Published online Dec 26, 2013. doi: 10.5662/wjm.v3.i4.65
Revised: November 6, 2013
Accepted: December 12, 2013
Published online: December 26, 2013
Bone marrow cells are capable of differentiation into liver cells. Therefore, transplantation of bone marrow cells has considerable potential as a future therapy for regeneration of damaged liver tissue. Autologous bone marrow infusion therapy has been applied to patients with liver cirrhosis, and improvement of liver function parameters has been demonstrated. In this review, we summarize clinical trials of regenerative therapy using bone marrow cells for advanced liver diseases including cirrhosis, as well as topics pertaining to basic in vitro or in vivo approaches in order to outline the essentials of this novel treatment modality.
Core tip: Bone marrow cells, which include multipotent progenitor cells, are capable of differentiation into liver cells. Autologous bone marrow infusion therapy has been applied to cirrhotic patients, and improvement of liver function parameters has been demonstrated. Although the efficacy of this treatment modality needs to be evaluated in more detail in a large number of patients, regenerative therapy using bone marrow cells for advanced liver diseases has considerable potential.
- Citation: Saito T, Tomita K, Haga H, Okumoto K, Ueno Y. Bone marrow cell-based regenerative therapy for liver cirrhosis. World J Methodol 2013; 3(4): 65-69
- URL: https://www.wjgnet.com/2222-0682/full/v3/i4/65.htm
- DOI: https://dx.doi.org/10.5662/wjm.v3.i4.65
Bone marrow cells (BMCs) are capable of differentiating into liver cells[1-4] because they include stem cells known as multipotent adult progenitor cells[5,6]. These cells have been shown to produce albumin when cultured with hepatocyte growth factor (HGF)[7] and various liver-specific proteins, including albumin, when cultured with mature hepatocytes[8]. Using cells obtained with a negatively selective magnetic cell separation system for efficient sorting of rat BMCs enriched with stem cells, we have shown that BMCs differentiate into cells expressing liver-specific genes when cultured with mature hepatocytes or HGF[9]. As there is now much evidence indicating that BMCs can differentiate into cells resembling liver cells in vitro[6-11], the characteristics of such BMCs are of great interest in the context of liver-regenerative medicine[12-14].
Liver cirrhosis is the end stage of chronic liver disease, and is associated with many serious systemic complications resulting from both liver failure and portal hypertension. This condition has a poor prognosis and is difficult to treat. Therefore, development of an effective liver-regenerative therapy for liver cirrhosis is an urgent priority. Liver transplantation is the only curative remedy for cirrhotic patients, but is associated with many problems such as donor shortage, surgical complications, rejection and high cost. As an alternative approach, regenerative cell therapy using stem cells is now attracting attention. Multipotent stem cells present in bone marrow are a particularly promising candidate for this purpose. In this review, we summarize clinical trials of liver-regenerative therapy using BMCs for advanced liver diseases including cirrhosis, as well as topics pertaining to basic in vitro or in vivo approaches in order to outline the essentials of this novel treatment modality.
Although BMCs can show liver cell lineage differentiation in vitro, an understanding of the dynamics of transplanted BMCs in vivo is essential for the development of BMC-based regenerative therapy. In this context, two important issues need to be clarified: (1) How do transplanted BMCs migrate to and engraft in the liver? and (2) Is there a relationship between the degree of liver damage and the extent of migration of transplanted cells? A previous study using model rats with carbon tetrachloride (CCl4)-induced liver injury has demonstrated that transplanted BMCs derived from transgenic rats expressing green fluorescent protein[15] in the spleen migrated to and remained in the periportal area of the recipient’s damaged liver[16]. These transplanted cells expressed liver cell markers such as alpha-fetoprotein as well as Notch signaling markers for stem cells, suggesting that the BMCs retained in the recipient liver possess the potential to differentiate into liver cells.
Migration of transplanted BMCs to the liver after injection into the spleen has been compared in two models of liver injury induced by administration of CCl4 and 2-acetylaminofluorene (2-AAF)[17], respectively, focusing particularly on differences in levels of liver mRNA for growth factors such as HGF and fibroblast growth factor (FGF), which have been shown to be responsible for efficient liver cell lineage differentiation of BMCs[9,18,19]. Interestingly, transplanted BMCs were found to engraft into CCl4-induced injured liver characterized by submassive hepatic necrosis and induction of high levels of HGF and FGF, but not into liver damaged by 2-AAF[20]. A higher degree of HGF induction is characteristic of more severe liver damage[21,22]. These findings suggest that transplanted BMCs migrate more effectively to a liver with greater damage, and that this transplantation approach would be clinically promising for treatment of advanced liver diseases. However, further studies are needed to clarify the factors produced by both BMCs and hepatocytes that contribute to better differentiation of BMCs into liver cells in vivo, thus improving the effectiveness of BMC transplantation.
The degree of liver function and fibrosis, as well as survival rate, have been shown to improve significantly after BMC transplantation in animal models of severe liver injury[23,24]. With regard to the mechanisms of liver regeneration resulting from BMC transplantation, many of the physiological and regenerative roles of transplanted BMCs remain unclear. However, it can be said with certainty that humoral factors produced in the liver during the regenerative process after BMC transplantation have a crucial role in both improvement of liver fibrosis and liver cell lineage differentiation of stem cells originating from BMCs and hepatic epithelial stem cells.
Improvement of liver fibrosis results from fibrolysis through the proteolytic action of BMC-induced factors. In this context, matrix metalloproteinase (MMP) activity is particularly noteworthy[25]. Sakaida et al[23] showed that BMC transplantation ameliorated liver fibrosis in the CCl4-induced liver-injury model, and that the fibrolytic change was attributable to MMP-9 secreted by BMCs that had migrated to fibrotic areas of the liver.
The liver cell lineage differentiation of BMCs occurs through the cooperative action of a variety of growth factors such as HGF or FGF induced in the injured liver[11,20,26]. Such differentiation may be accompanied by early elevation of the apolipoprotein A1 level in serum and liver[27]. Administration of FGF2 in combination with BMC transplantation synergistically ameliorates liver fibrosis in models of liver injury induced by CCl4[28]. In addition, in severe liver injury where hepatocyte proliferation is strongly inhibited, hepatic stem cells such as oval cells are induced and show differentiation toward a liver cell lineage, thus leading to liver regeneration[29,30].
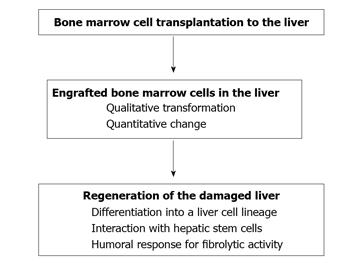
As BMC transplantation is successfully adaptable to cases of severe liver injury, it has been hypothesized that transplanted BMCs interact with hepatic epithelial stem cells and influence the subsequent proliferation and differentiation of stem cells. Studies of the interaction between BMCs and hepatic stem cells can provide new insight into the mechanisms of recovery from severe liver damage through liver regeneration after BMC transplantation. In this context, in vitro analysis using a system for co-culture of BMCs and an established epithelial hepatic stem cell line has been conducted. Haga et al[31] demonstrated that the expression of FGF2 mRNA was upregulated in BMCs co-cultured with hepatic stem cells, and that expression of mRNAs for both albumin and tyrosine aminotransferase, representative of mature hepatic cells, became detectable in hepatic stem cells after culture with FGF2 protein. Thus, BMCs stimulate both proliferation and differentiation of hepatic stem cells into the hepatocyte lineage, and FGF2 is one of the factors produced by interaction with BMCs, which stimulates such differentiation. Cross-talk between bone marrow stem cells and hepatic epithelial stem cells may underlie the process of liver regeneration, and this is an area of interest for future investigation. Figure 1 shows an overall representation of the putative action of transplanted BMCs in the regeneration of damaged liver.
BMC transplantation has received increasing attention as a promising therapy for advanced and severe liver diseases such as cirrhosis. Clinical trials of BMC administration to patients with advanced liver diseases have been performed, and improvement of liver function parameters such as the serum level of albumin, Child-Pugh score or Model for Endstage Liver Disease score have been reported[32-40]. Another study has shown that intraportal administration of autologous CD133+ BMCs and subsequent portal venous embolization of right liver segments resulted in a 2.5-fold increase in the mean proliferation rate of the left lateral segment, in comparison with controls not receiving BM transfusion[41]. These findings suggest that transplanted BMCs have a potential role in liver regeneration and proliferate in the recipient liver. Recently, autologous BMC transplantation - a technique named autologous BMC infusion (ABMi) therapy - has been applied to multi-center patients with liver cirrhosis due to hepatitis C[42], hepatitis B[43] and excess alcohol intake[44] using almost the same protocol, and a series of studies have demonstrated improvement of the serum albumin level, leading to improvement of the Child-Pugh score.
Although BMC administration for advanced liver diseases including cirrhosis is an attractive strategy in the field of cell therapy for liver regeneration, many concerns need to be addressed[45-47]. As in vitro and in vivo experiments have clearly shown, BMCs induce fibrolysis and show hepatocyte differentiation, and they may interact with hepatic epithelial stem cells to aid their differentiation into the hepatocyte lineage. However, it is still unclear how infused BMCs work to improve liver function in humans. A clinical trial of ABMi for patients with cirrhosis demonstrated that the number of AFP-positive cells increased significantly in the liver relative to the situation before ABMi[42]. In addition, ABMi appeared to induce hepatocyte proliferation in the liver, as expression of proliferating cell nuclear antigen, a marker of hepatocyte proliferation, was significantly increased after ABMi in comparison with the pretreatment situation. Although these findings suggest that transplanted BMCs have a potential role in liver regeneration and proliferate in the recipient liver, it remains unknown whether fully functional hepatocytes are induced by ABMi. The characteristics of stem cells present among BMCs that show hepatocyte differentiation require further elucidation.
The factors that determine the difference between effectiveness and non-effectiveness of ABMi are unclear. Collateral circulation resulting from the portal vein disorganization that characterizes liver cirrhosis may affect the flow and effective migration of infused BMCs to the liver, and thus migration of infused cells to the liver may partly depend on the portal venous pressure. In addition, the expression levels of cellular adhesion molecules associated with the attachment of infused cells to liver tissue may vary a great deal among patients. The long-term effectiveness of this therapy in terms of survival rate has not been demonstrated. These issues should be evaluated by a randomized controlled trial involving a large number of patients. Additionally, other issues that impact the efficacy of this therapy, i.e., the long-term culture conditions optimal for stocking BMCs for repeated infusion, the optimal cell population to employ, the optimal number of cells to infuse, the effectiveness of repeated infusion and the optimal route for cell delivery need to be investigated further.
In conclusion, regenerative therapy using BMCs for advanced liver diseases including cirrhosis has considerable potential. Further studies are needed to develop a better method of BMC transplantation that can contribute to improvement of liver function and to clarify the long-term effectiveness of this therapy.
P- Reviewers: Akyuz U, Bayraktar Y, Invernizzi P S- Editor: Gou SX L- Editor: A E- Editor: Lu YJ
| 1. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1795] [Cited by in RCA: 1669] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 2. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 749] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 3. | Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 696] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 4. | Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 851] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 5. | Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4340] [Cited by in RCA: 3918] [Article Influence: 170.3] [Reference Citation Analysis (0)] |
| 6. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [PubMed] |
| 7. | Oh SH, Miyazaki M, Kouchi H, Inoue Y, Sakaguchi M, Tsuji T, Shima N, Higashio K, Namba M. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem Biophys Res Commun. 2000;279:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Avital I, Inderbitzin D, Aoki T, Tyan DB, Cohen AH, Ferraresso C, Rozga J, Arnaout WS, Demetriou AA. Isolation, characterization, and transplantation of bone marrow-derived hepatocyte stem cells. Biochem Biophys Res Commun. 2001;288:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 175] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Okumoto K, Saito T, Hattori E, Ito JI, Adachi T, Takeda T, Sugahara K, Watanabe H, Saito K, Togashi H. Differentiation of bone marrow cells into cells that express liver-specific genes in vitro: implication of the Notch signals in differentiation. Biochem Biophys Res Commun. 2003;304:691-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Miyazaki M, Akiyama I, Sakaguchi M, Nakashima E, Okada M, Kataoka K, Huh NH. Improved conditions to induce hepatocytes from rat bone marrow cells in culture. Biochem Biophys Res Commun. 2002;298:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Okumoto K, Saito T, Hattori E, Ito JI, Suzuki A, Misawa K, Ishii R, Karasawa T, Haga H, Sanjo M. Differentiation of rat bone marrow cells cultured on artificial basement membrane containing extracellular matrix into a liver cell lineage. J Hepatol. 2005;43:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Mitaka T. Hepatic stem cells: from bone marrow cells to hepatocytes. Biochem Biophys Res Commun. 2001;281:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Faris RA, Konkin T, Halpert G. Liver stem cells: a potential source of hepatocytes for the treatment of human liver disease. Artif Organs. 2001;25:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Forbes S, Vig P, Poulsom R, Thomas H, Alison M. Hepatic stem cells. J Pathol. 2002;197:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Hakamata Y, Tahara K, Uchida H, Sakuma Y, Nakamura M, Kume A, Murakami T, Takahashi M, Takahashi R, Hirabayashi M. Green fluorescent protein-transgenic rat: a tool for organ transplantation research. Biochem Biophys Res Commun. 2001;286:779-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Okumoto K, Saito T, Hattori E, Ito JI, Suzuki A, Misawa K, Sanjyo M, Takeda T, Sugahara K, Saito K. Expression of Notch signalling markers in bone marrow cells that differentiate into a liver cell lineage in a rat transplant model. Hepatol Res. 2005;31:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Petersen BE, Zajac VF, Michalopoulos GK. Hepatic oval cell activation in response to injury following chemically induced periportal or pericentral damage in rats. Hepatology. 1998;27:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 162] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Sekhon SS, Tan X, Micsenyi A, Bowen WC, Monga SP. Fibroblast growth factor enriches the embryonic liver cultures for hepatic progenitors. Am J Pathol. 2004;164:2229-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Lange C, Bassler P, Lioznov MV, Bruns H, Kluth D, Zander AR, Fiegel HC. Hepatocytic gene expression in cultured rat mesenchymal stem cells. Transplant Proc. 2005;37:276-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Okumoto K, Saito T, Haga H, Hattori E, Ishii R, Karasawa T, Suzuki A, Misawa K, Sanjo M, Ito JI. Characteristics of rat bone marrow cells differentiated into a liver cell lineage and dynamics of the transplanted cells in the injured liver. J Gastroenterol. 2006;41:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Tsubouchi H, Kawakami S, Hirono S, Miyazaki H, Kimoto M, Arima T, Sekiyama K, Yoshiba M, Arakaki N, Daikuhara Y. Prediction of outcome in fulminant hepatic failure by serum human hepatocyte growth factor. Lancet. 1992;340:307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Maher JJ. Cell-specific expression of hepatocyte growth factor in liver. Upregulation in sinusoidal endothelial cells after carbon tetrachloride. J Clin Invest. 1993;91:2244-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 416] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 24. | Terai S, Sakaida I, Yamamoto N, Omori K, Watanabe T, Ohata S, Katada T, Miyamoto K, Shinoda K, Nishina H. An in vivo model for monitoring trans-differentiation of bone marrow cells into functional hepatocytes. J Biochem. 2003;134:551-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Haraguchi T, Tani K, Koga M, Oda Y, Itamoto K, Yamamoto N, Terai S, Sakaida I, Nakazawa H, Taura Y. Matrix metalloproteinases (MMPs) activity in cultured canine bone marrow stromal cells (BMSCs). J Vet Med Sci. 2012;74:633-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Ishikawa T, Terai S, Urata Y, Marumoto Y, Aoyama K, Sakaida I, Murata T, Nishina H, Shinoda K, Uchimura S. Fibroblast growth factor 2 facilitates the differentiation of transplanted bone marrow cells into hepatocytes. Cell Tissue Res. 2006;323:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Yokoyama Y, Terai S, Ishikawa T, Aoyama K, Urata Y, Marumoto Y, Nishina H, Nakamura K, Okita K, Sakaida I. Proteomic analysis of serum marker proteins in recipient mice with liver cirrhosis after bone marrow cell transplantation. Proteomics. 2006;6:2564-2570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Ishikawa T, Terai S, Urata Y, Marumoto Y, Aoyama K, Murata T, Mizunaga Y, Yamamoto N, Nishina H, Shinoda K. Administration of fibroblast growth factor 2 in combination with bone marrow transplantation synergistically improves carbon-tetrachloride-induced liver fibrosis in mice. Cell Tissue Res. 2007;327:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Shiota G, Kunisada T, Oyama K, Udagawa A, Nomi T, Tanaka K, Tsutsumi A, Isono M, Nakamura T, Hamada H. In vivo transfer of hepatocyte growth factor gene accelerates proliferation of hepatic oval cells in a 2-acetylaminofluorene/partial hepatectomy model in rats. FEBS Lett. 2000;470:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Hu Z, Evarts RP, Fujio K, Marsden ER, Thorgeirsson SS. Expression of hepatocyte growth factor and c-met genes during hepatic differentiation and liver development in the rat. Am J Pathol. 1993;142:1823-1830. [PubMed] |
| 31. | Haga H, Saito T, Okumoto K, Ugajin S, Sato C, Ishii R, Nishise Y, Ito J, Watanabe H, Saito K. Enhanced expression of fibroblast growth factor 2 in bone marrow cells and its potential role in the differentiation of hepatic epithelial stem-like cells into the hepatocyte lineage. Cell Tissue Res. 2011;343:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Lyra AC, Soares MB, da Silva LF, Fortes MF, Silva AG, Mota AC, Oliveira SA, Braga EL, de Carvalho WA, Genser B. Feasibility and safety of autologous bone marrow mononuclear cell transplantation in patients with advanced chronic liver disease. World J Gastroenterol. 2007;13:1067-1073. [PubMed] |
| 33. | Mohamadnejad M, Namiri M, Bagheri M, Hashemi SM, Ghanaati H, Zare Mehrjardi N, Kazemi Ashtiani S, Malekzadeh R, Baharvand H. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol. 2007;13:3359-3363. [PubMed] |
| 34. | Gordon MY, Levicar N, Pai M, Bachellier P, Dimarakis I, Al-Allaf F, M’Hamdi H, Thalji T, Welsh JP, Marley SB. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822-1830. [PubMed] |
| 35. | Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR, Levicar N, Tait P, Scott M, Marley SB, Jestice K. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | Han Y, Yan L, Han G, Zhou X, Hong L, Yin Z, Zhang X, Wang S, Wang J, Sun A. Controlled trials in hepatitis B virus-related decompensate liver cirrhosis: peripheral blood monocyte transplant versus granulocyte-colony-stimulating factor mobilization therapy. Cytotherapy. 2008;10:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 38. | Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, Baharvand H, Ghavamzadeh A, Malekzadeh R. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10:459-466. [PubMed] |
| 39. | Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, Telkabadi M, Atashi A, Honardoost M, Zali MR. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 40. | Amer ME, El-Sayed SZ, El-Kheir WA, Gabr H, Gomaa AA, El-Noomani N, Hegazy M. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011;23:936-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 41. | am Esch JS, Knoefel WT, Klein M, Ghodsizad A, Fuerst G, Poll LW, Piechaczek C, Burchardt ER, Feifel N, Stoldt V. Portal application of autologous CD133+ bone marrow cells to the liver: a novel concept to support hepatic regeneration. Stem Cells. 2005;23:463-470. [PubMed] |
| 42. | Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292-2298. [PubMed] |
| 43. | Kim JK, Park YN, Kim JS, Park MS, Paik YH, Seok JY, Chung YE, Kim HO, Kim KS, Ahn SH. Autologous bone marrow infusion activates the progenitor cell compartment in patients with advanced liver cirrhosis. Cell Transplant. 2010;19:1237-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Saito T, Okumoto K, Haga H, Nishise Y, Ishii R, Sato C, Watanabe H, Okada A, Ikeda M, Togashi H. Potential therapeutic application of intravenous autologous bone marrow infusion in patients with alcoholic liver cirrhosis. Stem Cells Dev. 2011;20:1503-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Kallis YN, Alison MR, Forbes SJ. Bone marrow stem cells and liver disease. Gut. 2007;56:716-724. [PubMed] |
| 46. | Lorenzini S, Andreone P. Stem cell therapy for human liver cirrhosis: a cautious analysis of the results. Stem Cells. 2007;25:2383-2384. [PubMed] |
| 47. | Terai S, Tanimoto H, Maeda M, Zaitsu J, Hisanaga T, Iwamoto T, Fujisawa K, Mizunaga Y, Matsumoto T, Urata Y. Timeline for development of autologous bone marrow infusion (ABMi) therapy and perspective for future stem cell therapy. J Gastroenterol. 2012;47:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |