Peer-review started: August 26, 2016
First decision: November 29, 2016
Revised: January 6, 2017
Accepted: January 16, 2017
Article in press: January 18, 2017
Published online: March 26, 2017
Processing time: 210 Days and 8.1 Hours
Autoantibodies can help clinicians to allow early detection of autoimmune diseases and their clinical manifestations, to determine effective monitoring of prognosis and the treatment response. From this point, they have a high impact in rheumatic disease management. When used carefully they allow rapid diagnosis and appropriate treatment. However, as they may be present in healthy population they may cause confusion for interpreting the situation. False positive test results may lead to wrong treatment and unnecessary anxiety for patients. Autoantibody positivity alone does not make a diagnosis. Similarly, the absence of autoantibodies alone does not exclude diagnosis. The success of the test is closely related to sensitivity, specificity and likelihood ratios. So, interpretation of these is very important for a proper laboratory evaluation. In conclusion, in spite of the remarkable advances in science and technology, a deeply investigated anamnesis and comprehensive physical examination still continue to be the best diagnostic method. The most correct approach is that clinicians apply laboratory tests to confirm or exclude preliminary diagnosis based on anamnesis and physical examination. This review will discuss these issues.
Core tip: Serological and proteomic biomarkers are useful in confirming clinically suspected preliminary diagnosis, monitoring the treatment response and prognosis of autoimmune diseases. Tests for acute phase proteins, rheumatoid factor, anti-citrullinated peptide antibodies and antinuclear antibodies, may support the diagnoses of rheumatic diseases. But these biomarkers should be used beside a careful anamnesis and detailed physical examination. Improper using of these tests may cause false-positive results and unnecessary harmful treatments. The sensitivity, specificity and likelihood ratios of the test must be known. If the test is highly specific, the diagnosis can be confirmed in case of positivity and if it is highly sensitive, the possible diagnosis can be excluded in case of negativity.
- Citation: Birtane M, Yavuz S, Taştekin N. Laboratory evaluation in rheumatic diseases. World J Methodol 2017; 7(1): 1-8
- URL: https://www.wjgnet.com/2222-0682/full/v7/i1/1.htm
- DOI: https://dx.doi.org/10.5662/wjm.v7.i1.1
When the organism’s own immune system elements attack its own tissue or cells it is called autoimmunity, with the antibodies formed called autoantibodies and the diseases occurring called autoimmune diseases. Autoantibodies can be successfully used to confirm the preliminary diagnosis of autoimmune diseases, to determine prognosis, identify disease activity, and to monitor the response to treatment and medication side effects. From this aspect, they have important roles in the management of rheumatic diseases. When used carefully they allow rapid diagnosis and appropriate treatment. However, in some situations instead of helping the clinician to reach a conclusion, they may cause even more confusion. This is because some positive autoantibodies for many autoimmune diseases may be encountered in healthy population. False positive test results may lead to inappropriate treatment and unnecessary anxiety for patients. Autoantibody positivity alone does not make a diagnosis. Similarly, the absence of autoantibodies alone does not exclude diagnosis. The success of the test is closely related to sensitivity, specificity and likelihood ratios. As a result, in spite of the remarkable advances in science and technology, a deeply investigated anamnesis and comprehensive physical examination still continue to be the best diagnostic method. The most correct approach is that clinicians apply laboratory tests to confirm or exclude preliminary diagnosis based on anamnesis and physical examination. Also common rheumatic diseases like osteoarthritis, rheumatoid arthritis (RA) and psoriatic arthritis (PsA) may be diagnosed without laboratory tests.
In this review we examine serologic and proteomic biomarkers used for diagnosis and monitoring of rheumatologic diseases and common errors in daily practice. This article also reviews the use of inflammatory activity tests currently available in health care.
One of the characteristic features of rheumatologic diseases is inflammation. The inflammation response developing secondary to tissue damage eliminates pathogens, limits injury and allows tissue regeneration. All of these changes are connected with increases [complement, ceruloplasmin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), ferritin, haptoglobin, fibrinogen, alpha-1 antitrypsin and amyloid A] or decreases (albumin, transferrin, and transthyretin) of some certain proteins. The serum levels of these markers are combined with clinical information and used to assess disease activity and treatment response. However, none of these markers are unique to a disease. In addition to rheumatic diseases they may increase with infections and malignancy. The most common tests used by clinicians are ESR and CRP.
The increase in acute phase proteins, especially fibrinogen, occurs with an increase in ESR in plasma concentrations. The protein with the most aggregation effect of all plasma proteins is fibrinogen. This is followed by albumin and globulins[1]. ESR is observed vertical to gravity in sodium citrate blood after being left for 1 h in Westergren or Wintrobe tubes. ESR is stated in mm (mm/h)[2]. ESR may increase during the acute phase response to RA, polymyalgia rheumatica (PMR), systemic lupus erythematosus (SLE) and vasculitis. The sensitivity of this test is high; however the specificity is very low. In 10% of RA patients and 20% of PMR patients ESR levels may be within normal limits[3,4]. It may increase in situations without accompanying inflammation. Additionally errors in the measurement technique (delay in evaluation, tube not held vertical, room temperature) and physiological factors (male sex, age, pregnancy) may cause deviations from the normal levels[5]. As an expected increase happens in ESR with ageing, it is necessary to make a correction for age. The formula (age + 10)/2 is used for women, with the formula age/2 for men. For all of these reasons attempting to monitor inflammation with ESR may not work sometimes[6].
This name was given due to the ability of the protein to precipitate with pneumococcal C polysaccharide. It is synthesized in the liver during the acute phase response and serum levels may increase up to 1000 times[7]. The causes to increase ESR also increase CRP. However, the increase and return to normal levels of CRP is more rapid and is not affected by age and sex. It begins to increase within the first 4-6 h after inflammation, peaks at 2-3 d and has a half-life of nearly 18 h[8]. It has both pro-inflammatory and anti-inflammatory effects[9,10].
As a general rule, CRP levels are staged as follows: Normal < 0.2 mg/dL, indeterminate = 0.2 mg/dL - 1.0 mg/dL and inflammatory > 1 mg/dL[2]. While high levels may indicate bacterial infection (> 10 mg/dL), there may be a slight increase observed in situations such as obesity, diabetes, smoking, hypertension, physical inactivity, alcohol, chronic tiredness and depression. Additionally examples of other diseases where CRP is used for diagnosis and monitoring include myocardial infarction and atherosclerosis[5]. In conclusion CRP, which increases in many inflammatory and non-inflammatory situations, has high sensitivity and lower specificity like ESR.
RA: CRP levels may be used to distinguish RA from osteoarthritis. However in some types of osteoarthritis CRP levels may increase. Due to the previously mentioned properties, CRP is more sensitive compared to ESR in terms of showing variation in disease activity[11]. Additionally CRP is proportionally better correlated to treatment response and radiologic progression than ESR[12]. In the early period of the disease, high CRP levels lead to the consideration that a progressive and erosive disease is present and prognosis may be bad. However, CRP levels within normal limits do not mean that there is no disease progression. In 10% of RA cases with active disease, acute phase reaction (APR) levels may be in normal limits[5]. In clinical practice CRP and ESR are used in scores and indices measuring disease activity.
Ankylosing spondylitis and PsA: Due to increased CRP levels in only 50%-70% of active AS patients, there is no linear correlation between symptoms and disease activity in the APR. The highest CRP levels are measured in patients with peripheral arthritis and uveitis[13]. However, there is no correlation between severity of enthesitis and ESR[14]. The BASDAI score is slightly better correlated to CRP values compared to ESR[15]. For evaluation of treatment response, the sensitivity and specificity of CRP and ESR are low. As a result to increase efficiency it is recommended to use both tests together[13,16].
Some composit measures, such as BASDAI have had limitations for the measurement of disease activity because it is a subjective measure with fully patient oriented and have lacked validity. Thus the Assessment of Spondylo Arthritis International Society proposed to use CRP which is an objective determinant of inflammation and developed ASDAS with higher construct validity[17]. This was the first to combine patient reported and objective parameters to understand the severity of disease activity.
PMR: This disease characteristically has high ESR and CRP levels. They have very good negative predictive values. And in EULAR/ACR 2012 provisional classification criteria they have been proposed as diagnostic parameters[18]. However, up to 20% of patients may have ESR at normal levels[19]. There is a very strong correlation between ESR-CRP and corticotherapy response. However, it should not be forgotten that steroid dose should be regulated according to the patient’s clinical symptoms and not ESR and CRP levels[2]. The steroid use has been detailed in EULAR/ACR 2015 recommendations[20].
SLE: In spite of active disease and increased ESR, CRP levels are frequently normal or slightly increased[21]. Increased ESR values may be the first indicator of disease. CRP increases in the presence of severe infection, synovitis and serositis. Slightly high CRP may be a precursor of atherosclerosis[9,22].
Rheumatoid factor (RF) is a specific antibody formed against Fc section of immunoglobulins. Though every class of these antibodies have Ig structure, the most common is IgM structure[23]. The role of RF in RA is not fully known. However, it may play a role in antigen presentation and amplification of the humoral response[2]. In nearly 70% of RA patients it is positive and may be an indicator of worse prognosis. High RF levels may show aggressive joint disease, rheumatoid nodules and accompanying extra-articular involvement[24]. RF positivity alone is not sufficient for diagnosis. In the healthy population 15% may be positive at low titrations and this rate increases with age[25]. Additionally in other autoimmune rheumatologic diseases including Sjogren’s syndrome, SLE, cryoglobulinemia, pulmonary diseases such as interstitial fibrosis and silicosis and various infectious diseases, RF may be positive[25,26]. Nearly 30% of RA patients are seronegative and this rate may increase to 50% in early RA[27]. As a result, negative RA may not exclude diagnosis. Due to contradictory results, it cannot be used for monitoring treatment response and disease[28]. Due to all of these reasons, only in patients where RA is a strong possibility after anamnesis and physical examination should RF be requested.
In a large proportion of RA patients IgG antibodies developed against citrulline peptides are encountered. Many studies have determined that the target of these antibodies is a type of protein, filaggrin. These antibodies are post translationally altered or target citrullinated filaggrin. The posttranslational citrullination procedure includes deiminization of arginine in certain polypeptides and is catalyzed by the peptidylarginine deiminase (PAD) enzyme. The result of this biochemical process is that arginines transform to citrullines. These changes in the structure of citrullinated peptides make them a target for the IgG antibodies in RA[29]. The pioneer of these antibodies identified in 1964 was anti-perinuclear factor. In the intervening period many different antibodies have been described and all of these are given the collective common name anti-citrullinated peptide antibodies (ACPAs). Anti-perinuclear factor, anti-keratin antibody, anti-fliaggrin, anti-Sa and anti-cyclic citrullinated peptide (anti-CCP) are the primary members of this family[30]. As anti-CCP has higher specificity compared to RF, it is more commonly used for RA diagnosis and has taken its place in new classification criteria[31]. The first generation anti-CCP test (anti-CCP1) had 96% specificity and 53% sensitivity for RA. The second generation anti-CCP test (anti-CCP2) had specificity of 99% and sensitivity of 61.6% for early RA, 75.2% for late RA and 71.7% for all RA patients[30]. Thus a test with similar sensitivity as RF but with higher specificity was obtained[32].
Anti-CCP antibodies occur years before the development of clinical symptoms and RA patients are divided into two groups as ACPA positive and ACPA negative[33,34]. In the early stages of disease the groups show similar characteristics, but with time the ACPA positive group are observed to have more erosion and the disease progresses more severely[35]. Some environmental factors, especially smoking, increase the risk of ACPA development. ACPA positivity increases the risk of cardiac disease[36,37]. In a study, researchers found ACPA-mediated activation of platelets. They have suggested that ACPA-mediated platelet activation may lead to increased vascular permeability and erosive damage[38,39].
Anti-CCP test should be requested for patients clinically suspected of RA. If it is positive once, there is no need for repeat because anti-CCP antibody titrations are not correlated with disease activity. As a result, it cannot be used to monitor the disease[40].
Antibodies generally developing against DNA, RNA, histones, centromeres, nucleolus and other nucleoproteins in the cell nucleus, sometimes targeting organelles, other cytoplasmic structures and even cell membrane are called anti-nuclear antibodies. Clinically the most commonly used antigens are DNA and RNA protein complexes[41].
When these antibodies are identified in blood they may indicate an emerging rheumatic disease, they may be determinants to make diagnosis and may provide important information related to prognosis.
There has been a clear change in antibodies to nuclear antigen (ANA) measurement techniques since lupus erythematosus (LE) cell was identified in 1940 to the present day when immunoflourescent (IF) techniques are used. Together with the variationin laboratory methods, the performance of the ANA test has changed. With an increase in sensitivity of the test, the probability of observing “ANA-negative lupus” has decreased; however the ANA positivity in healthy individuals has increased. As a result the cut/off value for the test has increased from 1/40 to 1/80[42].
ANA may be measured in two ways. The first ANA measurement assesses all generic antibodies and is a specific antibody assay that may be specific for other diseases[43]. Generic ANA measurement may be completed with IF and ELISA methods. If ANA is positive, specific antibodies may be researched with automated methods. IF is the gold standard for ANA identification. For those with clinical suspicion it is significant if identified at high titrations. A study conducted on healthy people found that at 1/40 dilution 31.7% were ANA positive, while this value was 13.3% for 1/80 dilution, 5.0% for 1/160 dilution and 3.3% for 1/320 dilution[44]. As a result, high titrations are clinically more significant. However, at high titrations correlation with disease activity and severity is not possible[41]. So it is not correct to attempt to monitor disease activity with ANA values[2].
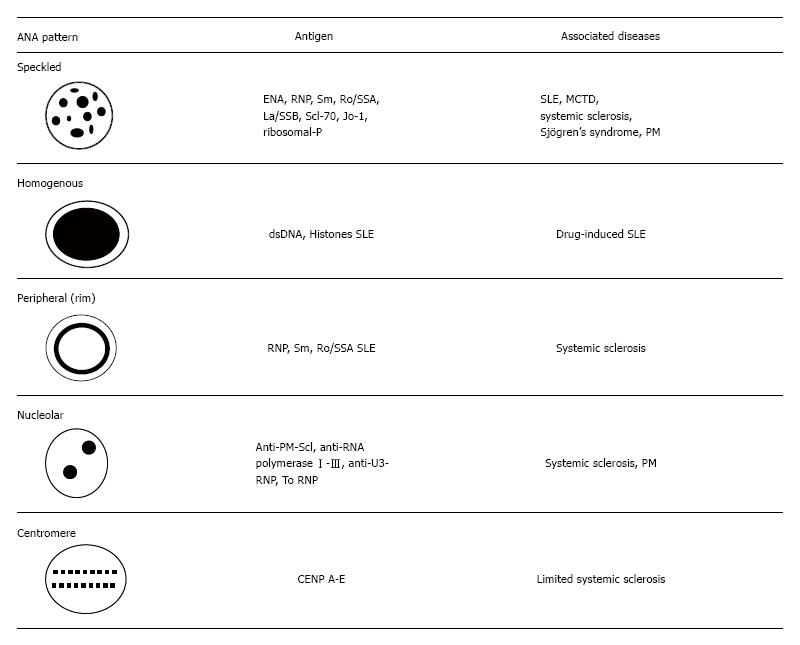
ANA staining patterns may provide an idea of specific disease by showing which specific antibodies entered a reaction with which region of the cell. These patterns are usually reported as either nuclear, centromere, or nucleolar. Homogenous, speckled, peripheral, and nucleolar staining patterns are more frequently encountered and have clinically important meanings. This is detailed in Figure 1[45]. However, it should not be forgotten that reporting of these staining patterns is closely related to the experience and competence of laboratory staff. To avoid this operator-dependent situation, automated tests have received attention and have been commonly used. These techniques are immunodiffusion, immunoprecipitation, radioimmunoassay, hemagglutination, enzyme immunoassay and enzyme-linked immunosorbent assay[2]. American College of Rheumatology points IF ANA as the gold standard for ANA testing because it still has more sensitivity than solid phase assays. Laboratories must indicate ANA testing method in their reports[46].
We know that two major types of antibodies exist in ANA, one including antibodies against DNA and histones which indicates SLE and drug-induced lupus erythematosus (DILE). The second group includes autoantibodies to extractable nuclear antigens. This group contains autoantibodies to Smith antigen (Sm) ribonucleoproteins (RNP), Ro/SSA or La/SSB, Scl-70, histidyl-tRNA synthetase (Jo-1), and PM1. Centromere protein (CENP)-B, topoisomerase-I (topo-I), RNA polymerase I-III (RNA-pol I-III), TM, MU, Mi-2, Ku and RA33 are also in this group and the number of new indicators are increasing day by day[45].
Basic statistics: The sensitivity of a test is the proportion of affected individuals with a positive test and the specificity is the proportion of unaffected individuals with a negative test. Tests with highest sensitivity or specificity have much potential to make differential diagnosis. If a test is highly specific, then positive results points the diagnosis in a high probability. Negatif reports of a highly sensitive test can almost exclude the diagnosis.
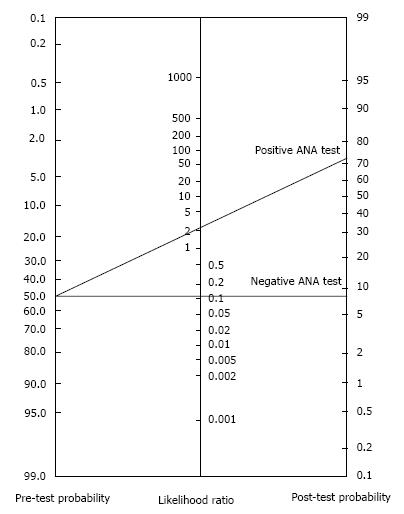
The likelihood ratio (LR) is one of the efficient ways to reach diagnostic accuracy taking using both sensitivity and specificity. A positive test with a positive LR for any disease indicates the multiplied probability of the diagnosis. A negative test with a negative LR for a disease shows the odds of the decreasing probability[47]. Taking a detailed history and performance of a carefull physical examination is very important to get the pretest probability of a RD. Then using this value, we can get the post test probability of a RD by processing the LR of a test by the help of LR normogram (Figure 2)[46].
An ANA test is not a routine test which is requested for any patient with a musculoskeletal symptom and must be used only if we suspect the existence of a RD. ANA test has a sensitivity of 93% for SLE and 85% for scleroderma. On the other hand specificity of ANA for the same diseases are much lower than sensitivity rates (SLE: 57%, scleroderma: 54%). So ANA negativity is an indicative finding to exclude SLE, however its positivity seems not to be so important to as the specificity is relatively lower. Similarly a negative ANA is more meaningful to rule out scleroderma while a positive report do not confirm diagnosis exactly although it supports[2,42].
For drug-induced SLE and mixed connective tissue disease (MCTD) ANA is a diagnostic criteria as the sensitivity is almost 100%[42]. The diseases with lower rates of ANA sensitivity are secondary Raynaud’s syndrome (64%), polymyositis/dermatomyositis (61%) and Sjögren’s syndrome (SS) (48%)[2,48,49]. ANA is useful in SS and idiopathic inflammatory myositis despite its relatively lower sensitivity for these diseases (40% and 70%). ANA is even worse in case of specificity with lower values[42].
For the diseases generally indicated by specific antibodies, contrary to generic ANA, specificity is more meaningful as they are extremely high unlike their sensitivity values. The most important of these antibodies are:
Anti-dsDNA antibodies: It is the diagnostic criteria of SLE (97.4% Specificity and 57.3% sensitivity, +LR: 16 and -LR: 0.49)[2,45].
Anti-Sm antibodies: Anti-Sm antibodies reveals mostly and only in SLE patients (sensitivity: 25%-30% and specificity: Very high)[2].
Anti-RNP antibodies: They can be shown in 30%-60% of SLE patients, however not specific enough. They have use in the diagnosis of MCTD. Anti-U1 RNP antibody is among the diagnostic criteria of MCTD[2].
Anti-histone antibodies: They are present in 95% of DILE patients and 50%-70% of those with SLE. A lot of patients revealing the antibodies are asymptomatic so, the positive sera does not always mean the disease exists[2].
Anti-chromatin (anti-nucleosome) antibody: Present in 50%-90% of SLE patients[50].
Anti Ro/SSA - anti La/SSB antibodies: They are often shown in SS and SLE patients and also are among the diagnostic criterion of SS[51]. And these antibodies may be encountered in SLE patients with negative ANA[2].
Anti-centromere antibodies: Three major centromere proteins exist: CENP-A, B, and C. The major target is CENP-B[52]. They have relation with limited cutaneous systemic sclerosis and the CREST syndrome[53]. The specificity in CREST syndrome is high, while sensitivity is lower. Anti-centromere antibodies can estimate the upcoming development of scleroderma in patients with Raynaud’s syndrome (+LR: 3.5). However, they are more discriminative for excluding CREST (-LR: 0.2).
Anti-Scl-70 antibodies: They are found in approximately 20%-40% of patients with systemic sclerosis. Their presence predicts pulmonary fibrosis, diffuse cutaneous involvement, and nephropathy. Although the sensitivity is low, specificity approaches 100%. It shown in patients with Raynaud’s syndrome, the diagnosis of scleroderma is highly probable as specificity is 98% and positive LR is 10. On the other hand the sensitivity is low (28%, negative LR is 0.7)[2].
Anti-nucleolar antibodies: The nucleolar IF pattern is very specific for scleroderma. Specific antibodies which form this pattern are anti-PM/Scl antibodies, anti-Th/To antibodies, anti-RNA polymerase I, anti-RNA polymerase III and anti-U3-RNP[54].
Other antibodies: The presence of anti-neutrophil cytoplasmic antibodies (ANCAs) is supportive in the diagnosis of vasculitic conditions. These antibodies demonstrate two forms of IF patterns: Cytoplasmic (cANCA) and perinuclear (pANCA). The cANCA has a high sensitivity and a low specificity (90%, 50% respectively) in Wegener’s granulomatosis[2]. The pANCA form is shown frequently in pauci-immune glomerulonephritis, microscopic polyangiitis, Churg-Strauss syndrome, and sometimes in Wegener’s granulomatosis[47,55].
The myositis-specific antibodies are not often used for the identification of inflammatory myopathies; but, they can provide evidence about the manifestations of the disease once the diagnosis is made[2]. In 25%-30% of the patients with dermatomyositis or polymyositis the Jo-1 ANA can be detected[56]. Anti-Mi2 antibodies are also seen in dermatomyositis and are a predictor of good prognosis. Anti-SRP is related with heart disease and is responsiveness to treatment. Anti-MAS is identified in rhabdomyolysis[2].
In conclusion, laboratory tests are useful for informing us for an emerging RD. They help diagnose a specific disease and can predict prognosis. An experienced clinician must first evaluate the patient with clinical approaches and then request meaningful laboratory tests as complementary diagnostic tools. Interpretation of laboratory tests necessitates to know the diagnostic power of each test.
Manuscript source: Invited manuscript
Specialty type: Clinical medicine
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: La Montagna G, Ni Y, Song J, Wang F S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Bochen K, Krasowska A, Milaniuk S, Kulczynska M, Prystupa A, Dzida G. Erythrocyte sedimentation rate-an old marker with new applications. JPCCR. 2011;5:50-55. |
| 2. | Colglazier CL, Sutej PG. Laboratory testing in the rheumatic diseases: a practical review. South Med J. 2005;98:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 3. | Amos RS, Crockson RA, Crockson AP, Walsh L, McConkey B. Rheumatoid arthritis: C-reactive protein and erythrocyte sedimentation rate during initial treatment. Br Med J. 1978;1:1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | González-Gay MA, Rodríguez-Valverde V, Blanco R, Fernández-Sueiro JL, Armona J, Figueroa M, Martínez-Taboada VM. Polymyalgia rheumatica without significantly increased erythrocyte sedimentation rate. A more benign syndrome. Arch Intern Med. 1997;157:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Neto R, Salles N, Carvalho JFd. The use of inflammatory laboratory tests in rheumatology. Rev Bras Reumatol. 2009;49:413-430. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Sox HC, Liang MH. The erythrocyte sedimentation rate. Guidelines for rational use. Ann Intern Med. 1986;104:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 188] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Gruys E, Toussaint MJ, Niewold TA, Koopmans SJ. Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B. 2005;6:1045-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 682] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 8. | Morley JJ, Kushner I. Serum C-reactive protein levels in disease. Ann N Y Acad Sci. 1982;389:406-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 263] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4404] [Cited by in RCA: 4612] [Article Influence: 177.4] [Reference Citation Analysis (0)] |
| 10. | Verma S, Li SH, Badiwala MV, Weisel RD, Fedak PW, Li RK, Dhillon B, Mickle DA. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation. 2002;105:1890-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 441] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 11. | Matsui T, Kuga Y, Kaneko A, Nishino J, Eto Y, Chiba N, Yasuda M, Saisho K, Shimada K, Tohma S. Disease Activity Score 28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients in Japan. Ann Rheum Dis. 2007;66:1221-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Emery P, Gabay C, Kraan M, Gomez-Reino J. Evidence-based review of biologic markers as indicators of disease progression and remission in rheumatoid arthritis. Rheumatol Int. 2007;27:793-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Ozgocmen S, Godekmerdan A, Ozkurt-Zengin F. Acute-phase response, clinical measures and disease activity in ankylosing spondylitis. Joint Bone Spine. 2007;74:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Kaya T, Bal S, Gunaydin R. Relationship between the severity of enthesitis and clinical and laboratory parameters in patients with ankylosing spondylitis. Rheumatol Int. 2007;27:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Yildirim K, Erdal A, Karatay S, Melikoğlu MA, Uğur M, Senel K. Relationship between some acute phase reactants and the Bath Ankylosing Spondylitis Disease Activity Index in patients with ankylosing spondylitis. South Med J. 2004;97:350-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Romero-Sánchez C, Robinson WH, Tomooka BH, Londoño J, Valle-Oñate R, Huang F, Deng X, Zhang L, Yang C, Yu DT. Identification of acute phase reactants and cytokines useful for monitoring infliximab therapy in ankylosing spondylitis. Clin Rheumatol. 2008;27:1429-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | van der Heijde D, Lie E, Kvien TK, Sieper J, Van den Bosch F, Listing J, Braun J, Landewé R. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68:1811-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 475] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 18. | Dasgupta B, Cimmino MA, Kremers HM, Schmidt WA, Schirmer M, Salvarani C, Bachta A, Dejaco C, Duftner C, Jensen HS. 2012 Provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheum. 2012;64:943-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 19. | Helfgott SM, Kieval RI. Polymyalgia rheumatica in patients with a normal erythrocyte sedimentation rate. Arthritis Rheum. 1996;39:304-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Dejaco C, Singh YP, Perel P, Hutchings A, Camellino D, Mackie S, Abril A, Bachta A, Balint P, Barraclough K. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheumatol. 2015;67:2569-2580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Gaitonde S, Samols D, Kushner I. C-reactive protein and systemic lupus erythematosus. Arthritis Rheum. 2008;59:1814-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | de Carvalho JF, Hanaoka B, Szyper-Kravitz M, Shoenfeld Y. C-Reactive protein and its implications in systemic lupus erythematosus. Acta Reumatol Port. 2006;32:317-322. [PubMed] |
| 23. | Renaudineau Y, Jamin C, Saraux A, Youinou P. Rheumatoid factor on a daily basis. Autoimmunity. 2005;38:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Vittecoq O, Pouplin S, Krzanowska K, Jouen-Beades F, Menard JF, Gayet A, Daragon A, Tron F, Le Loet X. Rheumatoid factor is the strongest predictor of radiological progression of rheumatoid arthritis in a three-year prospective study in community-recruited patients. Rheumatology (Oxford). 2003;42:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Castro C, Gourley M. Diagnostic testing and interpretation of tests for autoimmunity. J Allergy Clin Immunol. 2010;125:S238-S247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Wolfe F, Cathey MA, Roberts FK. The latex test revisited. Rheumatoid factor testing in 8,287 rheumatic disease patients. Arthritis Rheum. 1991;34:951-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Visser H. Early diagnosis of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2005;19:55-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Ateş A, Kinikli G, Turgay M, Akay G, Tokgöz G. Effects of rheumatoid factor isotypes on disease activity and severity in patients with rheumatoid arthritis: a comparative study. Clin Rheumatol. 2007;26:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Girbal-Neuhauser E, Durieux JJ, Arnaud M, Dalbon P, Sebbag M, Vincent C, Simon M, Senshu T, Masson-Bessière C, Jolivet-Reynaud C. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol. 1999;162:585-594. [PubMed] |
| 30. | van Venrooij WJ, van Beers JJ, Pruijn GJ. Anti-CCP antibodies: the past, the present and the future. Nat Rev Rheumatol. 2011;7:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 31. | Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6830] [Cited by in RCA: 6212] [Article Influence: 414.1] [Reference Citation Analysis (0)] |
| 32. | Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, Saigo K, Morinobu A, Koshiba M, Kuntz KM. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797-808. [PubMed] |
| 33. | Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1182] [Cited by in RCA: 1327] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 34. | van der Helm-van Mil AH, Huizinga TW. Advances in the genetics of rheumatoid arthritis point to subclassification into distinct disease subsets. Arthritis Res Ther. 2008;10:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Bos WH, Wolbink GJ, Boers M, Tijhuis GJ, de Vries N, van der Horst-Bruinsma IE, Tak PP, van de Stadt RJ, van der Laken CJ, Dijkmans BA. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: a prospective cohort study. Ann Rheum Dis. 2010;69:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Pedersen M, Jacobsen S, Klarlund M, Pedersen BV, Wiik A, Wohlfahrt J, Frisch M. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res Ther. 2006;8:R133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 259] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 37. | López-Longo FJ, Sánchez-Ramón S, Carreño L. The value of anti-cyclic citrullinated peptide antibodies in rheumatoid arthritis: do they imply new risk factors? Drug News Perspect. 2009;22:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 38. | Habets KL, Trouw LA, Levarht EW, Korporaal SJ, Habets PA, de Groot P, Huizinga TW, Toes RE. Anti-citrullinated protein antibodies contribute to platelet activation in rheumatoid arthritis. Arthritis Res Ther. 2015;17:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 39. | Habets KL, Huizinga TW, Toes RE. Platelets and autoimmunity. Eur J Clin Invest. 2013;43:746-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 40. | Aggarwal A. Role of autoantibody testing. Best Pract Res Clin Rheumatol. 2014;28:907-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Satoh M, Vázquez-Del Mercado M, Chan EK. Clinical interpretation of antinuclear antibody tests in systemic rheumatic diseases. Mod Rheumatol. 2009;19:219-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Kavanaugh A, Tomar R, Reveille J, Solomon DH, Homburger HA. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med. 2000;124:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Villalta D, Tozzoli R, Tonutti E, Bizzaro N. The laboratory approach to the diagnosis of autoimmune diseases: is it time to change? Autoimmun Rev. 2007;6:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, Gordon T, Hardin JA, Kalden JR, Lahita RG. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40:1601-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Kumar Y, Bhatia A, Minz RW. Antinuclear antibodies and their detection methods in diagnosis of connective tissue diseases: a journey revisited. Diagn Pathol. 2009;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis. 2010;69:1420-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 328] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 47. | McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 647] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 48. | American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Guidelines for immunologic laboratory testing in the rheumatic diseases: an introduction. Arthritis Rheum. 2002;47:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Birtane M. Diagnostic Role of Anti-Nuclear Antibodies in Rheumatic Diseases. Turk J Rheumatol. 2012;27:79-89. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Gómez-Puerta JA, Burlingame RW, Cervera R. Anti-chromatin (anti-nucleosome) antibodies. Lupus. 2006;15:408-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Bizzaro N, Tozzoli R, Shoenfeld Y. Are we at a stage to predict autoimmune rheumatic diseases? Arthritis Rheum. 2007;56:1736-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Nakano M, Ohuchi Y, Hasegawa H, Kuroda T, Ito S, Gejyo F. Clinical significance of anticentromere antibodies in patients with systemic lupus erythematosus. J Rheumatol. 2000;27:1403-1407. [PubMed] |
| 53. | Miyawaki S, Asanuma H, Nishiyama S, Yoshinaga Y. Clinical and serological heterogeneity in patients with anticentromere antibodies. J Rheumatol. 2005;32:1488-1494. [PubMed] |
| 54. | Reveille JD, Solomon DH. Evidence-based guidelines for the use of immunologic tests: anticentromere, Scl-70, and nucleolar antibodies. Arthritis Rheum. 2003;49:399-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 55. | Stone JH, Talor M, Stebbing J, Uhlfelder ML, Rose NR, Carson KA, Hellmann DB, Burek CL. Test characteristics of immunofluorescence and ELISA tests in 856 consecutive patients with possible ANCA-associated conditions. Arthritis Care Res. 2000;13:424-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Mammen AL. Autoimmune myopathies: autoantibodies, phenotypes and pathogenesis. Nat Rev Neurol. 2011;7:343-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |