Published online Dec 26, 2015. doi: 10.5662/wjm.v5.i4.196
Peer-review started: July 31, 2015
First decision: August 14, 2015
Revised: August 27, 2015
Accepted: October 12, 2015
Article in press: October 13, 2015
Published online: December 26, 2015
Processing time: 138 Days and 15.6 Hours
Although it is assumed that the combination of chemotherapy and radical surgery should be indicated in all newly diagnosed advanced-stage ovarian cancer patients, one of the main raised questions is how to select the best strategy of initial treatment in this group of patients, neoadjuvant chemotherapy followed by interval debulking surgery or primary debulking surgery followed by adjuvant chemotherapy. The selection criteria to offer one strategy over the other as well as a stepwise patient selection for initial treatment are described. Selecting the best strategy of treatment in newly diagnosed advanced stage ovarian cancer patients is a multifactorial and multidisciplinary decision. Several factors should be taken into consideration: (1) the disease factor, related to the extension and localization of the disease as well as tumor biology; (2) the patient factor, associated with patient age, poor performance status, and co-morbidities; and (3) institutional infrastructure factor, related to the lack of prolonged operative time, an appropriate surgical armamentarium, as well as well-equipped intensive care units with well-trained personnel.
Core tip: Selecting the best strategy of treatment in newly diagnosed advanced-stage ovarian cancer patients is a multifactorial and multidisciplinary decision. Surgeries performed by gynecologic oncologists with formal training in cytoreductive techniques at referral centers are crucial factors in obtaining better oncologic outcomes. However, other factors such as clinical status of the patients, the hospital’s infrastructure and equipment, as well as the tumor biology of each individual patient should also be taken into account before deciding on an initial strategy of treatment in women with advanced-stage ovarian cancer.
- Citation: Minig L, Zorrero C, Iserte PP, Poveda A. Selecting the best strategy of treatment in newly diagnosed advanced-stage ovarian cancer patients. World J Methodol 2015; 5(4): 196-202
- URL: https://www.wjgnet.com/2222-0682/full/v5/i4/196.htm
- DOI: https://dx.doi.org/10.5662/wjm.v5.i4.196
It is estimated that over 80% of women with ovarian cancer are diagnosed at advanced stages, when the disease is already extended in the abdominal cavity or beyond. Primary complete debulking surgery (PDS) followed by adjuvant chemotherapy is associated with the best oncological outcome and is considered, therefore, the standard of care[1]. Limitations, however, have been postulated with respect to this treatment strategy. First, patients with postoperative residual disease have no meaningful impact on overall survival (OS)[2-4]. Second, only in few cases is the complete primary cytoreduction rate acceptable, and only when the procedure is performed by experienced surgeons with extended formal training in cytoreductive techniques. Third, PDS is associated with a high incidence of postoperative complications[5,6].
Consequently, an alternative strategy based on neoadjuvant chemotherapy followed by interval debulking surgery (NACT-IDS) has been proposed[7]. Patients receive three to four courses of platinum-taxanes NACT and then, in the absence of progression disease, IDS is performed. The proposed advantages include a reduced risk of peri-operative morbidity, a higher rate of complete tumor resection, and a contention that deferring the initial attempt at surgical debulking does not compromise survival[8].
Nevertheless, there currently exist several controversies regarding the best strategy of treatment[9,10]. Although it is assumed that the combination of chemotherapy and radical surgery should be indicated in all newly diagnosed advanced-stage ovarian cancer patients, one of the main questions raised is how to select the best strategy for initial treatment in this group of patients, a topic that will be the focus of this review.
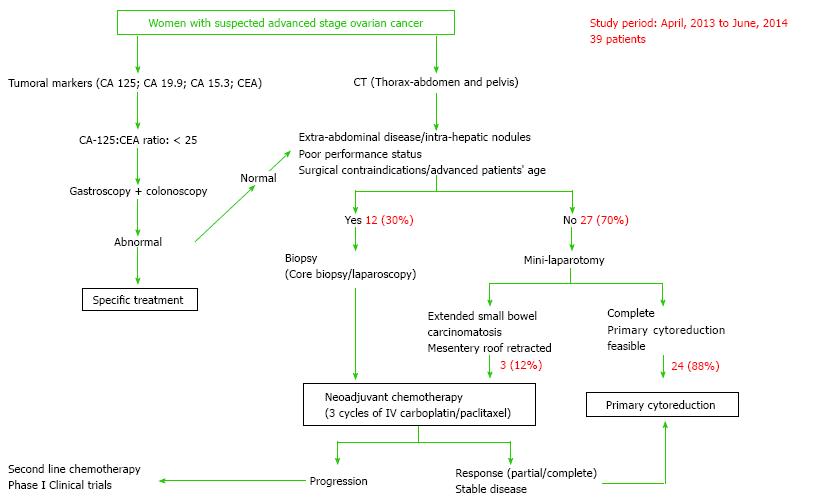
An algorithm of management for newly suspected or diagnosed advanced-stage ovarian cancer patients has been developed at the Instituto Valenciano de Oncologia (IVO), Valencia, Spain. Hence, patients are initially evaluated with computer tomography (CT) of thorax-abdomen and pelvis, plus tumor markers including CA-125, CA-19.9, and CEA.
Pre-operative tumor markers can also provide additional information to allow discrimination between an ovarian or extra-ovarian origin of peritoneal carcinomatosis. In this case, if the CA-125 (UI/mL)/CEA (ng/mL) ratio is < 25, mammography and endoscopy are mandatory to exclude primary breast, gastric, or colon cancer[11] (Figure 1). The main aim of the pre-operative CT scan is to localize intra-abdominal disease at non-resectable structures such as liver hilium, celiac trunk, superior mesenteric artery, supra-renal lymph node metastases, and intrahepatic metastases; as well as to identify extra-abdominal disease.
In cases of non-resectable disease at CT scan or in patients older than 75, with poor status or surgical contraindications, a core biopsy or a diagnostic laparoscopy with biopsy is performed to obtain a tissue sample. If the final diagnosis confirms an epithelial ovarian carcinoma, NACT is indicated. According to our series, a total of 30% of patients would receive NACT at this point. In the absence of the previously mentioned criteria, a mini-laparotomy is performed to rule out any extended small bowel carcinomatosis and mesentery roof retraction. These findings are present in 10% of cases, a tumor biopsy is performed and NACT is started in these patients a few days later (Figure 1). The remaining 90% of our patients undergo complete PDS, following the surgical step-wise description detailed elsewhere[12].
Patients undergoing NACT receive 3 courses of carboplatin/paclitaxel intravenously every 3 wk, and are then evaluated by using clinical examination, CA-125 and CT scan. Women with a partial response undergo IDS in an attempt to complete tumor resection. Second-line chemotherapy or inclusion in clinical trials is proposed to women with stable disease or progression to NACT.
Complete resection of all macroscopic disease at primary debulking surgery is the single most important independent prognostic factor in women with advanced ovarian cancer[2,4-6,13-15]. The definition of “optimal” cytoreduction has been the subject of debate for decades. Therefore, optimal residual disease, such as that measuring 1-2 cm in diameter, has been traditionally considered[16]. However, a significant improvement in survival after complete tumor resection at the time of primary surgical cytoreduction has been observed[2-4]. Thus, according to the last Gynecological Cancer Inter Group (GCIG) consensus conference, cytoreduction should be classified as “complete”, without residual disease”; or “incomplete”, if residual disease is left at the end of the surgery. In addition, the consensus established that the aim of surgical debulking should be to obtain a complete tumor resection[1]. The final decision as to whether or not to perform a tumor debulking depends on the surgeon’s training and confidence in the majority of her or his operations on patients[17]. A great body of evidence suggests that patients operated on by gynecologic oncologists with formal training in cytoreductive techniques are more likely to undergo a complete cytoreduction in comparison to those treated by general gynecologists or general surgeons, with significantly better oncologic outcome[18,19]. Therefore, the main worldwide consensus[1,12] states that gynecologic oncologists should make the decision regarding whether to start treatment with PDS or NACT in patients with advanced-stage ovarian cancer.
The decision regarding the initial strategy of treatment, based on NACT or PDS, in women with advanced ovarian cancer has been largely debated. A large meta-analysis involving 6885 patients in 53 studies after PDS demonstrated that each 10% increase in cytoreduction correlated with a 5.5% increase in median survival time. Patients with 75% or greater maximal cytoreductive efforts had a median survival of 37 mo compared with a 23 mo for patients with 25% or less maximal effort[14]. On the other hand, Bristow et al[20] studied 835 patients in 22 cohorts with advanced ovarian cancer who received NACT. The study showed a median OS of 24.5 mo, range 10-42 mo. Despite the fact that this rate was shorter than what was obtained after PDS, this comparison should be taken with caution given that a bias upon the selection of patients to receive NACT might exist. On the basis regarding the extension of the disease or performance status, within patients who underwent NACT might have a worse prognosis.
The results of the first randomized controlled trial (RCT) in patients with ovarian cancer FIGO stage IIIC-IV of the European Organization for Treatment and Research (EORTC) and the National Cancer Institute of Canada, comparing PDS vs NACT-IDS, were published in 2010[11]. The authors randomized 718 patients with stage IIIC-IV ovarian cancer, excluding IIIC by node metastases only. Surgical time was 180 min in both arms and the median OS and progression-free survival was 30 mo and 12 mo, respectively, in the two arms. One of the main criticisms of the EORTC trial was, however, that NACT was compared to a weak PDS arm. The study was conducted in non-selected centers, achieving a median OS of 30 mo, with a complete cytoreduction rate in the PDS arm of 21%. A similar RCT performed in 87 hospitals in the United Kingdom and New Zealand found the same results[21]. It is interesting to note that these rates are markedly inferior to the outcomes reported by other international multicenter studies[2-4]. When surgery is performed at referral oncologic centers by well-trained surgeons, the complete primary cytoreduction rate can be over 40%-50%, with a median 5-year OS of 50-60 mo[2,5,6].
Despite the fact that the radicalness of the surgery is the most important factor to obtain a better oncologic outcome, other issues should also be taken into account. These factors include: (1) the time since the first visit of the patient to the commencement of the treatment; (2) the time from the hospital discharge after primary or interval debulking surgery to the initiation of adjuvant chemotherapy. Median time should not exceed 40 d, a longer period of time is related with a high incidence of postoperative complications; (3) the number of cycles in relation to neoadjuvant chemotherapy, should not be more than four; and (4) the time from the end of neoadjuvant chemotherapy to interval debulking surgery.
Whether tumor biology or maximal up-front cytoreduction surgery is the most important determinant for better outcomes is being largely debated. At same time that some studies found cytoreduction removal of visible disease had a more significant impact on survival than the extent of the disease before surgery[22], other studies observed opposite results[23]. Thus, other factors should be taken into consideration in an attempt to classify ovarian tumors as with “bad” or “good” prognosis. Recent molecular studies, using microarray analysis, have associated overall survival with gene expression profiles in ovarian cancer patients after up-front surgical treatment[24]. Although future large analysis should confirm these findings, it should be expected that molecular studies using genes and proteomic pattern might represent the tools to select patients for the best individual treatment rather than to generalize one strategy over the other for all women with ovarian cancer.
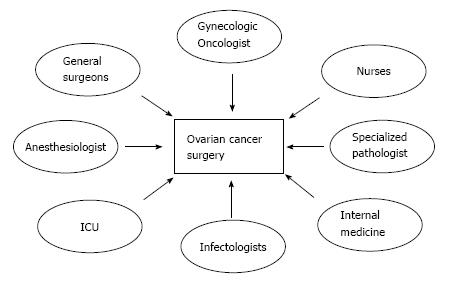
However, beyond the surgeon factor previously described, the cytoreduction rate is also associated to other variables such as: (1) the disease factor, related to the extension and localization of the disease as well as tumor biology; (2) the patient factor, associated with patient age, poor performance status, and co-morbidities; and (3) institutional infrastructure factor, related to the lack of prolonged operative time, an appropriate surgical armamentarium, as well as well-equipped Intensive Care Units with well-trained personnel[12,25,26] (Table 1). It is crucial, moreover, to establish an adequate ovarian cancer multidisciplinary surgical team that includes other specialists such as general surgeons, anesthesiologists, infectologists, etc. (Figure 2).
| Factor | Characteristic |
| Surgeon | Adequate skills and training in cytoreductive techniques |
| Disease | Extension and localization of the disease |
| Tumor biology | |
| Patient | Advanced age |
| Comorbidities | |
| Poor performance status | |
| Institutional infrastructure | Ovarian cancer multidisciplinary surgical team |
| Availability of prolonged operative time | |
| Appropriate surgical armamentarium | |
| Well-trained ICU personnel | |
| Well-equipped ICU capability |
CT scan is recommended as the most appropriate imaging test prior to treatment planning in women with a suspected advanced stage ovarian cancer[27]. However, limitations with CT scan have been associated with its inability to accurately predict extensive serosal and mesenteric disease[28], and as it was previously described, these anatomical localizations are major limits to obtaining a complete cytoreduction. In fact, several models were developed to predict suboptimal cytoreduction by using CT scan parameters, but with very poor outcome[28-30].
Clinical studies have also evaluated the role of positron emission tomography (PET) and PET/CT as part of pre-operative evaluation in women with advanced-stage ovarian cancer[31]. However, based on the available literature, there is still no evidence that PET or PET/CT works better than CT in detecting the extension of primary ovarian cancer[31].
Diffusion-weighted magnetic resonance imaging (DW-MRI) is another tool under investigation used to predict resectability in women with advanced-stage ovarian cancer. The evidence for using the DW-MRI in improving detection of the true extent of the disease seems promising. The utility of DW-MRI in predicting intra-abdominal spreading in women with ovarian cancer has recently been evaluated in some investigations[32-34]. A recent study of 32 patients with ovarian cancer found the main gains of using DW-MRI were the detection of bowel serosal and mesenteric disease, with an accuracy for detection of peritoneal disease of 91% on DW-MRI compared with 75% on CT and 71% on FDG-PET/CT[34]. The results of this technique do appear to be promising for improving the detection of small volume-diffuse peritoneal disease. This encouraged data from a small number of studies; however, it should be prospectively evaluated and validated with a larger sample of patients to establish stronger conclusions in this regard.
Additional clinical factors can help surgeons to identify high-risk patients with postoperative complications and mortality after primary cytoreductive surgery. Two studies tried to correlate clinical factors with increased risk for postoperative morbidity after primary cytoreduction[35,36]. The studies observed for those aged over 75 together with either FIGO stage III or IV and coincident comorbidity[35]; or aged over 75 combined with serum albumin < 3 g/dL or ASA score of at least 3 and high initial tumor burden (FIGO IV or high volume FIGO IIIC)[36] identifies a subgroup of 7%-12% of patients with advanced ovarian cancer where upfront debulking surgery is associated with unacceptably high rates of morbidity and peri-operative mortality.
The majority of women receive either NACT or PDS based upon tumor extension and on estimated tumor resectability[17]. As has been previously detailed, there is no current imaging tool that can predict complete cytoreduction in women with advanced-stage ovarian cancer. Therefore, a direct laparoscopic or laparotomic assessment of the abdominal cavity is sometimes needed.
A pre-treatment laparoscopic score to predict tumor resectability was developed at a referral Italian cancer center[37]. This model established a predictive index value (PIV) with punctuation between 0-2 if tumors were present or not in specific areas of the pelvis and the abdomen. A score of 2 corresponded when the parameters were present, and score of 0 when they were absent. The study found that a predictive index value ≥ 8 resulted in a predictive probability of cytoreduction to less than 1 cm of zero (specificity of 100%), thus, avoiding unnecessary laparotomies. The PIV of the laparoscopic evaluation was then validated at 4 Italian Satellite Centers[38] and, more recently, the prognostic value of the laparoscopy-based-score was also established[39]. However, despite the fact that this strategy seems to be promising, some open questions still need to be clarified before its implementation into clinical practice: (1) the definition of each item is subjective, including terms such as “Unresectable massive peritoneal involvement plus milliary pattern of distribution” or “Obvious neoplastic involvement of the gastric wall”; (2) the oncologic impact of the missed assessment of the retroperitoneum is unknown; and (3) the model does not take into consideration clinical factors such as age, performance status or comorbidity. There are currently three ongoing trials which will probably answer some of these questions[40].
By using our algorithm, the evaluation of complete resectability is performed by a periumbilical longitudinal 10-cm mini-laparotomy instead of laparoscopy. By this approach, a surgeon’s hand can be introduced into the abdominal cavity to carefully determine the extension of the disease on the liver surface, abdominal wall, hilium of the spleen and pancreatic tail, as well as the anterior stomach surface. In addition, this maneuver allows palpation of the most critical area of unresectability, such as liver hilium, celiac trunk, the mesenterium and the small bowel surface. This is a 40-min intervention with very low morbidity, allowing patients to start NACT 10-15 d later.
At our center, if complete tumor resectability is possible at the time of mini-laparotomy, patients undergo an immediate xipho-pubic midline incision with full exposure of the abomino-pelvic organs in order to establish the true extent of the disease. In this sense, before starting the removal of the disease, a stepwise systematic evaluation of the abdominal cavity is performed in order to avoid the so-called “point of no return” with unnecessary patient morbidity[12]. This standardized strategy has been well described previously[12], and includes two points of stop-or-go decisions. Initially, the ligamentum falciforme is resected, and the peritoneum of the paracolic gutters and the omentum are dissected from the transverse colon. Then, the lesser sac is opened allowing the evaluation of the pancreas, the truncus coeliacus, the liver, and the hepatoduodenal ligament with portal vein, hepatic artery and ductus choledochus. If a non-resectable tumor is present, surgery is stopped. If not, the second point of decision is the evaluation of the radix mesenterii and the small bowel surface by dissecting the adhesions and separating the small bowel from the colon and the greater omentum[12].
Surgical training plays a crucial role in treating women with advanced-stage ovarian cancer[18]. Given the complexity of surgical procedures in obtaining a complete primary cytoreduction, as well as its positive impact on OS, not surprisingly, many studies from several countries have shown better OS when ovarian cancer patients were initially operated by a gynecologic oncologist rather than general gynecologist[41-43] or general surgeon[44].
Several authors have proposed the centralization of care of ovarian cancer[14,18,42,45] as an approach for improving the quality of care and outcomes. The main demonstrated benefits include better optimal cytoreduction rate[42,45], better chemotherapeutic administration rate and schemes[44,45], and better overall quality of treatment; therefore, improving the patient’s quality of life. Thus, in comparison with unspecialized hospitals, patients who receive care at specialized centers may prolong their OS by almost a year[19,45]. Nevertheless, despite these clear advantages and according to population-based studies, fewer than 40% of patients with ovarian cancer have access to a specialized center in developed countries[43,44]. More recently, a study-population performed in California, United States, demonstrated that only 4% of women with advanced-stage ovarian cancer were operated on by high- volume physicians at high-volume teaching hospitals[19].
Selecting the best strategy for treatment in newly diagnosed advanced-stage ovarian cancer patients is a multifactorial and multidisciplinary decision. Surgeries performed by gynecologic oncologists with formal training in cytoreductive techniques at referral centers are crucial factors in obtaining better oncologic outcomes. However, other factors such as clinical status of the patients, hospital infrastructure and equipment, as well as tumor biology of each individual patient should also be taken into account before deciding on an initial strategy of treatment in women with advanced-stage ovarian cancer.
P- Reviewer: Hutz RJ, Peng Y, Wan TTH S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Stuart GC, Kitchener H, Bacon M, duBois A, Friedlander M, Ledermann J, Marth C, Thigpen T, Trimble E; participants of 4th Ovarian Cancer Consensus Conference (OCCC); Gynecologic Cancer Intergroup. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer. 2011;21:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 2. | du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009;115:1234-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1121] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 3. | Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, Colombo N, Fowler JM, Argenta PA, De Geest K. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419-1425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 574] [Cited by in RCA: 516] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 4. | Winter WE, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F, McGuire WP. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621-3627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 661] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 5. | Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, Sonoda Y, Levine DA, Hensley M, Barakat RR. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 447] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 6. | Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Surgical treatment of diaphragm disease correlates with improved survival in optimally debulked advanced stage ovarian cancer. Gynecol Oncol. 2006;100:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Vergote I, De Wever I, Tjalma W, Van Gramberen M, Decloedt J, van Dam P. Neoadjuvant chemotherapy or primary debulking surgery in advanced ovarian carcinoma: a retrospective analysis of 285 patients. Gynecol Oncol. 1998;71:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 252] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Huober J, Meyer A, Wagner U, Wallwiener D. The role of neoadjuvant chemotherapy and interval laparotomy in advanced ovarian cancer. J Cancer Res Clin Oncol. 2002;128:153-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Vergote I, Tropé CG, Amant F, Ehlen T, Reed NS, Casado A. Neoadjuvant chemotherapy is the better treatment option in some patients with stage IIIc to IV ovarian cancer. J Clin Oncol. 2011;29:4076-4078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | du Bois A, Marth C, Pfisterer J, Harter P, Hilpert F, Zeimet AG, Sehouli J. Neoadjuvant chemotherapy cannot be regarded as adequate routine therapy strategy of advanced ovarian cancer. Int J Gynecol Cancer. 2012;22:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ, Panici PB. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1666] [Cited by in RCA: 1763] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 12. | Vergote I, du Bois A, Amant F, Heitz F, Leunen K, Harter P. Neoadjuvant chemotherapy in advanced ovarian cancer: On what do we agree and disagree? Gynecol Oncol. 2013;128:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Zivanovic O, Eisenhauer EL, Zhou Q, Iasonos A, Sabbatini P, Sonoda Y, Abu-Rustum NR, Barakat RR, Chi DS. The impact of bulky upper abdominal disease cephalad to the greater omentum on surgical outcome for stage IIIC epithelial ovarian, fallopian tube, and primary peritoneal cancer. Gynecol Oncol. 2008;108:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 702] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 15. | Polterauer S, Vergote I, Concin N, Braicu I, Chekerov R, Mahner S, Woelber L, Cadron I, Van Gorp T, Zeillinger R. Prognostic value of residual tumor size in patients with epithelial ovarian cancer FIGO stages IIA-IV: analysis of the OVCAD data. Int J Gynecol Cancer. 2012;22:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Hoskins WJ, McGuire WP, Brady MF, Homesley HD, Creasman WT, Berman M, Ball H, Berek JS. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974-979; discussion 979-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 493] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 17. | Chi DS, Schwartz PE. Cytoreduction vs. neoadjuvant chemotherapy for ovarian cancer. Gynecol Oncol. 2008;111:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Vernooij F, Heintz P, Witteveen E, van der Graaf Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: a systematic review. Gynecol Oncol. 2007;105:801-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 186] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Bristow RE, Chang J, Ziogas A, Randall LM, Anton-Culver H. High-volume ovarian cancer care: survival impact and disparities in access for advanced-stage disease. Gynecol Oncol. 2014;132:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 2006;103:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 275] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 21. | Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, Luesley D, Perren T, Bannoo S, Mascarenhas M. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 945] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 22. | Luyckx M, Leblanc E, Filleron T, Morice P, Darai E, Classe JM, Ferron G, Stoeckle E, Pomel C, Vinet B. Maximal cytoreduction in patients with FIGO stage IIIC to stage IV ovarian, fallopian, and peritoneal cancer in day-to-day practice: a Retrospective French Multicentric Study. Int J Gynecol Cancer. 2012;22:1337-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Crawford SC, Vasey PA, Paul J, Hay A, Davis JA, Kaye SB. Does aggressive surgery only benefit patients with less advanced ovarian cancer? Results from an international comparison within the SCOTROC-1 Trial. J Clin Oncol. 2005;23:8802-8811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Yang JY, Yoshihara K, Tanaka K, Hatae M, Masuzaki H, Itamochi H; Cancer Genome Atlas (TCGA) Research Network, Takano M, Ushijima K, Tanyi JL, Coukos G, Lu Y, Mills GB, Verhaak RG. Predicting time to ovarian carcinoma recurrence using protein markers. J Clin Invest. 2013;123:3740-3750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Salani R, Bristow RE. Surgical management of epithelial ovarian cancer. Clin Obstet Gynecol. 2012;55:75-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Brand AH. Ovarian cancer debulking surgery: a survey of practice in Australia and New Zealand. Int J Gynecol Cancer. 2011;21:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Redman C, Duffy S, Bromham N, Francis K; Guideline Development Group. Recognition and initial management of ovarian cancer: summary of NICE guidance. BMJ. 2011;342:d2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Nelson RC, Chezmar JL, Hoel MJ, Buck DR, Sugarbaker PH. Peritoneal carcinomatosis: preoperative CT with intraperitoneal contrast material. Radiology. 1992;182:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Bristow RE, Duska LR, Lambrou NC, Fishman EK, O’Neill MJ, Trimble EL, Montz FJ. A model for predicting surgical outcome in patients with advanced ovarian carcinoma using computed tomography. Cancer. 2000;89:1532-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Dowdy SC, Mullany SA, Brandt KR, Huppert BJ, Cliby WA. The utility of computed tomography scans in predicting suboptimal cytoreductive surgery in women with advanced ovarian carcinoma. Cancer. 2004;101:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Hynninen J, Kemppainen J, Lavonius M, Virtanen J, Matomäki J, Oksa S, Carpén O, Grénman S, Seppänen M, Auranen A. A prospective comparison of integrated FDG-PET/contrast-enhanced CT and contrast-enhanced CT for pretreatment imaging of advanced epithelial ovarian cancer. Gynecol Oncol. 2013;131:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Low RN, Barone RM. Combined diffusion-weighted and gadolinium-enhanced MRI can accurately predict the peritoneal cancer index preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol. 2012;19:1394-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Espada M, Garcia-Flores JR, Jimenez M, Alvarez-Moreno E, De Haro M, Gonzalez-Cortijo L, Hernandez-Cortes G, Martinez-Vega V, Sainz De La Cuesta R. Diffusion-weighted magnetic resonance imaging evaluation of intra-abdominal sites of implants to predict likelihood of suboptimal cytoreductive surgery in patients with ovarian carcinoma. Eur Radiol. 2013;23:2636-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Michielsen K, Vergote I, Op de Beeck K, Amant F, Leunen K, Moerman P, Deroose C, Souverijns G, Dymarkowski S, De Keyzer F. Whole-body MRI with diffusion-weighted sequence for staging of patients with suspected ovarian cancer: a clinical feasibility study in comparison to CT and FDG-PET/CT. Eur Radiol. 2014;24:889-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 35. | Thrall MM, Goff BA, Symons RG, Flum DR, Gray HJ. Thirty-day mortality after primary cytoreductive surgery for advanced ovarian cancer in the elderly. Obstet Gynecol. 2011;118:537-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Aletti GD, Eisenhauer EL, Santillan A, Axtell A, Aletti G, Holschneider C, Chi DS, Bristow RE, Cliby WA. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol Oncol. 2011;120:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 37. | Fagotti A, Ferrandina G, Fanfani F, Ercoli A, Lorusso D, Rossi M, Scambia G. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Ann Surg Oncol. 2006;13:1156-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 272] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 38. | Fagotti A, Vizzielli G, De Iaco P, Surico D, Buda A, Mandato VD, Petruzzelli F, Ghezzi F, Garzarelli S, Mereu L. A multicentric trial (Olympia-MITO 13) on the accuracy of laparoscopy to assess peritoneal spread in ovarian cancer. Am J Obstet Gynecol. 2013;209:462.e1-462.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Fagotti A, Vizzielli G, Fanfani F, Costantini B, Ferrandina G, Gallotta V, Gueli Alletti S, Tortorella L, Scambia G. Introduction of staging laparoscopy in the management of advanced epithelial ovarian, tubal and peritoneal cancer: impact on prognosis in a single institution experience. Gynecol Oncol. 2013;131:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Rutten MJ, Gaarenstroom KN, Van Gorp T, van Meurs HS, Arts HJ, Bossuyt PM, Ter Brugge HG, Hermans RH, Opmeer BC, Pijnenborg JM. Laparoscopy to predict the result of primary cytoreductive surgery in advanced ovarian cancer patients (LapOvCa-trial): a multicentre randomized controlled study. BMC Cancer. 2012;12:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Engelen MJ, Kos HE, Willemse PH, Aalders JG, de Vries EG, Schaapveld M, Otter R, van der Zee AG. Surgery by consultant gynecologic oncologists improves survival in patients with ovarian carcinoma. Cancer. 2006;106:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 42. | Paulsen T, Kjaerheim K, Kaern J, Tretli S, Tropé C. Improved short-term survival for advanced ovarian, tubal, and peritoneal cancer patients operated at teaching hospitals. Int J Gynecol Cancer. 2006;16 Suppl 1:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Junor EJ, Hole DJ, McNulty L, Mason M, Young J. Specialist gynaecologists and survival outcome in ovarian cancer: a Scottish national study of 1866 patients. Br J Obstet Gynaecol. 1999;106:1130-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 177] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 44. | Earle CC, Schrag D, Neville BA, Yabroff KR, Topor M, Fahey A, Trimble EL, Bodurka DC, Bristow RE, Carney M. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006;98:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 313] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 45. | Tingulstad S, Skjeldestad FE, Hagen B. The effect of centralization of primary surgery on survival in ovarian cancer patients. Obstet Gynecol. 2003;102:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |