Published online Dec 26, 2013. doi: 10.5662/wjm.v3.i4.39
Revised: December 4, 2013
Accepted: December 12, 2013
Published online: December 26, 2013
Genes can be transferred using viral or non-viral vectors. Non-viral methods that use plasmid DNA and short interference RNA (siRNA) have advantages, such as low immunogenicity and low likelihood of genomic integration in the host, when compared to viral methods. Non-viral methods have potential merit, but their gene transfer efficiency is not satisfactory. Therefore, new methods should be developed. Low-frequency ultrasound irradiation causes mechanical perturbation of the cell membrane, allowing the uptake of large molecules in the vicinity of the cavitation bubbles. The collapse of these bubbles generates small transient holes in the cell membrane and induces transient membrane permeabilization. This formation of small pores in the cell membrane using ultrasound allows the transfer of DNA/RNA into the cell. This phenomenon is known as sonoporation and is a gene delivery method that shows great promise as a potential new approach in gene therapy. Microbubbles lower the threshold of cavity formation. Complexes of therapeutic genes and microbubbles improve the transfer efficiency of genes. Diagnostic ultrasound is potentially a suitable sonoporator because it allows the real-time monitoring of irradiated fields.
Core tip: Ultrasound causes cavitation bubbles to form cell membrane pores through which DNA/RNA are transferred. This phenomenon is known as sonoporation. Microbubbles lower the threshold of cavity formation. Sonoporation is less toxic and not associated with tumorigenicity as compared with retroviral and adenoviral vectors. Sonoporation does not require surgical procedure and enhances gene transfer with lipofection. Current limitations of sonoporation are low efficiency of gene transfer and damage of target cells are The use of complexes with chemicals and diagnostic ultrasound are promising approaches to overcome these limitations.
- Citation: Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Sueishi M. Sonoporation: Gene transfer using ultrasound. World J Methodol 2013; 3(4): 39-44
- URL: https://www.wjgnet.com/2222-0682/full/v3/i4/39.htm
- DOI: https://dx.doi.org/10.5662/wjm.v3.i4.39
Gene therapy is a promising approach to treat diseases and is applicable to tissue engineering controlling differentiation of cells to form tissues[1,2]. Therapeutic genes are transferred using viral or non-viral vectors. Viral vectors are mainly retroviral vectors, adenoviral vectors and adeno-associated vectors. Retroviral vectors are the cause of tumorigenicity[3]. Adenoviral vectors provoke a severe systemic immune response[4]. Adeno-associated vectors do not cause major immune response, but they are not permissive to some types of cells[5]. On the other hand, plasmid DNA does not induce host immune responses because exogenous proteins such as viral capsid proteins are not produced[6]. Moreover, plasmid DNA rarely integrates with the host genome upon introduction into target cells and not associated with tumorigenicity[3,7]. Plasmid DNA is safe for gene transfer therapy, but its transfer efficiency is low. Methods should therefore be developed to improve transfection efficiency of plasmid DNA. Irradiation with low-output intensity ultrasound causes mechanical perturbation in the vicinity of cavitation bubbles. Collapse of the bubbles generates small transient holes in the cell membrane and induces cell membrane permeabilization. The membrane poration (cavitation) increases the efficiency of drug and gene delivery. This phenomenon, sonoporation, is a gene delivery technique that could potentially be used for gene therapy. Sonoporation, a method for targeted drug delivery and non-viral gene transfection, has new and advantageous possibilities. Sonoporation stimulates endocytosis of adno-associated virus and enhances efficiency of gene transfer[8,9]. However, no clinical trials using sonoporation have been reported to date because it is not yet a satisfactory technique for efficient and reliable gene transfer. In this chapter, we will review the potential application of sonoporation in gene therapy, with a focus on mircobubbles as a drug delivery agent. We will also discuss other non-viral delivery methods. Finally, we will outline the future direction of ultrasound-assisted gene delivery aiming at improvement of sonoporation and enhancement of gene expression for clinical applications.
In this section, we review non-viral gene delivery methods. Gene therapy requires the delivery of nucleic acid material to target tissues and its entry into target cells. Given that DNA and the cell membrane are both negatively charged, electrostatic forces result in the repulsion of DNA by the cell membrane. To overcome this limitation, a physical or chemical approach needs to be applied. Another restriction of gene transfer is the rapid degradation of DNA by nucleases in the plasma after systemic administration.
Electroporation is useful for gene transfer to primary cells. Primary cells are not introduced with genes with lipofection. Electroporation is the only method to introduce genes to cells, auch as normal human dermal fibroblasts[10]. Electroporation, in which high-voltage electrical currents create transient pores on the cell membranes, allows the transport of nucleic acid material into the cell[11]. Therapeutic genes are successfully transferred in vivo[12]. The electroporation method, however, has limitations such as short range of gene transfer, necessity of a surgical procedure, and tissue damage. Distance between electrodes normally requires 1 cm and is not suitable for a large area. Placement of internal electrodes requires a surgical procedure, and high voltage can damage tissue when applied. Therefore, less invasive methods are desirable.
Chemical non-viral vectors have been studied because they are generally considered safer than viral vectors. In addition to safety, liposomes can transfer larger genes with less toxicity and are relatively easy to prepare. Cationic lipids and polymers form complexes with negatively charged DNA. The complexes protect the DNA from nucleases and increase the effectiveness of transfection through the cell membrane. One major problem with lipofection is low efficiency of gene transfer. Transfection efficiency of lipofection is improved by condensing DNA with a double chain monovalent quaternary ammonium lipid[13]. Still genes are not introduced to target cells efficiently. More efficient methods have been waited.
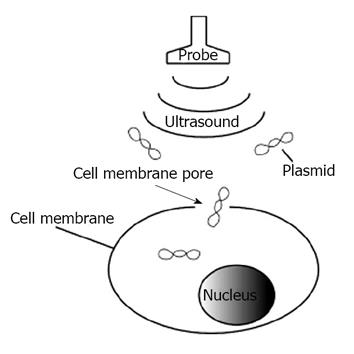
Sonoporation refers to the formation of small pores in cell membranes by using ultrasound for the transfer of nucleic acid materials (Figure 1). The biological effects of ultrasound are categorized as thermal and non-thermal. Non-thermal effects are composed of mechanical perturbation in the vicinity of bubbles. Cavitation bubbles cause membrane poration[14]. High speed camera images reveal that the cell membrane is fractionated, and cavitation bubbles are formed[15]. The cavitation bubbles induce cell death or permeability to allow the entry of a drug or genes into the cells. Sonoporation is similar to electroporation, wherein DNA is driven by an electrical force along the electric field. Sonoporation is mediated by passive diffusion. The transfer efficiency depends on ultrasound frequency and intensity[16]. The major advantages of sonoporation are its non-invasiveness and ability to transfer genes to internal organs without a surgical procedure[17]. Targeted gene transfer can be facilitated by ultrasound irradiation of selected tissues after systemic administration[18].
Drug delivery with ultrasound was first reported by Tachibana et al[16]. The delivery of insulin on the skin surface when exposed to ultrasound energy in the range of 3000-5000 Pa or 5000-8000 Pa at 48 kHz for 5 min decreased blood glucose levels to 22.4% of the control in 120 min. It was postulated that insulin absorption increased with ultrasound vibration after intradermal injection. This report is interesting because it demonstrates the potential of ultrasound as a method to improve absorption of therapeutic materials. With ultrasound, chemotherapeutic agents are more efficiently absorbed in the mouse xenograft model of cancer[19].
In vitro experiments are simplified models to investigate the mechanisms of sonoporation in vivo. Fechheimer et al[20] transferred plasmid DNA encoding the G418 resistance gene to cultured mouse fibroblasts by sonoporation. Colonies were observed after G418 was added to the media. This was the first report demonstrating that plasmid DNA can be transferred to cells in vitro by using ultrasound. Kim et al[21] also reported that rat joint cells can be successfully transfected with plasmid DNA. Plasmid DNA encoding green fluorescent protein (GFP) was injected as a reporter of expression into the left ventricle of mice percutaneously[22]. Mice were irradiated with transthoracic ultrasound at 1 MHz for 1 min. Histological examination showed GFP expression in the subendocardial myocardium. Intraventricular co-injection of siRNA and GFP exhibited reduced expression of GFP in the coronary artery. These data indicate that plasmid DNA and siRNA can be introduced into cells in vivo by sonoporation.
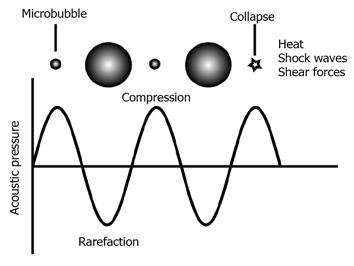
Biophysical effects of ultrasound include cavitation, radiation pressure, and microstreaming[23]. Cavitation refers to the growth and collapse of microbubbles. Radiation pressure is the force in the irradiation field. Microstreaming is the shear forces that exist near the microbubbles. Formation of cavitation increases with a rising ultrasound intensity while the frequency decreases[24]. Mechanical index (MI) is defined as PRP/√F0 (PRP: peak rarefactional pressure; √F0: transmission center frequency)[25]. MI should be lower than 1.9 for clinical use of ultrasound. Forsberg et al[26] reported that cavitation is unlikely to occur at an MI of less than 0.7. Cavitation has been well investigated with ultrasound contrast agents because microbubbles lower the threshold of cavitation[27]. Zhou et al[28] developed a device to observe the behavior of a single bubble near the cell membrane. The motion of a single bubble was monitored with a high-speed camera. Cell membrane disruption was assessed by monitoring the transmembrane current. This study showed that a single microbubble expands and contracts with ultrasound irradiation. When the bubble collapses, the cell membrane is ruptured and a pore is generated. Changes in membrane permeability are directly correlated with the formation of pores. Carugo et al[29] reported that a standing wave is involved in sonoporation in the absence of microbubbles.
The presence of microbubbles reduces the threshold of cavitation. Microbubble contrast agents are spheres filled with gas and stabilized with shells. Their size ranges between 1 and 10 μm[30]. They are small enough to circulate in blood vessels, but do not exit from the vessels. The contrast agents scatter ultrasound stronger than the surrounding blood and tissue. Thus, they are used as contrast agents for daily clinical practice. Tachibana et al[31] reported that albumin microbubbles (Albumex) accelerate thrombolysis by ultrasound. This was the first report that microbubbles improve the effects of ultrasound in tissues for purposes other than diagnostic imaging. Transfection of plasmid DNA into rat joint cells was improved in the presence of Albumex[21]. This report paved the way for the utilization of ultrasound contrast agents in sonoporation. Microbubbles expand and contract in response to compression and rarefaction of ultrasound (Figure 2). The microbubbles collapse at the high-pressure phase, emitting shock waves that perturb the cell membrane and increase permeability. Qiu et al[32] reported that pores on the cell membrane, generated by sonoporation with microbubbles, ranged from 100 nm to 1.25 μm in size. Their experiments used 1 MHz ultrasound at low acoustic pressures from 0.05 to 0.3 MPa. The pores generated with sonoporation were examined by scanning electron microscopy. The size of the pores enlarged with increased acoustic pressure or longer treatment. They concluded that the pores formed with shear stress. Liquid microjet, visualized with a high-speed camera in cultured cells exposed to single-shot short-pulsed ultrasound has been demonstrated[33]. Contrast agents are shells containing gas. Ultrasound scatters on the surface of contrast agents, and are visible as high echo on the display of diagnostic ultrasound. Physical and biological characteristics of contrast agents are basically the same as those of microbubbles. When a contrast agent (Levovist) was added to the media, the jet caused cell membrane damage. Interestingly, the cell membrane repair process suggests that the Ca2+-independent and Ca2+-trigger mechanisms are involved in rapid resealing. Positively charged microbubbles are more efficient in gene transfer than neutral ones[34]. It is hypothesized that positively charged microbubbles are more close to the cell membrane to enable more efficient gene transfer. Plasmids are introduced into cells in vitro and in vivo with sonoporation with microbubbles more efficiently than liposome that is commonly used for transfection[35,36].
To date, no clinical trials have been reported using sonoporation. There are several reasons for this, including low transfer efficiency and difficulty in monitoring irradiated fields using sonoporators.
Sonoporation harbors complicated aspects. Gene transfer efficiency is low with sonoporation. 37.5-50 μg of plasmid should be applied to a single rat with sonoporation in vivo[34]. This limitation is one of the reasons that sonoporation has not been applied clinically as mentioned above. Another limitation is cell damage caused with sonoporation. Miller et al[37] irradiated cells with 2.25 MHz continuous ultrasound for 1 min. When an ultrasound contrast agent (Definity, a perflutren lipid microsphere injectable suspension; Bristol Myers Squibb Medical Imaging, N. Billerica, MA) was added to the culture media, the irradiated cells underwent apoptosis. Enzymatic activity and mitochondrial membrane are changed after sonoporation[38]. Stresses to endoplasmic reticulum and mitochondria trigger apoptosis[39,40]. Sonoporation delays DNA synthesis to arrest cell cycle[41]. It should be noted that sonoporation itself may cause apoptosis when it is applied to cancer with therapeutic genes[37].
Microbubbles are expected to improve gene transfer at lower MI for the safety of patients; therefore, microbubbles may be suitable for sonoporation in human trials. Microbubbles are destroyed more efficiently with decreased pulse frequency and increased acoustic pressure and pulse length[42]. Care should be taken when applying in vitro data to in vivo studies because the effects of MI on microbubble destruction might not be similar[43]. Standing wave may be another option to improve sonoporation. Real-time monitoring of the irradiated field is desirable to introduce therapeutic genes to target tissues. Diagnostic ultrasound could therefore be used as a sonoporator.
Wang et al[27] analyzed the protection of plasmid DNA xenograft tumors in mouse hind limb after intravenous administration of a complex of plasmid DNA (luciferase) with cationic or neutral microbubbles. Luciferase activity was 3.8-fold stronger in complexes with cationic microbubbles than in those with neutral microbubbles. Factors influencing the transfection efficiency were analyzed, and the superiority of cationic microbubbles was more evident at lower doses of microbubbles and plasmid DNA[34]. This strategy appears suitable for this application because nucleic acids are negatively charged. It is expected that plasmid DNA or siRNA will interact and form better complexes with cationic microbubbles than with neutral microbubbles.
It is difficult to modify surfaces of microbubbles with functional molecules for targeting. On the other hand, liposomes are easy to modify for targeting. Suzuki et al[44] developed polyethylene glycol-modified liposomes containing perfluoropropane, a contrast agent for ultrasound imaging. These so-called “bubble liposomes”, significantly improved the transfection efficiency of plasmid DNA encoding luciferase into cultured cells. The luciferase reporter plasmid was injected into the femoral artery of mice. Two days after ultrasound irradiation, a signal was detected along the artery. These data suggest that the bubble liposome is a candidate for gene delivery both in vitro and in vivo. Bubble liposomes were transferred with the interleukin-12 gene in xenograft ovarian cancer mice, and the tumor sizes were reduced[45]. These animal experiments demonstrate that bubble liposomes may be applicable to clinical studies.
Sonoporators are typically used for sonoporation. One of the problems with sonoporators is that the irradiation field cannot be monitored. Diagnostic ultrasound is widely used in daily clinical practice. Diagnostic ultrasound is equipped with a display for image diagnosis. If diagnostic ultrasound could be utilized for sonoporation, therapeutic genes can be accurately introduced into the target fields. Miller and Quddus transfected cultured cells with plasmid DNA using diagnostic ultrasound[46]. They used a 3.5 MHz curved linear transducer of diagnostic ultrasound. A contrast agent of ultrasound (Optison) was added to the media. Although Optison may improve transfection efficiency, even without contrast agents, plasmid DNA was successfully introduced into cultured cells by using diagnostic ultrasound[47]. SiRNA of frizzled (Fz)-9, a receptor of the Wnt signaling pathway, transferred into cultured cells using diagnostic ultrasound suppresses cell proliferation[48]. SiRNA of Fz-9 transferred into cultured cells using lipofection also suppresses cell proliferation[49]. These 2 papers indicate that transfection efficiency of siRNA with diagnostic ultrasound is comparable with that of lipofection. Wang et al[27] investigated cell permeability of Evans Blue in vivo with diagnostic ultrasound. They observed the dye in xenograft hepatoma. Interestingly, they reported that efficiency of Evans Blue transfer was affected by MI sonication duration and dye dose. These data clearly demonstrate that ultrasound causes sonoporation, a biological process that is a promising new approach for the delivery of DNA/RNA for gene therapy.
Sonoporation is able to introduce plasmids to cells. Sonoporation is less toxic method of gene transfer as compared with retro viral vectors and adenoviral vectors because plasmids hardly causes immune response and are not associated with tumorigenicity. Sonoporation does not require surgical procedure and enhances gene transfer with lipofection. Current limitations of sonoporation are low efficiency of gene transfer and damage of target cells are the use of complexes with chemicals and diagnostic ultrasound are promising approaches to overcome these limitations.
P- Reviewers: Ghatak S, Klein RL, Kiselev SL S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | Mellott AJ, Forrest ML, Detamore MS. Physical non-viral gene delivery methods for tissue engineering. Ann Biomed Eng. 2013;41:446-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Feichtinger GA, Hofmann AT, Slezak P, Schuetzenberger S, Kaipel M, Schwartz E, Neef A, Nomikou N, Nau T, van Griensven M. Sonoporation Increases Therapeutic Efficacy of Inducible and Constitutive BMP2/7 In Vivo Gene Delivery. Hum Gene Ther Methods. 2013;Nov 27. [PubMed] |
| 3. | Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 640] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 4. | Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 994] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 5. | Jin L, Li F, Wang H, Li Y, Wei F, Du L. Ultrasound-targeted microbubble destruction enhances gene transduction of adeno-associated virus in a less-permissive cell type, NIH/3T3. Mol Med Rep. 2013;8:320-326. [PubMed] |
| 6. | Wells DJ. Electroporation and ultrasound enhanced non-viral gene delivery in vitro and in vivo. Cell Biol Toxicol. 2010;26:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Ledwith BJ, Manam S, Troilo PJ, Barnum AB, Pauley CJ, Griffiths TG, Harper LB, Beare CM, Bagdon WJ, Nichols WW. Plasmid DNA vaccines: investigation of integration into host cellular DNA following intramuscular injection in mice. Intervirology. 2000;43:258-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Geers B, Lentacker I, Alonso A, Sanders NN, Demeester J, Meairs S, De Smedt SC. Elucidating the mechanisms behind sonoporation with adeno-associated virus-loaded microbubbles. Mol Pharm. 2011;8:2244-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Jin LF, Li F, Wang HP, Wei F, Qin P, Du LF. Ultrasound targeted microbubble destruction stimulates cellular endocytosis in facilitation of adeno-associated virus delivery. Int J Mol Sci. 2013;14:9737-9750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14323] [Article Influence: 842.5] [Reference Citation Analysis (0)] |
| 11. | Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1:841-845. [PubMed] |
| 12. | Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26:5896-5903. [PubMed] |
| 13. | Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413-7417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3413] [Cited by in RCA: 3409] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 14. | Miller DL, Pislaru SV, Greenleaf JE. Sonoporation: mechanical DNA delivery by ultrasonic cavitation. Somat Cell Mol Genet. 2002;27:115-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (1)] |
| 15. | Xu Z, Raghavan M, Hall TL, Chang CW, Mycek MA, Fowlkes JB, Cain CA. High speed imaging of bubble clouds generated in pulsed ultrasound cavitational therapy--histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:2091-2101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Tachibana K, Tachibana S. Transdermal delivery of insulin by ultrasonic vibration. J Pharm Pharmacol. 1991;43:270-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Wang W, Li W, Ma N, Steinhoff G. Non-viral gene delivery methods. Curr Pharm Biotechnol. 2013;14:46-60. [PubMed] |
| 18. | Unger EC, Hersh E, Vannan M, Matsunaga TO, McCreery T. Local drug and gene delivery through microbubbles. Prog Cardiovasc Dis. 2001;44:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 188] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Tomizawa M, Ebara M, Saisho H, Sakiyama S, Tagawa M. Irradiation with ultrasound of low output intensity increased chemosensitivity of subcutaneous solid tumors to an anti-cancer agent. Cancer Lett. 2001;173:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Fechheimer M, Boylan JF, Parker S, Sisken JE, Patel GL, Zimmer SG. Transfection of mammalian cells with plasmid DNA by scrape loading and sonication loading. Proc Natl Acad Sci USA. 1987;84:8463-8467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 211] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Kim HJ, Greenleaf JF, Kinnick RR, Bronk JT, Bolander ME. Ultrasound-mediated transfection of mammalian cells. Hum Gene Ther. 1996;7:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 222] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Tsunoda S, Mazda O, Oda Y, Iida Y, Akabame S, Kishida T, Shin-Ya M, Asada H, Gojo S, Imanishi J. Sonoporation using microbubble BR14 promotes pDNA/siRNA transduction to murine heart. Biochem Biophys Res Commun. 2005;336:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | O’Brien WD. Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol. 2007;93:212-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 443] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 24. | Newman CM, Bettinger T. Gene therapy progress and prospects: ultrasound for gene transfer. Gene Ther. 2007;14:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 260] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 25. | Meltzer RS. Food and Drug Administration ultrasound device regulation: the output display standard, the “mechanical index,” and ultrasound safety. J Am Soc Echocardiogr. 1996;9:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Forsberg F, Shi WT, Merritt CR, Dai Q, Solcova M, Goldberg BB. On the usefulness of the mechanical index displayed on clinical ultrasound scanners for predicting contrast microbubble destruction. J Ultrasound Med. 2005;24:443-450. [PubMed] |
| 27. | Wang G, Zhuo Z, Xia H, Zhang Y, He Y, Tan W, Gao Y. Investigation into the impact of diagnostic ultrasound with microbubbles on the capillary permeability of rat hepatomas. Ultrasound Med Biol. 2013;39:628-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Zhou Y, Yang K, Cui J, Ye JY, Deng CX. Controlled permeation of cell membrane by single bubble acoustic cavitation. J Control Release. 2012;157:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Carugo D, Ankrett DN, Glynne-Jones P, Capretto L, Boltryk RJ, Zhang X, Townsend PA, Hill M. Contrast agent-free sonoporation: The use of an ultrasonic standing wave microfluidic system for the delivery of pharmaceutical agents. Biomicrofluidics. 2011;5:44108-4410815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Sirsi S, Borden M. Microbubble Compositions, Properties and Biomedical Applications. Bubble Sci Eng Technol. 2009;1:3-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 409] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 31. | Tachibana K, Tachibana S. Albumin microbubble echo-contrast material as an enhancer for ultrasound accelerated thrombolysis. Circulation. 1995;92:1148-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 218] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Qiu Y, Zhang C, Tu J, Zhang D. Microbubble-induced sonoporation involved in ultrasound-mediated DNA transfection in vitro at low acoustic pressures. J Biomech. 2012;45:1339-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Kudo N, Okada K, Yamamoto K. Sonoporation by single-shot pulsed ultrasound with microbubbles adjacent to cells. Biophys J. 2009;96:4866-4876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Panje CM, Wang DS, Pysz MA, Paulmurugan R, Ren Y, Tranquart F, Tian L, Willmann JK. Ultrasound-mediated gene delivery with cationic versus neutral microbubbles: effect of DNA and microbubble dose on in vivo transfection efficiency. Theranostics. 2012;2:1078-1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Zheng X, Ji P, Hu J. Sonoporation using microbubbles promotes lipofectamine-mediated siRNA transduction to rat retina. Bosn J Basic Med Sci. 2011;11:147-152. [PubMed] |
| 36. | Li YH, Jin LF, Du LF, Shi QS, Liu L, Jia X, Wu Y, Li F, Wang HH. Enhancing HSP70-ShRNA transfection in 22RV1 prostate cancer cells by combination of sonoporation, liposomes and HTERT/CMV chimeric promoter. Int J Oncol. 2013;43:151-158. [PubMed] |
| 37. | Miller DL, Dou C. Induction of apoptosis in sonoporation and ultrasonic gene transfer. Ultrasound Med Biol. 2009;35:144-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Van Ruijssevelt L, Smirnov P, Yudina A, Bouchaud V, Voisin P, Moonen C. Observations on the viability of C6-glioma cells after sonoporation with low-intensity ultrasound and microbubbles. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:34-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Zhong W, Sit WH, Wan JM, Yu AC. Sonoporation induces apoptosis and cell cycle arrest in human promyelocytic leukemia cells. Ultrasound Med Biol. 2011;37:2149-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Zhong W, Chen X, Jiang P, Wan JM, Qin P, Yu AC. Induction of endoplasmic reticulum stress by sonoporation: linkage to mitochondria-mediated apoptosis initiation. Ultrasound Med Biol. 2013;39:2382-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Chen X, Wan JM, Yu AC. Sonoporation as a cellular stress: induction of morphological repression and developmental delays. Ultrasound Med Biol. 2013;39:1075-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Yeh CK, Su SY. Effects of acoustic insonation parameters on ultrasound contrast agent destruction. Ultrasound Med Biol. 2008;34:1281-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Forsberg F, Merton DA, Goldberg BB. In vivo destruction of ultrasound contrast microbubbles is independent of the mechanical index. J Ultrasound Med. 2006;25:143-144. [PubMed] |
| 44. | Suzuki R, Takizawa T, Negishi Y, Hagisawa K, Tanaka K, Sawamura K, Utoguchi N, Nishioka T, Maruyama K. Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound. J Control Release. 2007;117:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 45. | Suzuki R, Namai E, Oda Y, Nishiie N, Otake S, Koshima R, Hirata K, Taira Y, Utoguchi N, Negishi Y. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J Control Release. 2010;142:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 46. | Miller DL, Quddus J. Sonoporation of monolayer cells by diagnostic ultrasound activation of contrast-agent gas bodies. Ultrasound Med Biol. 2000;26:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 119] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Tomizawa M, Shinozaki F, Sugiyama T, Yamamoto S, Sueishi M, Yoshida T. Plasmid DNA introduced into cultured cells with diagnostic ultrasound. Oncol Rep. 2012;27:1360-1364. [PubMed] |
| 48. | Tomizawa M, Shinozaki F, Sugiyama T, Yamamoto S, Sueishi M, Yoshida T. Short interference RNA introduced into cultured cells with diagnostic ultrasound. Oncol Rep. 2012;27:65-68. [PubMed] |
| 49. | Fujimoto T, Tomizawa M, Yokosuka O. SiRNA of frizzled-9 suppresses proliferation and motility of hepatoma cells. Int J Oncol. 2009;35:861-866. [PubMed] |