Published online Dec 20, 2025. doi: 10.5662/wjm.v15.i4.107664
Revised: April 21, 2025
Accepted: June 10, 2025
Published online: December 20, 2025
Processing time: 130 Days and 6.8 Hours
Micronutrients are fundamental to support and maintain normal physiological function. Deficiencies of these nutrients are a growing public health concern with potentially devastating consequences. An adequate diet of whole foods is the primary source of micronutrients; supplementation is sometimes necessary. Both deficiency and excess of these nutrients have adverse effects. Common defi
Core Tip: Micronutrient deficiency is an emerging public health concern affecting more than 2 billion people globally. These deficiencies can contribute to poor growth and intellectual disabilities, which increase the risk of morbidity and mortality, particularly in developing countries. Risk factors vary by micronutrient deficiency. Early recognition and intervention by clinicians drastically decrease global mortality. This article reviews various micronutrient deficiencies, their clinical features, and treatments in detail.
- Citation: English K, Uwibambe C, Daniels P, Dzukey E. Scoping review of micronutrient imbalances, clinical manifestations, and interventions. World J Methodol 2025; 15(4): 107664
- URL: https://www.wjgnet.com/2222-0682/full/v15/i4/107664.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i4.107664
Nutrition is a modifiable factor that can be targeted to promote optimal health and reduce disease risk throughout a lifetime[1]. A healthy diet contains all the resources needed to meet the body’s metabolic demands[1,2]. Nutrients (macronutrients and micronutrients) are essential molecules required to sustain a healthy and prolonged life[2,3]. Macronutrients (carbohydrates, lipids, and protein) are needed in significant amounts to facilitate hormone creation, synthesis of molecules, energy production, and regulation of metabolic pathways[4]. In smaller quantities, micronutrients such as minerals, vitamins, and antioxidants are needed to aid biochemical processes such as enzyme reactions, protection against oxidative stress, and gene transcription[5,6].
Micronutrient deficiencies (MNDs) occur when an individual, regardless of reason, does not consume an adequate amount of food to meet their energy requirements[5-7]. Patients at risk include older adults, individuals with alcohol use disorders, pregnant women, vegans or vegetarians, and persons with increased requirements due to medical conditions or long-term drug use that alters metabolism, absorption, and excretion[7-9]. A sufficient amount is needed to support physiological processes and metabolism[10]. However, inadequate and excessive intake can be harmful and should be avoided[8-10]. MNDs continue to affect more than 2 billion people worldwide despite advances in technology and science[11]. In this article, we provide a scoping review of MNDs with a particular focus on fat and water-soluble vitamins, their clinical manifestations, and treatment. A few micronutrients in excess were expanded upon.
Micronutrients are vitamins and minerals needed by the body in small quantities to meet its daily metabolic needs[11,12]. Deficiency occurs with low dietary intake of these compounds, and it is particularly prevalent in the developing world, affecting an estimated 2 billion people worldwide[11,13]. A lack of iron, folate, and vitamin B12 can result in anemia, which affects an estimated 42% of children under 5 years of age and approximately 40% of pregnant women[14,15]. Prevalence rates of MND are typically higher across South Asia and sub-Saharan Africa[16,17].
More than half of children under age five and more than two-thirds of women of reproductive age are deficient in at least one micronutrient[14-18]. Iron is the most common MND worldwide, which can result in anemia[19]. The World Health Organization (WHO) estimates that 25% of people worldwide have anemia and iron deficiency, the most common cause, is responsible for 50% of all cases[20,21]. Approximately 1.2 billion people worldwide suffer from iron deficiency anemia, with a higher prevalence in women and children[22,23].
These deficiencies are particularly prevalent in low-income countries, suggesting that poor sanitation, poor diet, and inadequate health care are major contributory factors[11,13,16,18]. Anemia and iodine deficiency disorders separately affect approximately 2 billion people in both developed and developing countries, and vitamin A deficiency is prevalent in up to 12% of low-income nations[13-19]. Muthayya et al[24] defined the hidden hunger index based on the national estimates of the prevalence of stunting, anemia due to iron deficiency, and low serum retinol concentration. Hot spots were found in South Asia and sub-Saharan Africa, where the highest prevalence of exists. MNDs are more common in developing countries, and clinical outcomes are more severe, given the lack of resources, including the quality of healthcare within these areas[12,14,16,18,20,22].
Fat-soluble vitamins, including A, D, E, and K, are energy-independent molecules that are essential to maintain adequate bodily function[25]. Their intake is almost exclusively from diet[26]. As such, deficiencies in fat-soluble vitamins are more likely seen in developing countries with limited resources than in industrialized nations[27]. These vitamins are classically absorption dependent on bile emulsification, pancreatic secretions, and a functional ileum[28]. Due to this fact, certain patient groups are at a higher risk for deficiencies, including those with liver disease, malabsorption syndromes with steatorrhea (i.e., celiac disease and cystic fibrosis) intensive care unit patients, and patients with chronic mineral oil intake[29-31]. Toxicity is less common for fat-soluble vitamins compared to water-soluble as fat-soluble molecules accumulate in fat[32]. The clinical manifestations of fat-soluble vitamin deficiencies and their dietary sources can be seen below.
Vitamin A is a general term for a group of fat-soluble compounds consisting primarily of retinol and retinyl esters[33]. This class of molecules are responsible for immune function, reproductive growth, and cellular signaling and differentiation[33,34]. Vitamin A is essential for the differentiation of epithelial cells into specialized tissues (mucus-secreting and pancreatic cells), playing a crucial role in the normal formation and maintenance of organs such as the lungs, heart, and eye[33-35]. It is also critical for vision, as it serves as an essential component of rhodopsin, the light-sensitive protein found in the rod cells of the retina that plays a pivotal role in the process of visual transduction[36,37].
Dietary vitamin A can be found in animal products, including kidney, liver, dairy products, and eggs[38]. They additionally can be seen as provitamin A (β-carotene) in plant sources, such as fruits and leafy vegetables[38,39]. Patients with vitamin A deficiency may present clinically with reduced night vision, dry eyes, keratomalacia, hyperkeratotic papular lesions, immunosuppression with recurrent infections, and dry skin[38-41]. Ophthalmological examination reveals conjunctival lesions with triangular perilimbal debris[41,42]. In healthy adults, vitamin A's recommended dietary allowance (RDA) is 700 mcg per day for women and 900 mcg per day for men. In pregnant women, the RDA is 770 mcg per day, while in children, it ranges between 300 to 900 mcg per day.
Toxicity: Excessive consumption of vitamin A can lead to acute and chronic toxicity[43]. This is characterized by elevated levels of > 80 mcg/dL in the body, known as hypervitaminosis A[43,44]. Acute vitamin A toxicity leads to nausea, vomiting, and elevated intracranial pressure, which can present clinically with blurred vision and vertigo[45]. Patients with chronic toxicity, in contrast, present clinically with alopecia, xerosis cutis, idiopathic intracranial hypertension, and hepatomegaly/hepatoxicity[45,46].
Vitamin D is a fat-soluble entity that plays a vital role in calcium regulation and bone metabolism[47]. It has also been shown to play a key part in muscle, cardiovascular, immune, and nervous system functions[47-49]. Vitamin D is known as an immunomodulatory hormone, as experimental studies have shown that the active form [1,25-dihydroxyvitamin D3 = 1,25(OH)2 D3] exerts immunological activities on components of the adaptive and innate immune system[50]. There have been observed associations between low levels of serum 25-hydroxyvitamin D [25(OH)D] and the development of autoimmune disorders such as multiple sclerosis, type 1 diabetes, rheumatoid arthritis, and others[51].
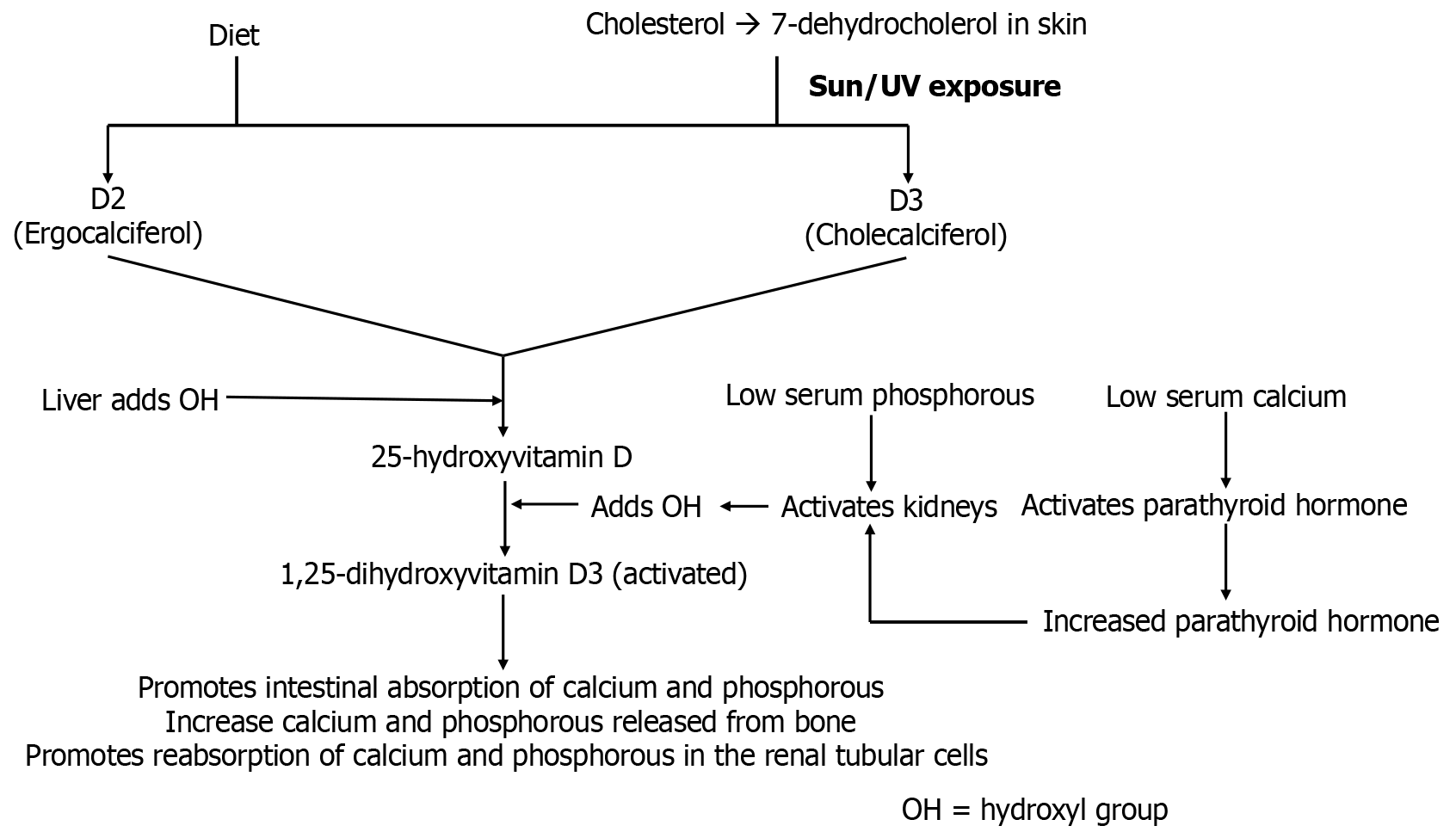
Vitamin D is obtained primarily from diet in the form of fish, milk, plants, fungi, and yeast [ergocalciferol (D2) and cholecalciferol (D3)][52]. Cholecalciferol can additionally be synthesized from skin exposure to the sun[53]. Both forms are converted to the storage form [25(OH)D] of vitamin D in the liver and then subsequently to the active form, 1,25(OH)2 D3 (calcitriol) in the kidney that increases intestinal absorption of calcium, promotes bone resorption, and decreases renal excretion of calcium and phosphate (Figure 1)[54,55]. Deficiency of vitamin D can be caused by malabsorption, reduced sun exposure, chronic kidney disease, poor diet, and advanced liver disease[56]. In children, deficiency can lead to rickets, a condition characterized by a defect in mineralization of the epiphyseal plates, which can present clinically with craniotabes, rickety rosary, spinal column deformity, genu varum, and other musculoskeletal findings (i.e., bone softening, gait disturbance, and growth retardation)[57,58]. In adults, it may lead to osteomalacia, characterized by abnormal bone mineralization, which may present clinically with bone pain, muscle weakness, and hypocalcemic tetany[59].
Recommendations regarding daily vitamin D intake vary by age. The Endocrine Society defines vitamin D [25(OH)D] deficiency as less than 30 ng/mL[60]. To maintain levels within the preferred range of 40 to 60 ng/mL, the organization recommends an intake of 400 to 1000 international units (IU) daily for infants below 1 year old, 600 to 1000 IU per day for children and adolescents up to 18 years old, and 1500 to 2000 IU for all adults. Oral vitamin D should be given to breastfed infants[61]. Darker skin and prematurity notably predispose to deficiency[62].
Toxicity: Elevated levels of vitamin D, also known as hypervitaminosis D, are related to excessive long-term intake, disruptions in the metabolic pathway, or concurrent disease that produces the active vitamin D metabolite[63]. This condition presents a range of clinical symptoms[63]. These include abdominal pain, polyuria, polydipsia, confusion, apathy, and loss of appetite[32,63]. Laboratory assessment typically shows hypercalcemia, hypercalciuria, and 25(OH)D concentrations > 150 ng/mL (> 375 nmol/L)[32,63,64]. Hypervitaminosis D can also be seen in granulomatous diseases that increase vitamin D levels by activating epithelioid macrophages[65].
Vitamin E is the combined name for a group of fat-soluble vitamins with distinct antioxidant properties[66]. They act as antioxidants that protect red blood cells (RBCs) and neuronal membranes from free radical damage[66,67]. Naturally occurring vitamin E exists in several chemical forms that possess different levels of biological activity[68]. The α-tocopherol is the only form recognized to meet human metabolic needs[69]. Vitamin E is found in avocadoes, soybeans, nuts, olive oil, and leafy vegetables[68,69].
Clinical manifestations of Vitamin E deficiency include acanthocytosis, muscle weakness, ataxia, decreased proprioception and vibration, and hemolytic anemia[70]. Demyelination of the spinocerebellar tract and the posterior columns is responsible for ataxia and loss of proprioception and vibration, respectively[71,72]. These neurological symptoms may appear similar to those of patients with vitamin B12 deficiency. However, megaloblastic anemia and elevated serum methylmalonic acid levels are absent[69-72]. The RDA for patients aged 14 years and older is 15 mg of α-tocopherol.
Toxicity: Vitamin E toxicity is associated with an elevated bleeding risk[73]. High-dose supplementation is known to alter the metabolism of vitamin K, which can enhance the anticoagulation effect in patients on warfarin[74]. In infants, toxicity is associated with the risk of enterocolitis[75].
Vitamin K is a group of molecules, including phylloquinone (vitamin K1) and a series of menaquinones (vitamin K2), that are naturally present in some foods[76]. Menaquinones are designated as MK-4 through MK-13 based on the length of their unsaturated isoprenyl side chains, with MK-4, MK-7, and MK-9 being the most well-studied menaquinones[77]. Phylloquinone is found in green leafy vegetables and is the primary dietary source of vitamin K[78]. Menaquinone is primarily synthesized in the human gut by bacteria[77,79]. Vitamin K is activated by epoxide reductase to the reduced form, which serves as a cofactor for the γ-carboxylation of glutamic acid residues, that is essential for the maturation of clotting factors II, VII, IX, X, protein C and S[77-81].
Clinical manifestations of vitamin K deficiency include neonatal hemorrhage, as neonates possess sterile intestines and are unable to synthesize vitamin K[82]. Labs commonly show elevated prothrombin, activated partial thromboplastin, and normal bleeding time[82,83]. The absence of vitamin K in the human body can result in a range of complications, including impaired bone development, bleeding disorders, and elevated risk for cardiovascular disease[82-85]. For adult men and women, the RDA of vitamin K is 120 and 90 mcg per day, respectively.
Water-soluble vitamins are a group of organic compounds that are required in small amounts by the human body to prevent disorders of metabolism[86]. Given their chemical properties, regular intake is required to avoid nutritional deficiency. The water-soluble vitamins include vitamin C and vitamin B complex (thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, biotin, folate, cobalamin, and ascorbic acid). These vitamins are readily excreted from the body except cobalamin (B12) and folate (B9)[87]. B12 is stored in the liver for approximately 3-4 years, while B9 is stored in the same organ for 3-4 months[88]. B-complex deficiencies often result in dermatitis, glossitis, and diarrhea[88,89]. These compounds can also be coenzymes (i.e., ascorbic acid) or precursors to coenzymes [i.e., flavin adenine dinucleotide (FAD), nicotinamide adenine dinucleotide+ (NAD+)][88-90]. The clinical manifestations of water-soluble vitamin deficiencies and their dietary sources can be seen below.
Vitamin B1, or thiamine, is a water-soluble compound that is a cofactor for various enzymes important in energy metabolism and glucose breakdown[91]. As such, it plays a critical role in cell growth, development, and function[91,92]. Approximately 80% of thiamin in the adult human body is thiamine pyrophosphate, the main metabolically active form of thiamine[91-93]. It can be found in nuts, poultry, soybeans, fortified foods, and peas[92-94]. Thiamine status is often measured indirectly by assaying transketolase activity following B1 administration[95]. An increase in RBC transketolase activity following thiamine administration suggests deficiency[93-95].
Inadequate levels of thiamine results in impaired glucose breakdown, which leads to adenosine triphosphate (ATP) depletion[91-94]. As such, highly aerobic tissues (i.e., the heart and brain) are affected more acutely[91-95]. Risk factors for deficiency include malnutrition and chronic alcohol overuse[96]. Insufficient levels of this vitamin can lead to several notable disorders, including Wernicke encephalopathy, Korsakoff syndrome, Wernicke-Korsakoff syndrome, and dry and wet beriberi[91-96].
Wernicke encephalopathy is an acute reversible life-threatening neurological condition due to B1 deficiency in the setting of alcohol use that may present clinically with confusion, ophthalmoplegia/nystagmus, and ataxia[97]. Thiamine deficiency can also lead to Korsakoff syndrome, which is an amnestic disorder due to chronic alcohol use that presents clinically with confabulations, memory loss, and personality changes[97,98]. Wernicke-Korsakoff syndrome appears clinically as a combination of both disorders due to damage to the mammillary bodies and the medial dorsal nucleus of the thalamus[99]. Inadequate thiamine levels can also cause dry beriberi syndrome, which presents clinically with polyneuropathy and symmetric muscle wasting, as well as wet beriberi, which causes high-output heart failure due to systemic vasodilation[100,101]. The RDA for adult males and females is 1.2 and 1.0 mg per day, respectively.
Riboflavin is a water-soluble vitamin that serves as an essential component of two major coenzymes, FAD and flavin mononucleotide[102,103]. These enzymes play a significant part in energy production, cellular function, and growth[102-104]. FAD and flavin mononucleotide are used as cofactors in redox reactions (i.e., succinate dehydrogenase reaction in the Krebs cycle)[105]. Riboflavin also plays a role as an antioxidant, as it helps in the regeneration of glutathione[106]. It can be found in fortified grains, dairy products, and certain fruits and vegetables[102-106].
Riboflavin deficiency can cause cheilosis and corneal vascularization. The RDA for women and men is 0.9 to 1.1 and 1.1 to 1.3 mg per day, respectively.
Niacin is a water-soluble B vitamin that is naturally present in many foods[107]. Body tissues convert niacin to its metabolically active form, NAD, which serves as a catalyst for more than 400 enzymes in the body[107]. NAD is also converted into another active form, nicotinamide adenine dinucleotide phosphate (NADP), which facilitates reactions in all tissues except skeletal muscle[108]. NAD is primarily involved in catabolic reactions that transform potential energy in proteins, fats, and carbohydrates to ATP[109]. NADP, in contrast, facilitates anabolic reactions such as fatty acid and cholesterol synthesis and plays a role in maintaining cellular antioxidant function[110]. Both are used in redox reactions and are cofactors for many dehydrogenases[107-110]. Niacin can be found in milk, meat, legumes, and fish[108,109]. Tryptophan is also a precursor for niacin to create NAD, so tryptophan is considered a dietary source of niacin[111].
Deficiencies are more common in developing countries due to insufficient dietary intake[108-112]. The lack of niacin and its precursor tryptophan leads to the manifestation of pellagra, which may be due to inadequate intake, Hartnup disease, malignant carcinoid syndrome, or isoniazid deficiency[113-115]. Symptoms of niacin deficiency include diarrhea, dementia (also hallucinations), and dermatitis (C3/C4 dermatome circumferential rash and hyperpigmentation on sun-exposed limbs)[108,110,113,115]. Hartnup disease is a rare autosomal recessive disorder due to a deficiency of neutral amino acid transporters in the proximal renal tubular cells and enterocytes[116]. This leads to neutral aminoaciduria and decreased absorption from the gut, resulting in little or no tryptophan for conversion to niacin, ultimately leading to pellagra-like symptoms. The RDA of niacin is 14 mg per day for women and 16 mg per day for men.
Toxicity: If niacin is consumed at high doses through supplements, it can lead to niacin toxicity. High doses of nicotinic acid may present clinically with facial flushing, hyperglycemia, hepatotoxicity, and hyperuricemia[117].
Pantothenic acid is a water-soluble compound that is naturally present in some foods and available as a dietary supplement[118]. The main function of vitamin B5 is in the synthesis of coenzyme A (CoA) and acyl carrier protein[118,119]. It also serves as a component of fatty acid synthase[119]. Pantothenic acid is found in milk, vegetables, eggs, chicken, and others[118-120].
Deficiency of B5 may lead to dermatitis, enteritis, alopecia, and adrenal insufficiency, which may lead to burning feet syndrome, distal paresthesias, and dysesthesia[119-121]. The RDA for adult men and women is 5 mg per day.
Pyridoxine is a group of water-soluble vitamins that are naturally present in many foods[122]. Pyridoxal 5’ phosphate and pyridoxamine 5’ phosphate are the active coenzyme forms of B6[123]. Pyridoxal 5’ phosphate is a cofactor that is used in several metabolic processes, including transamination, decarboxylation, and phosphorylation reactions[124]. It also plays a vital role in protein, carbohydrate, lipid metabolism, and the creation of RBCs[122-124]. Pyridoxine can be found in liver, poultry, and fortified cereals[122,123].
Patients with pyridoxine deficiency may present clinically with convulsions, hyperirritability, and peripheral neuropathy[125]. Some patients may appear with sideroblastic anemia due to impaired hemoglobin synthesis and iron excess[126]. The RDA for adults is 1.75 mg per day.
Biotin is a water-soluble compound that plays a role in energy metabolism and the regulation of oxidative stress[127]. It serves as a cofactor for several carboxylation enzymes, including pyruvate carboxylase, acetyl-CoA carboxylase, and propionyl-CoA carboxylase[127,128]. The vitamin can be found in many foods, such as egg yolk, liver, wheat, dairy, rice, and spinach[127,128].
Biotin deficiency is relatively rare[129]. It is commonly caused by long-term antibiotic use or excessive ingestion of raw egg whites, as raw egg whites contain a large volume of avidin, which binds to biotin[129,130]. Patients with a deficiency in this vitamin may clinically present with dermatitis, enteritis, and alopecia[127-130]. Intake recommendations range between 5 and 35 mcg per day.
Folate is a water-soluble B molecule that is present in multiple foods and is available as a dietary supplement[131]. Folate in foods is in the tetrahydrofolate form and usually possesses additional glutamate residues, making them polyglutamates[131,132]. Folic acid is the completely oxidized monoglutamate form of the vitamin that is used in most dietary supplements and fortified foods[133]. Folate plays a crucial role in the methylation/1-carbon transfer reactions necessary for deoxyribonucleic acid (DNA) and ribonucleic acid synthesis and metabolism of amino acids[131-133]. One of the most important folate-dependent reactions is the methylation of deoxyuridylate to thymidylate in the formation of DNA, which is required for proper cell division[134]. Impairment in this reaction initiates several steps that can lead to megaloblastic anemia, one of the hallmarks of folate deficiency[135]. Folate can be found in leafy green vegetables, eggs, and milk[132-136]. It is also produced by the guy microbiota, and a small, reserved pool is stored primarily in the liver[131-135].
Risk factors for folate deficiency include pregnancy, chronic alcohol use, digestive disorders such as Crohn’s disease, and several medications (i.e., phenytoin, trimethoprim, and methotrexate)[131,136,137]. These patients may clinically present with glossitis, megaloblastic anemia, and oral ulcers[134-137]. Folate deficiency before and during pregnancy increases the risk of neural tube defects[138]. A healthy adult requires 400 mcg per day, with higher intakes recommended during pregnancy to prevent neural tube defects.
Vitamin B12 is a water-soluble vitamin that is required for the development, myelination, and function of the central nervous system[139]. Cobalamin is also essential for RBC formation and DNA synthesis[139,140]. It serves as a cofactor for methionine synthase and L-methylmalonyl-CoA mutase[141]. Methionine synthase catalyzes the conversion of homocysteine to methionine[142]. Methionine is needed for the creation of S-adenosylmethionine, which is a universal methyl donor for several substrates, including ribonucleic acid, DNA, proteins, and lipids[141,142]. L-methylmalonyl-CoA mutase converts L-methylmalonyl-CoA to succinyl-CoA in the metabolism of propionate. B12 is found in animal products (i.e., meat, dairy, eggs) and is synthesized only by intestinal microbiota[141-143]. The site of synthesis in humans is distal to the site of absorption[144]. Thus, B12 must be consumed via animal products[143,144].
Humans possess a very large reserve pool of B12 that is primarily stored in the liver[145]. Deficiency can be caused by a lack of intrinsic factor (i.e., pernicious anemia, gastric bypass surgery), malabsorption (i.e., enteritis, sprue, achlorhydria, alcohol overuse, bacterial overgrowth, Diphyllobothrium latum), insufficient intake (i.e., veganism), absence of ileum (surgical resection, i.e., Crohn’s) and certain medications (i.e., metformin)[146-149]. Patients with deficiency may present with megaloblastic anemia, paresthesias, and subacute combined degeneration in severe cases (degeneration of dorsal columns, lateral corticospinal tracts, and spinocerebellar tracts) due to abnormal myelin[141,143,144,148-150]. B12 deficiency is associated with elevated serum homocysteine and methylmalonic acid levels and secondary folate deficiency[146-151]. A prolonged state of insufficiency can lead to irreversible nerve damage. The RDA of cobalamin for adults is 2.4 mcg per day.
Vitamin C, also known as L-ascorbic acid, is a water-soluble vitamin that is present in certain foods[152]. Unlike most animals, humans cannot produce endogenous vitamin C, so it is essentially a dietary component[153]. Vitamin C is required for the biosynthesis of L-carnitine, collagen, and certain neurotransmitters[154]. It is necessary for the hydroxylation of lysine and proline in collagen synthesis, which plays a vital role in wound healing[152-154]. The vitamin is also necessary for dopamine β-hydroxylase, which converts dopamine to norepinephrine[155]. It additionally serves as an antioxidant and has been shown to regenerate other antioxidants within the body, including vitamin E[156,157]. In addition to its synthetic and antioxidant properties, vitamin C facilitates iron absorption and plays an important role in immune function[158]. It is primarily found in fruits and vegetables, such as citrus, potatoes, berries, and green leafy vegetables[155-159].
Severe vitamin C deficiency can result in scurvy, a disorder characterized by swollen gums, easy bruising, petechiae, anemia, hemarthrosis, poor wound healing, corkscrew hair, and perifollicular and subperiosteal hemorrhages[160]. This simultaneously results in a weakened immune system[160,161]. The recommended intake may vary based on age and gender but typically falls between 40 and 120 mg per day.
Toxicity: Excess vitamin C intake can lead to nausea, vomiting, fatigue, diarrhea, and calcium oxalate stones[162,163]. Elevated levels can also increase iron toxicity in predisposed individuals by increasing dietary iron absorption (i.e., it can worsen transfusion-related iron overload or hemochromatosis)[164,165].
Before treatment, a practitioner must obtain a diagnosis. This begins with attaining a good clinical history from the patient, including their surgical past and dietary habits. Based on clinical suspicion, the provider should perform a complete physical examination to look for the clinical signs described above. The next step includes a series of direct and indirect assays performed on the blood to determine vitamin levels, as seen in Table 1. Some deficiencies are clinically diagnosed, and laboratory assessment further confirms suspicion[166-168]. Biomarker limitations in a lab setting stem from elements such as cost, assay standardization, variability in measurement, and sampling handling. These host of factors can ultimately affect biomarker reliability and accuracy, especially in low-income countries that may not have adequate resources. Due to this fact, clinicians should not diagnose MND, or excess solely based on laboratory values.
| Vitamin | Diagnostic test | Specimen type | Diagnostic values |
| A | Vitamin A (retinol) level | Serum | Low |
| D | Total 25-hydroxyvitamin D level | Serum | Low |
| E | Vitamin E (α-tocopherol) level | Serum | Low |
| K1 | PT/INR | Serum | Prolonged/elevated |
| Thiamine2 | Thiamine, whole blood | Whole blood EDTA | Low |
| Riboflavin | Riboflavin level or urinary excretion rate | Plasma or urine | Low |
| Niacin | Niacin level or urinary excretion of N1-methylnicotinamide | Serum or urine | Low |
| Pantothenic acid | Pantothenic acid level | Serum | Low |
| Pyridoxine3 | Pyridoxal phosphate | Serum | Low |
| Biotin | Biotin level | Serum | Low |
| Folate | Vitamin B12 and folate levels | Serum | Low |
| Cobalamin4 | Vitamin B12 and folate levels | Serum | Low |
| Ascorbic acid5 | Ascorbic acid level | Serum | Low |
Treatment of fat-soluble vitamin deficiencies depends on multiple factors, including the severity, deficiency type, and the patient's individual needs (i.e., pregnancy, growth, and underlying disease)[169]. The most common method for the treatment of fat-soluble vitamin deficiency is supplementation with specific preparations of vitamins A, D, E, or K[33,47,68,166,168,170]. These vitamins can be taken in the form of capsules, tablets, or drinkable preparations, but they can also be administered intravenously (IV) or intramuscularly (IM), depending on the severity of the deficiency[26-36,41-47,168]. There are a wide variety of therapeutic regimens that have been proposed by several academic societies (i.e., internal medicine, endocrine, rheumatology, and others)[30,165-170]. It is important to emphasize that the suggested therapeutic regimens are mostly based on studies that do not meet evidence-based medicine criteria and are rather a combination of suggestions from multiple medical societies from mostly the United States and Europe (Table 2).
| Vitamin | Clinical manifestations of deficiency | Recommended treatment |
| A | Nyctalopia, xerosis cutis, xeropthalmia, immunosuppression, keratomalacia, and conjunctival squamous metaplasia Bitot spots | Vitamin A palmitate 60000 IU (18000 mcg RAE) orally once per day for 2 days, followed by 4500 IU once a day |
| D | Bone pain, muscle weakness, depression, hair loss, and hypocalcemic tetany | Ergocalciferol (vitamin D2) 50000 IU orally every 7 days for 8 weeks |
| E | Hemolytic anemia, muscle weakness, ataxia, and decreased proprioception and vibration | α-tocopherol is 15 to 25 mg/kg orally once daily or 200 IU of mixed tocopherols daily. Injections should be used to treat neuropathy and patients with malabsorption syndromes |
| K | Increased risk for bleeding | Phytonadione 1 to 10 mg orally for nonemergent correction of prolonged INR in patients taking anticoagulants. When partial correction of INR is desirable (i.e., prosthetic heart valve), 1 to 2.5 mg doses should be used |
| Thiamine | Wernicke encephalopathy: Confusion, ophthalmoplegia, nystagmus, and ataxia. Korsakoff syndrome: Confabulations, personality changes, and permanent memory loss. Wernicke-Korsakoff syndrome: Presentation is a combination of Wernicke and Korsakoff syndrome. Dry beriberi: Polyneuropathy and symmetric muscle wasting. Wet beriberi: High-output heart failure | IV thiamine 250-500 mg/day should be given for 3-5 days, followed by oral thiamine 250-300 mg/day for chronic alcoholics and other patients at risk for deficiency. Patients with suspected Wernicke receive 500 mg of thiamine diluted in 50-100 mL of normal saline infused over 30 minutes three times daily for three days, followed by 250 mg IV daily for 3 to 5 days or until clinical improvement. 100 mg IV once per day for several days in patients with edema and congestion due to cardiovascular beriberi |
| Riboflavin | Cheilosis and corneal vascularization | 5 to 30 mg orally once per day until recovery. Other water-soluble vitamins should also be given |
| Niacin | Diarrhea, dermatitis, and dementia (also hallucinations) | Nicotinamide 250 to 500 mg orally once per day |
| Pantothenic acid | Enteritis, dermatitis, alopecia, and adrenal insufficiency may lead to “burning feet syndrome” | Pantothenic acid 5-10 mg orally once per day |
| Pyridoxine | Hyperirritability, peripheral neuropathy, convulsions, and sideroblastic anemia | Pyridoxine 50-100 mg orally once per day. Patients taking isoniazid should be given 30-50 mg orally once a day |
| Biotin | Isolated deficiency is relatively rare. Enteritis, dermatitis, and alopecia | Biotin 5-20 mg orally once per day |
| Folate | Megaloblastic anemia, glossitis, fatigue, and pale skin | Folic acid 400-1000 mcg orally once per day. Women who plan on becoming pregnant should be supplemented with 400 to 800 mcg once per day |
| Cobalamin | Megaloblastic anemia, paresthesias, depression/anxiety, pale skin, cognitive dysfunction, and SCD | Vitamin B12 1000-2000 mcg orally once per day in patients who are significantly deficient or possess neurologic symptoms. For severe deficiency, 1 mg IM 1-4 times per week for several weeks until anemia and symptoms resolve |
| Ascorbic acid | Scurvy-swollen gums, petechiae, anemia, poor wound healing, hemarthrosis, “corkscrew” hair, and perifollicular and subperiosteal hemorrhages | For scurvy, use 500-1000 mg orally once per day for 1-2 weeks until symptoms and signs resolve |
A multitude of studies have concluded that vitamin A supplementation reduces childhood morbidity and mortality, especially in patients with serum retinol concentrations less than 20 mcg/dL[171-173]. In regions with a high prevalence of vitamin A deficiency, the WHO recommends a one-time dose of 100000 IU in children 6 to 11 months, followed by 200,000 IU every 4 to 6 months up to age five[174,175]. For pregnant women who are at risk, the WHO recommends 10000 IU daily or 25,000 IU weekly for 12 weeks[176]. There are currently no international guidelines for the treatment of asymptomatic vitamin A deficiency for adults in resource-rich regions.
The most cost-effective method of replenishing 25(OH)D levels in patients with vitamin D deficiency is oral ergocalciferol at 50000 IU per week for eight weeks[177]. The optimal time for rechecking vitamin D stores after therapy is not defined, but the goal is to achieve at least a minimum of 30 ng/mL[177,178]. Serum 25(OH)D levels should be obtained again after completion of therapy, and if values have not reached or surpassed the minimum, a second eight-week course should be prescribed. If the target goal is still not achieved, the most likely cause is nonadherence or malabsorption. Patients with suspected malabsorption should be referred to a gastroenterologist for further evaluation. Once levels are replete, vitamin D should be instituted at 800 to 2000 IU daily from supplemental and dietary sources[177-179].
Vitamin E deficiency is extremely rare in humans and is mainly caused by irregularities in dietary fat absorption or metabolism[180]. A supplementation of 15 to 25 mg/kg/day or 200 IU of tocopherols can be used in patients who are deficient[180-182]. IM or subcutaneous injection can be used in patients who have oral ingestion and/or intestinal issues. The treatment of vitamin K deficiency is limited to neonates and anticoagulation overdoses (i.e., warfarin)[183-185].
The treatment of thiamine deficiency is based on the severity and the clinical presentation of the patient. In patients at high risk for thiamine deficiency, such as chronic alcoholics, IV thiamine 250-500 mg/day should be given for 3-5 days, followed by oral thiamine 250-300 mg/day[186,187]. Patients with suspected Wernicke encephalopathy should receive 500 mg of thiamine diluted in 50-100 mL of normal saline infused over 30 minutes three times daily for 3 days, followed by 250 mg IV daily for 3 to 5 days or until clinical improvement[188,189].
Vitamin B12 deficiency can be treated with oral therapy or IM injections of cyanocobalamin[190,191]. More than 8% of the standard injectable dose of 1 mg is absorbed, which allows for swift replacement in patients with severe neurologic symptoms or deficiency[190-192]. Guidelines from the British Society for Hematology recommend IM injections three times per week for two weeks in patients without neurologic deficits and every other day for up to three weeks or until improvement in patients with deficits[191]. In general, patients with irreversible causes of B12 deficiency should remain on lifelong therapy, whereas those with reversible causes should be treated until the deficiency is corrected or until symptoms resolve[193,194]. Oral high-dose B12 (1-2 mg daily) is equally effective to IM for correcting neurologic symptoms and anemia in patients with no contraindications to oral therapy[193-195].
Folate levels below two ng/mL are generally considered deficient[196,197]. All patients with folate deficiency should receive supplemental folic acid for treatment[198,199]. Oral folic acid (1-5 mg/day) typically suffice for management[198-200]. Other forms of therapy, including IV, IM, or subcutaneous, can be used in patients who are intolerant to oral medications. The duration of treatment is based on whether the cause of the initial deficiency persists. Patients with short gut or malabsorption syndromes typically require long-term management[196,198,199,201]. It is essential to obtain B12 levels in patients who are folate deficient as folic acid supplementation may correct the anemia but worsen the neurological signs and symptoms of B12 deficiency if present[202,203].
In summary, micronutrients are essential to sustain life, and nutrition is a modifiable risk factor that can be targeted to optimize health and well-being. Nutritional deficiencies can often lead to loss of bodily functions, developmental failure, and other medical conditions, including vision loss and lack of immunity. The major causes of nutritional deficiencies are insufficient food intake, malabsorption, and consumption of diets lacking essential nutrients. MNDs are the most prevalent type of nutritional deficiency and are a leading cause of worldwide morbidity and mortality. Deficiency in either fat or water-soluble vitamins can lead to serious conditions such as vision loss, pellagra, beriberi, recurrent infections, and decreased cognitive function, among others. Developing countries are most susceptible to these deficiencies, and diagnosis starts with a good clinical history and physical examination. Prompt recognition and treatment can drastically reduce morbidity and mortality, especially in developing countries.
| 1. | Díaz JR, de las Cagigas A, Rodríguez R. Micronutrient deficiencies in developing and affluent countries. Eur J Clin Nutr. 2003;57 Suppl 1:S70-S72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Cena H, Calder PC. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients. 2020;12:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 537] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 3. | Savarino G, Corsello A, Corsello G. Macronutrient balance and micronutrient amounts through growth and development. Ital J Pediatr. 2021;47:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 4. | Carreiro AL, Dhillon J, Gordon S, Higgins KA, Jacobs AG, McArthur BM, Redan BW, Rivera RL, Schmidt LR, Mattes RD. The Macronutrients, Appetite, and Energy Intake. Annu Rev Nutr. 2016;36:73-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Shenkin A. The key role of micronutrients. Clin Nutr. 2006;25:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Woodside JV, McCall D, McGartland C, Young IS. Micronutrients: dietary intake v. supplement use. Proc Nutr Soc. 2005;64:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Maggini S, Óvári V, Ferreres Giménez I, Pueyo Alamán MG. Benefits of micronutrient supplementation on nutritional status, energy metabolism, and subjective wellbeing. Nutr Hosp. 2021;38:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Steele E, Liu D, Omer E. Managing Micronutrient Deficiencies in High-Risk Patients: No Small Feat! Curr Nutr Rep. 2024;13:668-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Petry N, Jallow B, Sawo Y, Darboe MK, Barrow S, Sarr A, Ceesay PO, Fofana MN, Prentice AM, Wegmüller R, Rohner F, Phall MC, Wirth JP. Micronutrient Deficiencies, Nutritional Status and the Determinants of Anemia in Children 0-59 Months of Age and Non-Pregnant Women of Reproductive Age in The Gambia. Nutrients. 2019;11:2275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Shenkin A. Micronutrients in health and disease. Postgrad Med J. 2006;82:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Hibberd MC, Wu M, Rodionov DA, Li X, Cheng J, Griffin NW, Barratt MJ, Giannone RJ, Hettich RL, Osterman AL, Gordon JI. The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Sci Transl Med. 2017;9:eaal4069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 167] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 12. | Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci U S A. 2006;103:17589-17594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 13. | Ramakrishnan U. Prevalence of micronutrient malnutrition worldwide. Nutr Rev. 2002;60:S46-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 166] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Sun J, Wu H, Zhao M, Magnussen CG, Xi B. Prevalence and changes of anemia among young children and women in 47 low- and middle-income countries, 2000-2018. EClinicalMedicine. 2021;41:101136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Smith C, Teng F, Branch E, Chu S, Joseph KS. Maternal and Perinatal Morbidity and Mortality Associated With Anemia in Pregnancy. Obstet Gynecol. 2019;134:1234-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 16. | Wieringa FT, Dijkhuizen MA, Berger J. Micronutrient deficiencies and their public health implications for South-East Asia. Curr Opin Clin Nutr Metab Care. 2019;22:479-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Smith G. Micronutrient Fortification of Food: Issues for Asia. J Nutr Sci Vitaminol (Tokyo). 2015;61 Suppl:S183-S185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Stevens GA, Beal T, Mbuya MNN, Luo H, Neufeld LM; Global Micronutrient Deficiencies Research Group. Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: a pooled analysis of individual-level data from population-representative surveys. Lancet Glob Health. 2022;10:e1590-e1599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 239] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 19. | Percy L, Mansour D, Fraser I. Iron deficiency and iron deficiency anaemia in women. Best Pract Res Clin Obstet Gynaecol. 2017;40:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1390] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 21. | Mantadakis E, Chatzimichael E, Zikidou P. Iron Deficiency Anemia in Children Residing in High and Low-Income Countries: Risk Factors, Prevention, Diagnosis and Therapy. Mediterr J Hematol Infect Dis. 2020;12:e2020041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 22. | Al-Naseem A, Sallam A, Choudhury S, Thachil J. Iron deficiency without anaemia: a diagnosis that matters. Clin Med (Lond). 2021;21:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 23. | Camaschella C. Iron deficiency. Blood. 2019;133:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 367] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 24. | Muthayya S, Rah JH, Sugimoto JD, Roos FF, Kraemer K, Black RE. The global hidden hunger indices and maps: an advocacy tool for action. PLoS One. 2013;8:e67860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 25. | Stevens SL. Fat-Soluble Vitamins. Nurs Clin North Am. 2021;56:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Borel P, Dangles O, Kopec RE. Fat-soluble vitamin and phytochemical metabolites: Production, gastrointestinal absorption, and health effects. Prog Lipid Res. 2023;90:101220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 27. | Prentice AM, Paul AA. Fat and energy needs of children in developing countries. Am J Clin Nutr. 2000;72:1253S-1265S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Schmidt DR, Holmstrom SR, Fon Tacer K, Bookout AL, Kliewer SA, Mangelsdorf DJ. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J Biol Chem. 2010;285:14486-14494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 29. | Fabisiak N, Fabisiak A, Watala C, Fichna J. Fat-soluble Vitamin Deficiencies and Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2017;51:878-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Andrès E, Lorenzo-Villalba N, Terrade JE, Méndez-Bailon M. Fat-Soluble Vitamins A, D, E, and K: Review of the Literature and Points of Interest for the Clinician. J Clin Med. 2024;13:3641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 31. | Veraldi S, Pietrobattista A, Liccardo D, Basso MS, Mosca A, Alterio T, Cardile S, Benedetti S, Della Corte C, Candusso M. Fat soluble vitamins deficiency in pediatric chronic liver disease: The impact of liver transplantation. Dig Liver Dis. 2020;52:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Marcinowska-Suchowierska E, Kupisz-Urbańska M, Łukaszkiewicz J, Płudowski P, Jones G. Vitamin D Toxicity-A Clinical Perspective. Front Endocrinol (Lausanne). 2018;9:550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 257] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 33. | Carazo A, Macáková K, Matoušová K, Krčmová LK, Protti M, Mladěnka P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients. 2021;13:1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 34. | Huang Z, Liu Y, Qi G, Brand D, Zheng SG. Role of Vitamin A in the Immune System. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 298] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 35. | Dawson MI. The importance of vitamin A in nutrition. Curr Pharm Des. 2000;6:311-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Faustino JF, Ribeiro-Silva A, Dalto RF, Souza MM, Furtado JM, Rocha Gde M, Alves M, Rocha EM. Vitamin A and the eye: an old tale for modern times. Arq Bras Oftalmol. 2016;79:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Miyazono S, Isayama T, Delori FC, Makino CL. Vitamin A activates rhodopsin and sensitizes it to ultraviolet light. Vis Neurosci. 2011;28:485-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Ross AC. Diet in vitamin A research. Methods Mol Biol. 2010;652:295-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Grune T, Lietz G, Palou A, Ross AC, Stahl W, Tang G, Thurnham D, Yin SA, Biesalski HK. Beta-carotene is an important vitamin A source for humans. J Nutr. 2010;140:2268S-2285S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 319] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 40. | Sommer A. Vitamin a deficiency and clinical disease: an historical overview. J Nutr. 2008;138:1835-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 41. | Patil S, Zamwar UM, Mudey A. Etiology, Epidemiology, Pathophysiology, Signs and Symptoms, Evaluation, and Treatment of Vitamin A (Retinol) Deficiency. Cureus. 2023;15:e49011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 42. | Ge H, Di G, Song P, Han W, Chen P, Wang Y. Role of vitamin A on the ocular surface. Exp Eye Res. 2025;250:110179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Cheruvattath R, Orrego M, Gautam M, Byrne T, Alam S, Voltchenok M, Edwin M, Wilkens J, Williams JW, Vargas HE. Vitamin A toxicity: when one a day doesn't keep the doctor away. Liver Transpl. 2006;12:1888-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Silverman AK, Ellis CN, Voorhees JJ. Hypervitaminosis A syndrome: a paradigm of retinoid side effects. J Am Acad Dermatol. 1987;16:1027-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 77] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006;83:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 343] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 46. | Chen G, Weiskirchen S, Weiskirchen R. Vitamin A: too good to be bad? Front Pharmacol. 2023;14:1186336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 47. | Pludowski P, Grant WB, Karras SN, Zittermann A, Pilz S. Vitamin D Supplementation: A Review of the Evidence Arguing for a Daily Dose of 2000 International Units (50 µg) of Vitamin D for Adults in the General Population. Nutrients. 2024;16:391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 48. | Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 851] [Cited by in RCA: 743] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 49. | Cui X, Eyles DW. Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders. Nutrients. 2022;14:4353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 50. | Zhao R, Zhang W, Ma C, Zhao Y, Xiong R, Wang H, Chen W, Zheng SG. Immunomodulatory Function of Vitamin D and Its Role in Autoimmune Thyroid Disease. Front Immunol. 2021;12:574967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 51. | Sîrbe C, Rednic S, Grama A, Pop TL. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int J Mol Sci. 2022;23:9784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 129] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 52. | Benedik E. Sources of vitamin D for humans. Int J Vitam Nutr Res. 2022;92:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 53. | Knuschke P. Sun Exposure and Vitamin D. Curr Probl Dermatol. 2021;55:296-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 1157] [Article Influence: 105.2] [Reference Citation Analysis (1)] |
| 55. | Gallieni M, Cozzolino M, Fallabrino G, Pasho S, Olivi L, Brancaccio D. Vitamin D: physiology and pathophysiology. Int J Artif Organs. 2009;32:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Karagol C, Duyan Camurdan A. Evaluation of vitamin D levels and affecting factors of vitamin D deficiency in healthy children 0-18 years old. Eur J Pediatr. 2023;182:4123-4131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Fischer PR, Almasri NI. Nutritional rickets - Vitamin D and beyond. J Steroid Biochem Mol Biol. 2022;219:106070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Sahay M, Sahay R. Rickets-vitamin D deficiency and dependency. Indian J Endocrinol Metab. 2012;16:164-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 59. | Arboleya L, Braña I, Pardo E, Loredo M, Queiro R. Osteomalacia in Adults: A Practical Insight for Clinicians. J Clin Med. 2023;12:2714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 60. | Giustina A, Bilezikian JP, Adler RA, Banfi G, Bikle DD, Binkley NC, Bollerslev J, Bouillon R, Brandi ML, Casanueva FF, di Filippo L, Donini LM, Ebeling PR, Fuleihan GE, Fassio A, Frara S, Jones G, Marcocci C, Martineau AR, Minisola S, Napoli N, Procopio M, Rizzoli R, Schafer AL, Sempos CT, Ulivieri FM, Virtanen JK. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr Rev. 2024;45:625-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 87] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 61. | Lin CH, Lin CY, Sung YH, Li ST, Cheng BW, Weng SL, Chang SJ, Lee HC, Lee YJ, Ting WH, Chang HY, Wu YL, Lin CS. Effect of Oral Vitamin D3 Supplementation in Exclusively Breastfed Newborns: Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. J Bone Miner Res. 2022;37:786-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Libon F, Cavalier E, Nikkels AF. Skin color is relevant to vitamin D synthesis. Dermatology. 2013;227:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 63. | Morita R, Yamamoto I, Takada M, Ohnaka Y, Yuu I. [Hypervitaminosis D]. Nihon Rinsho. 1993;51:984-988. [PubMed] |
| 64. | Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S-586S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 588] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 65. | Kamphuis LS, Bonte-Mineur F, van Laar JA, van Hagen PM, van Daele PL. Calcium and vitamin D in sarcoidosis: is supplementation safe? J Bone Miner Res. 2014;29:2498-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Mustacich DJ, Bruno RS, Traber MG. Vitamin E. Vitam Horm. 2007;76:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005;25:151-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 381] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 68. | Sen CK, Khanna S, Rink C, Roy S. Tocotrienols: the emerging face of natural vitamin E. Vitam Horm. 2007;76:203-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 69. | Khadangi F, Azzi A. Vitamin E - The Next 100 Years. IUBMB Life. 2019;71:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | Lobo LMC, Hadler MCCM. Vitamin E deficiency in childhood: a narrative review. Nutr Res Rev. 2023;36:392-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 71. | Tanyel MC, Mancano LD. Neurologic findings in vitamin E deficiency. Am Fam Physician. 1997;55:197-201. [PubMed] |
| 72. | Satya-Murti S, Howard L, Krohel G, Wolf B. The spectrum of neurologic disorder from vitamin E deficiency. Neurology. 1986;36:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 65] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Corrigan JJ Jr, Marcus FI. Coagulopathy associated with vitamin E ingestion. JAMA. 1974;230:1300-1301. [PubMed] |
| 74. | Traber MG. Vitamin E and K interactions--a 50-year-old problem. Nutr Rev. 2008;66:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Finer NN, Peters KL, Hayek Z, Merkel CL. Vitamin E and necrotizing enterocolitis. Pediatrics. 1984;73:387-393. [PubMed] |
| 76. | Gröber U, Reichrath J, Holick MF, Kisters K. Vitamin K: an old vitamin in a new perspective. Dermatoendocrinol. 2014;6:e968490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 77. | Sato T, Inaba N, Yamashita T. MK-7 and Its Effects on Bone Quality and Strength. Nutrients. 2020;12:965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 78. | Yoshida M, Booth SL, Meigs JB, Saltzman E, Jacques PF. Phylloquinone intake, insulin sensitivity, and glycemic status in men and women. Am J Clin Nutr. 2008;88:210-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 79. | Fenn K, Strandwitz P, Stewart EJ, Dimise E, Rubin S, Gurubacharya S, Clardy J, Lewis K. Quinones are growth factors for the human gut microbiota. Microbiome. 2017;5:161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 80. | Tie JK, Stafford DW. Structure and function of vitamin K epoxide reductase. Vitam Horm. 2008;78:103-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Hao Z, Jin DY, Stafford DW, Tie JK. Vitamin K-dependent carboxylation of coagulation factors: insights from a cell-based functional study. Haematologica. 2020;105:2164-2173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Araki S, Shirahata A. Vitamin K Deficiency Bleeding in Infancy. Nutrients. 2020;12:780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 83. | Puckett RM, Offringa M. Prophylactic vitamin K for vitamin K deficiency bleeding in neonates. Cochrane Database Syst Rev. 2000;2000:CD002776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Kaesler N, Schurgers LJ, Floege J. Vitamin K and cardiovascular complications in chronic kidney disease patients. Kidney Int. 2021;100:1023-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 85. | Tsugawa N, Shiraki M. Vitamin K Nutrition and Bone Health. Nutrients. 2020;12:1909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 86. | Bruno EJ Jr, Ziegenfuss TN. Water-soluble vitamins: research update. Curr Sports Med Rep. 2005;4:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 87. | Coelho SC, Estevinho BN, Rocha F. Recent Advances in Water-Soluble Vitamins Delivery Systems Prepared by Mechanical Processes (Electrospinning and Spray-Drying Techniques) for Food and Nutraceuticals Applications-A Review. Foods. 2022;11:1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Sugihara T, Koda M, Okamoto T, Miyoshi K, Matono T, Oyama K, Hosho K, Okano JI, Isomoto H, Murawaki Y. Falsely Elevated Serum Vitamin B(12) Levels Were Associated with the Severity and Prognosis of Chronic Viral Liver Disease. Yonago Acta Med. 2017;60:31-39. [PubMed] |
| 89. | Tanaka K, Ao M, Kuwabara A. Insufficiency of B vitamins with its possible clinical implications. J Clin Biochem Nutr. 2020;67:19-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Hanna M, Jaqua E, Nguyen V, Clay J. B Vitamins: Functions and Uses in Medicine. Perm J. 2022;26:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 78] [Reference Citation Analysis (0)] |
| 91. | Mrowicka M, Mrowicki J, Dragan G, Majsterek I. The importance of thiamine (vitamin B1) in humans. Biosci Rep. 2023;43:BSR20230374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 92. | Manzetti S, Zhang J, van der Spoel D. Thiamin function, metabolism, uptake, and transport. Biochemistry. 2014;53:821-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 93. | Subki A, Ho CL, Ismail NFN, Zainal Abidin AA, Balia Yusof ZN. Identification and characterisation of thiamine pyrophosphate (TPP) riboswitch in Elaeis guineensis. PLoS One. 2020;15:e0235431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 94. | Frank LL. Thiamin in Clinical Practice. JPEN J Parenter Enteral Nutr. 2015;39:503-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 95. | Jones KS, Parkington DA, Cox LJ, Koulman A. Erythrocyte transketolase activity coefficient (ETKAC) assay protocol for the assessment of thiamine status. Ann N Y Acad Sci. 2021;1498:77-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 96. | Whitfield KC, Bourassa MW, Adamolekun B, Bergeron G, Bettendorff L, Brown KH, Cox L, Fattal-Valevski A, Fischer PR, Frank EL, Hiffler L, Hlaing LM, Jefferds ME, Kapner H, Kounnavong S, Mousavi MPS, Roth DE, Tsaloglou MN, Wieringa F, Combs GF Jr. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430:3-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 97. | Habas E, Farfar K, Errayes N, Rayani A, Elzouki AN. Wernicke Encephalopathy: An Updated Narrative Review. Saudi J Med Med Sci. 2023;11:193-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 98. | Arts NJ, Walvoort SJ, Kessels RP. Korsakoff's syndrome: a critical review. Neuropsychiatr Dis Treat. 2017;13:2875-2890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 99. | Wijnia JW. A Clinician's View of Wernicke-Korsakoff Syndrome. J Clin Med. 2022;11:6755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 100. | Shible AA, Ramadurai D, Gergen D, Reynolds PM. Dry Beriberi Due to Thiamine Deficiency Associated with Peripheral Neuropathy and Wernicke's Encephalopathy Mimicking Guillain-Barré syndrome: A Case Report and Review of the Literature. Am J Case Rep. 2019;20:330-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 101. | Lei Y, Zheng MH, Huang W, Zhang J, Lu Y. Wet beriberi with multiple organ failure remarkably reversed by thiamine administration: A case report and literature review. Medicine (Baltimore). 2018;97:e0010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 102. | Suwannasom N, Kao I, Pruß A, Georgieva R, Bäumler H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int J Mol Sci. 2020;21:950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 103. | Hustad S, McKinley MC, McNulty H, Schneede J, Strain JJ, Scott JM, Ueland PM. Riboflavin, flavin mononucleotide, and flavin adenine dinucleotide in human plasma and erythrocytes at baseline and after low-dose riboflavin supplementation. Clin Chem. 2002;48:1571-1577. [PubMed] |
| 104. | Powers HJ, Corfe BM, Nakano E. Riboflavin in development and cell fate. Subcell Biochem. 2012;56:229-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 105. | Aigrain L, Pompon D, Truan G. Role of the interface between the FMN and FAD domains in the control of redox potential and electronic transfer of NADPH-cytochrome P450 reductase. Biochem J. 2011;435:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 106. | Olfat N, Ashoori M, Saedisomeolia A. Riboflavin is an antioxidant: a review update. Br J Nutr. 2022;128:1887-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 107. | Petrović K, Djoković R, Cincović M, Hristovska T, Lalović M, Petrović M, Majkić M, Došenović Marinković M, Anđušić L, Devečerski G, Stojanović D, Štrbac F. Niacin Status Indicators and Their Relationship with Metabolic Parameters in Dairy Cows during Early Lactation. Animals (Basel). 2022;12:1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 108. | Kirkland JB. Niacin status impacts chromatin structure. J Nutr. 2009;139:2397-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 109. | Dhuguru J, Dellinger RW, Migaud ME. Defining NAD(P)(H) Catabolism. Nutrients. 2023;15:3064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 110. | Kern SE, Price-Whelan A, Newman DK. Extraction and measurement of NAD(P)(+) and NAD(P)H. Methods Mol Biol. 2014;1149:311-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 111. | Shibata K. Nutritional factors that regulate on the conversion of L-tryptophan to niacin. Adv Exp Med Biol. 1999;467:711-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 112. | Malfait P, Moren A, Dillon JC, Brodel A, Begkoyian G, Etchegorry MG, Malenga G, Hakewill P. An outbreak of pellagra related to changes in dietary niacin among Mozambican refugees in Malawi. Int J Epidemiol. 1993;22:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 113. | Ahmed A, Acharya S, Shukla S, Ahmed A, Ravikumar V. Beyond the Bottle: Niacin Deficiency and Chronic Alcoholism. Cureus. 2023;15:e49482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 114. | Seow HF, Bröer S, Bröer A, Bailey CG, Potter SJ, Cavanaugh JA, Rasko JE. Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nat Genet. 2004;36:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 177] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 115. | Shah GM, Shah RG, Veillette H, Kirkland JB, Pasieka JL, Warner RR. Biochemical assessment of niacin deficiency among carcinoid cancer patients. Am J Gastroenterol. 2005;100:2307-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 116. | Tahmoush AJ, Alpers DH, Feigin RD, Armbrustmacher V, Prensky AL. Hartnup disease. Clinical, pathological, and biochemical observations. Arch Neurol. 1976;33:797-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 117. | Song WL, FitzGerald GA. Niacin, an old drug with a new twist. J Lipid Res. 2013;54:2586-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 118. | Tahiliani AG, Beinlich CJ. Pantothenic acid in health and disease. Vitam Horm. 1991;46:165-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 117] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 119. | Leonardi R, Jackowski S. Biosynthesis of Pantothenic Acid and Coenzyme A. EcoSal Plus. 2007;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 214] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 120. | Freese R, Aarsland TE, Bjørkevoll M. Pantothenic acid - a scoping review for Nordic Nutrition Recommendations 2023. Food Nutr Res. 2023;67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 121. | Tang J, Feng Y, Zhang B, Wu Y, Guo Z, Liang S, Zhou Z, Xie M, Hou S. Severe pantothenic acid deficiency induces alterations in the intestinal mucosal proteome of starter Pekin ducks. BMC Genomics. 2021;22:491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 122. | Vrolijk MF, Opperhuizen A, Jansen EHJM, Hageman GJ, Bast A, Haenen GRMM. The vitamin B6 paradox: Supplementation with high concentrations of pyridoxine leads to decreased vitamin B6 function. Toxicol In Vitro. 2017;44:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 123. | Agnihotri G, Liu HW. PLP and PMP radicals: a new paradigm in coenzyme B6 chemistry. Bioorg Chem. 2001;29:234-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 124. | Ngo HP, Nguyen DQ, Park H, Park YS, Kwak K, Kim T, Lee JH, Cho KS, Kang LW. Conformational change of organic cofactor PLP is essential for catalysis in PLP-dependent enzymes. BMB Rep. 2022;55:439-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 125. | Sousou JM, Griffith EM, Marsalisi C, Reddy P. Pyridoxine Deficiency and Neurologic Dysfunction: An Unlikely Association. Cureus. 2023;15:e47647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 126. | Sawhney A, Singhal S, Patel R. Isolated Pyridoxine Deficiency Presenting as Peripheral Neuropathy Post-chemotherapy. Cureus. 2022;14:e26725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 127. | Soleymani T, Lo Sicco K, Shapiro J. The Infatuation With Biotin Supplementation: Is There Truth Behind Its Rising Popularity? A Comparative Analysis of Clinical Efficacy versus Social Popularity. J Drugs Dermatol. 2017;16:496-500. [PubMed] |
| 128. | Tong L. Structure and function of biotin-dependent carboxylases. Cell Mol Life Sci. 2013;70:863-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 129. | Zempleni J, Hassan YI, Wijeratne SS. Biotin and biotinidase deficiency. Expert Rev Endocrinol Metab. 2008;3:715-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 130. | Stratton SL, Henrich CL, Matthews NI, Bogusiewicz A, Dawson AM, Horvath TD, Owen SN, Boysen G, Moran JH, Mock DM. Marginal biotin deficiency can be induced experimentally in humans using a cost-effective outpatient design. J Nutr. 2012;142:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 131. | Ebara S. Nutritional role of folate. Congenit Anom (Kyoto). 2017;57:138-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 132. | Fox JT, Stover PJ. Folate-mediated one-carbon metabolism. Vitam Horm. 2008;79:1-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 290] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 133. | Ristow KA, Gregory JF 3rd, Damron BL. Effects of dietary fiber on the bioavailability of folic acid monoglutamate. J Nutr. 1982;112:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 134. | Ly A, Hoyt L, Crowell J, Kim YI. Folate and DNA methylation. Antioxid Redox Signal. 2012;17:302-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 135. | Green R, Datta Mitra A. Megaloblastic Anemias: Nutritional and Other Causes. Med Clin North Am. 2017;101:297-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 136. | Shohag MJ, Wei Y, Yu N, Lu L, Zhang J, He Z, Patring J, Yang X. Folate content and composition of vegetables commonly consumed in China. J Food Sci. 2012;77:H239-H245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 137. | Allen LH. Causes of vitamin B12 and folate deficiency. Food Nutr Bull. 2008;29:S20-34; discussion S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 197] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 138. | US Preventive Services Task Force, Barry MJ, Nicholson WK, Silverstein M, Chelmow D, Coker TR, Davis EM, Donahue KE, Jaén CR, Li L, Ogedegbe G, Rao G, Ruiz JM, Stevermer J, Tsevat J, Underwood SM, Wong JB. Folic Acid Supplementation to Prevent Neural Tube Defects: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA. 2023;330:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 139. | Smith AD, Warren MJ, Refsum H. Vitamin B(12). Adv Food Nutr Res. 2018;83:215-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 140. | Ge Y, Zadeh M, Mohamadzadeh M. Vitamin B12 Regulates the Transcriptional, Metabolic, and Epigenetic Programing in Human Ileal Epithelial Cells. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 141. | Froese DS, Fowler B, Baumgartner MR. Vitamin B(12), folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J Inherit Metab Dis. 2019;42:673-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 248] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 142. | Goulding CW, Postigo D, Matthews RG. Cobalamin-dependent methionine synthase is a modular protein with distinct regions for binding homocysteine, methyltetrahydrofolate, cobalamin, and adenosylmethionine. Biochemistry. 1997;36:8082-8091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 143. | Ledley FD, Lumetta M, Nguyen PN, Kolhouse JF, Allen RH. Molecular cloning of L-methylmalonyl-CoA mutase: gene transfer and analysis of mut cell lines. Proc Natl Acad Sci U S A. 1988;85:3518-3521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 144. | Seetharam B, Alpers DH. Absorption and transport of cobalamin (vitamin B12). Annu Rev Nutr. 1982;2:343-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 145. | GLASS GB. Deposition and storage of vitamin B12 in the normal and diseased liver. Gastroenterology. 1959;36:180-90; discussion 190. [PubMed] |
| 146. | Htut TW, Thein KZ, Oo TH. Pernicious anemia: Pathophysiology and diagnostic difficulties. J Evid Based Med. 2021;14:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 147. | Guéant JL, Guéant-Rodriguez RM, Alpers DH. Vitamin B12 absorption and malabsorption. Vitam Horm. 2022;119:241-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 148. | Jimenez JA, Rodriguez S, Gamboa R, Rodriguez L, Garcia HH; Cysticercosis Working Group in Peru. Diphyllobothrium pacificum infection is seldom associated with megaloblastic anemia. Am J Trop Med Hyg. 2012;87:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 149. | Ramzan NUH, Shahjahan K, Dhillon RA, Khan NTA, Hashmat MB, Anwer MU, Ahmed D, Afzal F, Tahir MM, Muzaffar A. Vitamin B12 Deficiency in Patients Taking Metformin: Pathogenesis and Recommendations. Cureus. 2024;16:e68550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 150. | Jhunjhnuwala D, Tanglay O, Briggs NE, Yuen MTY, Huynh W. Prognostic indicators of subacute combined degeneration from B12 deficiency: A systematic review. PM R. 2022;14:504-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 151. | Stabler SP. Clinical practice. Vitamin B12 deficiency. N Engl J Med. 2013;368:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 754] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 152. | Carr AC, Maggini S. Vitamin C and Immune Function. Nutrients. 2017;9:1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 662] [Cited by in RCA: 988] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 153. | Carr AC, Vissers MC. Synthetic or food-derived vitamin C--are they equally bioavailable? Nutrients. 2013;5:4284-4304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 154. | DePhillipo NN, Aman ZS, Kennedy MI, Begley JP, Moatshe G, LaPrade RF. Efficacy of Vitamin C Supplementation on Collagen Synthesis and Oxidative Stress After Musculoskeletal Injuries: A Systematic Review. Orthop J Sports Med. 2018;6:2325967118804544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 155. | Menniti FS, Knoth J, Diliberto EJ Jr. Role of ascorbic acid in dopamine beta-hydroxylation. The endogenous enzyme cofactor and putative electron donor for cofactor regeneration. J Biol Chem. 1986;261:16901-16908. [PubMed] |
| 156. | Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, Thorp JM Jr, Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Harper M, Smith WJ, Saade G, Sorokin Y, Anderson GB; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362:1282-1291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 302] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 157. | Gęgotek A, Skrzydlewska E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants (Basel). 2022;11:1993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 158. | Li N, Zhao G, Wu W, Zhang M, Liu W, Chen Q, Wang X. The Efficacy and Safety of Vitamin C for Iron Supplementation in Adult Patients With Iron Deficiency Anemia: A Randomized Clinical Trial. JAMA Netw Open. 2020;3:e2023644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 159. | Doseděl M, Jirkovský E, Macáková K, Krčmová LK, Javorská L, Pourová J, Mercolini L, Remião F, Nováková L, Mladěnka P, On Behalf Of The Oemonom. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients. 2021;13:615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 226] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 160. | Gandhi M, Elfeky O, Ertugrul H, Chela HK, Daglilar E. Scurvy: Rediscovering a Forgotten Disease. Diseases. 2023;11:78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 161. | Reikersdorfer KN, Singh A, Young JD, Batty MB, Steele AE, Yuen LC, Momtaz DA, Weissert JN, Liu DS, Hogue GD. The Troubling Rise of Scurvy: A Review and National Analysis of Incidence, Associated Risk Factors, and Clinical Manifestations. J Am Acad Orthop Surg Glob Res Rev. 2024;8:e24.00162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 162. | Sestili MA. Possible adverse health effects of vitamin C and ascorbic acid. Semin Oncol. 1983;10:299-304. [PubMed] |
| 163. | Ferraro PM, Curhan GC, Gambaro G, Taylor EN. Total, Dietary, and Supplemental Vitamin C Intake and Risk of Incident Kidney Stones. Am J Kidney Dis. 2016;67:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 164. | Lynch SR, Cook JD. Interaction of vitamin C and iron. Ann N Y Acad Sci. 1980;355:32-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 149] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 165. | Lane DJ, Richardson DR. The active role of vitamin C in mammalian iron metabolism: much more than just enhanced iron absorption! Free Radic Biol Med. 2014;75:69-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 166. | Ceglie G, Macchiarulo G, Marchili MR, Marchesi A, Rotondi Aufiero L, Di Camillo C, Villani A. Scurvy: still a threat in the well-fed first world? Arch Dis Child. 2019;104:381-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 167. | Allen LH. Micronutrients - Assessment, Requirements, Deficiencies, and Interventions. N Engl J Med. 2025;392:1006-1016. [PubMed] [DOI] [Full Text] |
| 168. | Selbuz S, Kırsaçlıoğlu CT, Kuloğlu Z, Yılmaz M, Penezoğlu N, Sayıcı U, Altuntaş C, Kansu A. Diagnostic Workup and Micronutrient Deficiencies in Children With Failure to Thrive Without Underlying Diseases. Nutr Clin Pract. 2019;34:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 169. | Argao EA, Heubi JE. Fat-soluble vitamin deficiency in infants and children. Curr Opin Pediatr. 1993;5:562-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 170. | Siener R, Machaka I, Alteheld B, Bitterlich N, Metzner C. Effect of Fat-Soluble Vitamins A, D, E and K on Vitamin Status and Metabolic Profile in Patients with Fat Malabsorption with and without Urolithiasis. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 171. | Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ. 2011;343:d5094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 172. | Ramakrishnan U, Martorell R. The role of vitamin A in reducing child mortality and morbidity and improving growth. Salud Publica Mex. 1998;40:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 173. | Sommer A, Tarwotjo I, Djunaedi E, West KP Jr, Loeden AA, Tilden R, Mele L. Impact of vitamin A supplementation on childhood mortality. A randomised controlled community trial. Lancet. 1986;1:1169-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 378] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 174. | Vitamin A supplementation: who, when and how. Community Eye Health. 2013;26:71. [PubMed] |
| 175. | Benn CS, Martins C, Rodrigues A, Jensen H, Lisse IM, Aaby P. Randomised study of effect of different doses of vitamin A on childhood morbidity and mortality. BMJ. 2005;331:1428-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 176. | McGuire S. WHO Guideline: Vitamin A supplementation in pregnant women. Geneva: WHO, 2011; WHO Guideline: Vitamin A supplementation in postpartum women. Geneva: WHO, 2011. Adv Nutr. 2012;3:215-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 177. | Bader DA, Abed A, Mohammad BA, Aljaberi A, Sundookah A, Habash M, Alsayed AR, Abusamak M, Al-Shakhshir S, Abu-Samak M. The Effect of Weekly 50,000 IU Vitamin D(3) Supplements on the Serum Levels of Selected Cytokines Involved in Cytokine Storm: A Randomized Clinical Trial in Adults with Vitamin D Deficiency. Nutrients. 2023;15:1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 178. | AlAteeq MA, AlShail A, AlZahrani A, AlNafisah O, Masuadi E, Alshahrani A. Effect of Monthly and Bi-Monthly 50,000 International Units (IU) Maintenance Therapy With Vitamin D3 on Serum Level of 25-Hydroxyvitamin D in Adults: A Randomized Controlled Trial. Cureus. 2021;13:e13929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 179. | Dědečková E, Viták R, Jirásko M, Králová M, Topolčan O, Pecen L, Fürst T, Brož P, Kučera R. Vitamin D(3) Supplementation: Comparison of 1000 IU and 2000 IU Dose in Healthy Individuals. Life (Basel). 2023;13:808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 180. | Traber MG. Vitamin E inadequacy in humans: causes and consequences. Adv Nutr. 2014;5:503-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 181. | Mittal M, Flora SJ. Vitamin E supplementation protects oxidative stress during arsenic and fluoride antagonism in male mice. Drug Chem Toxicol. 2007;30:263-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 182. | Sethi R, Takeda N, Nagano M, Dhalla NS. Beneficial effects of vitamin E treatment in acute myocardial infarction. J Cardiovasc Pharmacol Ther. 2000;5:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 183. | Hand I, Noble L, Abrams SA. Vitamin K and the Newborn Infant. Pediatrics. 2022;149:e2021056036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 184. | Mihatsch WA, Braegger C, Bronsky J, Campoy C, Domellöf M, Fewtrell M, Mis NF, Hojsak I, Hulst J, Indrio F, Lapillonne A, Mlgaard C, Embleton N, van Goudoever J; ESPGHAN Committee on Nutrition. Prevention of Vitamin K Deficiency Bleeding in Newborn Infants: A Position Paper by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2016;63:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 185. | Crowther MA, Ageno W, Garcia D, Wang L, Witt DM, Clark NP, Blostein MD, Kahn SR, Vesely SK, Schulman S, Kovacs MJ, Rodger MA, Wells P, Anderson D, Ginsberg J, Selby R, Siragusa S, Silingardi M, Dowd MB, Kearon C. Oral vitamin K versus placebo to correct excessive anticoagulation in patients receiving warfarin: a randomized trial. Ann Intern Med. 2009;150:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 186. | Dervaux A, Laqueille X. [Thiamine (vitamin B1) treatment in patients with alcohol dependence]. Presse Med. 2017;46:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 187. | Osiezagha K, Ali S, Freeman C, Barker NC, Jabeen S, Maitra S, Olagbemiro Y, Richie W, Bailey RK. Thiamine deficiency and delirium. Innov Clin Neurosci. 2013;10:26-32. [PubMed] |
| 188. | Praharaj SK, Munoli RN, Shenoy S, Udupa ST, Thomas LS. High-dose thiamine strategy in Wernicke-Korsakoff syndrome and related thiamine deficiency conditions associated with alcohol use disorder. Indian J Psychiatry. 2021;63:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 189. | Nishimoto A, Usery J, Winton JC, Twilla J. High-dose Parenteral Thiamine in Treatment of Wernicke's Encephalopathy: Case Series and Review of the Literature. In Vivo. 2017;31:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 190. | Obeid R, Andrès E, Češka R, Hooshmand B, Guéant-Rodriguez RM, Prada GI, Sławek J, Traykov L, Ta Van B, Várkonyi T, Reiners K; The Vitamin B Consensus Panelists Group. Diagnosis, Treatment and Long-Term Management of Vitamin B12 Deficiency in Adults: A Delphi Expert Consensus. J Clin Med. 2024;13:2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 191. | Abdelwahab OA, Abdelaziz A, Diab S, Khazragy A, Elboraay T, Fayad T, Diab RA, Negida A. Efficacy of different routes of vitamin B12 supplementation for the treatment of patients with vitamin B12 deficiency: A systematic review and network meta-analysis. Ir J Med Sci. 2024;193:1621-1639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 192. | Wolffenbuttel BH, Owen PJ, Ward M, Green R. Vitamin B(12). BMJ. 2023;383:e071725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 193. | Wolffenbuttel BHR, McCaddon A, Ahmadi KR, Green R. A Brief Overview of the Diagnosis and Treatment of Cobalamin (B12) Deficiency. Food Nutr Bull. 2024;45:S40-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 194. | Stover PJ. Vitamin B12 and older adults. Curr Opin Clin Nutr Metab Care. 2010;13:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 195. | Vidal-Alaball J, Butler CC, Cannings-John R, Goringe A, Hood K, McCaddon A, McDowell I, Papaioannou A. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst Rev. 2005;CD004655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 196. | Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am J Clin Nutr. 2011;94:666S-672S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 197. | Ho CH, Yuan CC, Yeh SH. Serum folate levels in normal full-term pregnant Chinese women. Acta Obstet Gynecol Scand. 1988;67:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 198. | Donnelly JG. Folic acid. Crit Rev Clin Lab Sci. 2001;38:183-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 199. | Shulpekova Y, Nechaev V, Kardasheva S, Sedova A, Kurbatova A, Bueverova E, Kopylov A, Malsagova K, Dlamini JC, Ivashkin V. The Concept of Folic Acid in Health and Disease. Molecules. 2021;26:3731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 200. | Finkelstein JL, Cuthbert A, Weeks J, Venkatramanan S, Larvie DY, De-Regil LM, Garcia-Casal MN. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2024;8:CD004736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 201. | Okereke OI, Cook NR, Albert CM, Van Denburgh M, Buring JE, Manson JE. Effect of long-term supplementation with folic acid and B vitamins on risk of depression in older women. Br J Psychiatry. 2015;206:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 202. | Shane B, Stokstad EL. Vitamin B12-folate interrelationships. Annu Rev Nutr. 1985;5:115-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 215] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 203. | Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 430] [Article Influence: 23.9] [Reference Citation Analysis (0)] |