Patch tests in adverse cutaneous drug reaction. Indian J Dermatol Venereol Leprol. 2002;68:13-15. [PubMed] |
| 13. | Hirsch LJ, Arif H, Nahm EA, Buchsbaum R, Resor SR, Bazil CW. Cross-sensitivity of skin rashes with antiepileptic drug use. Neurology. 2008;71:1527-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Barbaud A, Collet E, Milpied B, Assier H, Staumont D, Avenel-Audran M, Grange A, Amarger S, Girardin P, Guinnepain MT. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 269] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 15. | Schaub N, Bircher AJ. Severe hypersensitivity syndrome to lamotrigine confirmed by lymphocyte stimulation in vitro. Allergy. 2000;55:191-193. [PubMed] |
| 16. | Barbaud A, Trechot P, Reichert-Penetrat S, Commun N, Schmutz JL. Relevance of skin tests with drugs in investigating cutaneous adverse drug reactions. Contact Dermatitis. 2001;45:265-268. [PubMed] |
| 17. | Gonçalo M, Bruynzeel D. Patch testing in adverse drug reactions. Contact Dermatitis. 5th edn. Berlin Heidelberg: Springer 2011; 475-491. |