Published online Jun 20, 2025. doi: 10.5662/wjm.v15.i2.99580
Revised: October 11, 2024
Accepted: November 4, 2024
Published online: June 20, 2025
Processing time: 125 Days and 1.9 Hours
Epilepsy impacts millions of people, with many not responding to existing treatments. Some evidence links neuroinflammatory processes to epilepsy. Statins exhibit anti-inflammatory and neuroprotective properties, potentially offering antiepileptic effects.
To evaluate the anticonvulsant effects of rosuvastatin in animal models of epilepsy.
Ninety-six albino mice were divided into 16 groups. In the maximal electroshock seizure (MES) model, eight groups received intraperitoneal vehicle, carbama
In the MES model, rosuvastatin exhibited protection against THLE in a small percentage of mice. Rosuvastatin shortens the duration of THLE in a dose-dependent manner. However, none of these were statistically significant com
Rosuvastatin enhanced the anticonvulsant effects of carbamazepine and valproate. Further studies are required to explore the antiepileptic potential of rosuvastatin at various doses, durations, dosage forms, routes and models.
Core Tip: Many patients do not benefit from the available antiepileptics. Statins are known for their pleiotropic effects, which include neuroprotective properties. We investigated whether rosuvastatin, a potent statin, has anticonvulsant properties on its own or if it potentiates the effects of standard anticonvulsants. The study used maximal electroshock and pentylenetetrazol seizure models in albino mouse We observed that rosuvastatin potentiated some of the anticonvulsant effects of the standard antiepileptics carbamazepine and valproate.
- Citation: Tayal V, Mandal A, Haque M I, Mishra A, Kalra BS, Roy V. Anticonvulsant potential of rosuvastatin in combination with carbamazepine and valproate in animal models of epilepsy. World J Methodol 2025; 15(2): 99580
- URL: https://www.wjgnet.com/2222-0682/full/v15/i2/99580.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i2.99580
Epilepsy, a prevalent neurological disorder affecting more than 50 million individuals globally, is characterized by abnormal brain activity leading to seizures and various neurological manifestations[1]. Despite the availability of numerous antiepileptic drugs, a considerable proportion of patients remain unresponsive to standard treatment and even add-on therapies[2]. The ongoing pursuit of innovative anticonvulsant strategies underscores the intricate nature of epilepsy and the imperative need for novel therapeutic interventions.
Recent evidence has linked neuroinflammatory processes to epilepsy, emphasizing the role of inflammation in determining the seizure threshold and recurrence. Clinical observations support the notion that inflammation contributes to epilepsy, as evidenced by the prevalence of seizures in patients with autoimmune disorders, increased proinflammatory cytokines in febrile seizures, and the anticonvulsant efficacy of steroids in infantile spasms. Experimental studies conducted in rodent models have provided additional support for the notion that brain inflammation facilitates seizures and neuronal hyper-excitability[3,4]. This insight has sparked interest in pharmacological strategies targeting neuroinflammation for potential antiepileptic effects.
Statins, inhibitors of the 3-hydroxy-3-methylglutaryl Coenzyme A reductase enzyme, exhibit pleiotropic effects, including antithrombotic, anti-inflammatory, and antioxidative effects, in addition to their primary role in reducing cholesterol levels. A substantial body of evidence supports the neuroprotective effects of statins in various neuropathological conditions, including stroke, traumatic brain injury, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and epilepsy[5]. Statins have been shown to suppress the expression of pro-inflammatory genes, as well as the release of pro-inflammatory cytokines, chemokines, adhesion molecules, and matrix metalloproteinases. Furthermore, preliminary research suggests that statins' net effect on neurosteroids may contribute to their antiepileptic properties[6,7].
Research on the antiepileptic effects of statins has focused predominantly on atorvastatin, revealing its protective role in chemically induced convulsions and audiogenic seizures[8,9]. Rosuvastatin, a potent statin, has been shown to reduce neuroinflammation, improve blood-brain barrier (BBB) integrity, and increase endothelial nitric oxide synthase mRNA expression[10]. A recent study showed that rosuvastatin has protective effect against pentylenetetrazol (PTZ)-induced seizures, increasing current electroshock-induced seizures, and PTZ-induced status epilepticus[11]. Hence, this study was undertaken to explore the protective effects of rosuvastatin against PTZ seizures and maximal electroshock induced seizures and examine its interaction with commonly prescribed antiepileptic drugs.
The study was conducted on albino mice at the Central Animal Facility and Department of Pharmacology, Maulana Azad Medical College (MAMC), New Delhi. Approval for the study was obtained from the Institutional Animal Ethics Committee MAMC, New Delhi.
A total of 96 mice were used for this study. Albino mice aged 2-3 months and weighing 20-30 g were procured from the CSIR Institute of Genomics and Integrative Biology. The mice were housed in polypropylene cages under controlled environmental conditions, with a 12-hour light-dark cycle and free access to food and water. Animals were acclimatized to laboratory conditions before the experiments.
Drugs (rosuvastatin, carbamazepine, and valproate) were procured from MedChemExpress Pvt. Ltd. PTZ and polypropylene glycol were provided by Sun Pharmaceutical Industries Ltd.
The 96 mice were randomly divided into 16 groups. Randomization was performed using computer-generated random number tables. For the maximal electroshock seizure (MES) model, various treatments were administered intraperitoneally to eight groups of six mice each. The control group received vehicle (polypropylene glycol) at a dose of 10 mg/kg. The three groups received carbamazepine at doses of 4 mg/kg, 6 mg/kg, and 8 mg/kg, respectively. Another three groups received rosuvastatin at doses of 10 mg/kg, 20 mg/kg, and 30 mg/kg. The final group received a combination of carbamazepine and rosuvastatin at doses of 4 mg/kg and 10 mg/kg, respectively. In the PTZ seizure model study, eight groups of six mice each were similarly treated by intraperitoneal injection. The control group received vehicle (polypropylene glycol) at a dose of 10 mg/kg. Three groups received valproate at doses of 100 mg/kg, 200 mg/kg, and 400 mg/kg, respectively. Another three groups received rosuvastatin at doses of 10 mg/kg, 20 mg/kg, and 30 mg/kg. The final group received a combination of valproate and rosuvastatin at doses of 100 mg/kg and 10 mg/kg, respectively.
Two experimental seizure models were used: (1) The MES model (8 groups); and (2) The PTZ model (8 groups).
MES was induced via an electroconvulsiometer with transauricular electrodes after 40 minutes of intraperitoneal administration of the vehicle or test drugs. A current of 30 milliamperes was applied at a frequency of 50 Hz for a period of 0.2 seconds. The outcomes measured were seizure protection [presence or absence of tonic hind limb extension (THLE)], mean duration of THLE, mean duration of seizure activity, and percentage mortality.
PTZ (dose 30 mg/kg) was administered intraperitoneally to the mice to induce seizures after 40 minutes of intraperitoneal administration of vehicle or test drugs. The outcomes measured were seizure onset latency (the time to whole-body clonus event, determined after the intraperitoneal administration of PTZ), the mean duration of tonic-clonic convulsions, and percentage mortality.
The sample size of 6 mice per group was determined on the basis of a power of 80%, with the type 1 error rate set at 5%, the duration of PTZ-induced seizure standard deviation of 18.05, and an effect size of 30[12]. The data were compiled and analyzed using MS Excel Office 365 and Statistical Package for the Social Sciences version 25. Fisher's exact test was used to compare proportions. Continuous variables were analyzed via the Kruskal-Wallis test for multigroup analysis. Post hoc analysis for intergroup comparisons was performed via Dunn’s post hoc test. A P value of ≤ 0.05 was considered significant.
Seizure protection from MES: All the mice in the control group exhibited maximal electroshock induced seizures characterized by THLE. All the mice in groups given carbamazepine at various doses and in combination with rosuvastatin showed 100% protection against THLE. THLE was absent in 1 of the 6 mice given rosuvastatin alone at a dose of 10 mg/kg, whereas it was absent in 2 of the 6 mice in the rosuvastatin 20 mg/kg and 30 mg/kg groups. However, this protection was not statistically significant compared with that of control group (Table 1).
Duration of THLE: The mean duration of THLE in the control group was 6.73 seconds ± 0.51 seconds. THLE was not observed in groups administered carbamazepine either alone or in combination with rosuvastatin. We observed a shorter mean duration of THLE in the groups of mice pretreated with rosuvastatin, i.e., 5.8 seconds ± 1.18 seconds in the rosuvastatin 10 mg/kg group, 4.47 seconds ± 1.41 seconds in the rosuvastatin 20 mg/kg group and 4.93 seconds ± 1.58 seconds in the rosuvastatin 30 mg/kg group. However, these reductions were not statistically significant compared with those in the control group (Table 2).
| Treatment groups | Mean duration of tonic hind limb extension (seconds) | SEM |
| Vehicle (control group) | 6.73 | 0.51 |
| Carbamazepine 4 mg/kg | 0a | 0 |
| Carbamazepine 6 mg/kg | 0a | 0 |
| Carbamazepine 8 mg/kg | 0a | 0 |
| Rosuvastatin 10 mg/kg | 5.80 | 1.18 |
| Rosuvastatin 20 mg/kg | 4.47 | 1.41 |
| Rosuvastatin 30 mg/kg | 4.93 | 1.58 |
| Rosuvastatin 10 mg/kg + carbamazepine 4 mg/kg | 0a | 0 |
Duration of seizure activity: The mean duration of seizure activity in the control group of mice was 22.01 seconds ± 1.09 seconds. A dose-dependent decrease in the duration of seizure activity was observed with carbamazepine. The mean duration of seizure activity was 14.37 seconds ± 0.68 seconds, 11.63 seconds ± 0.39 seconds and 10.33 seconds ± 0.58 seconds in mice pretreated with carbamazepine at 4 mg/kg, 6 mg/kg, and 8 mg/kg, respectively. Compared with that in the control group, the duration of seizure activity in the 8 mg/kg carbamazepine group was significantly shorter.
In mice administered rosuvastatin 10 mg/kg, 20 mg/kg, and 30 mg/kg, the mean duration of seizure activity was similar to that of the control group. The combined administration of rosuvastatin 10 mg/kg and carbamazepine 4 mg/kg resulted in a significant reduction in the mean seizure duration (10.20 seconds ± 0.60 seconds), whereas the reduction in seizure duration in the mice treated with carbamazepine (4 mg/kg) alone was not statistically significant compared with that in the control group (Table 3).
| Treatment groups | Mean duration of seizure (seconds) | SEM |
| Vehicle (control group) | 22.00 | 1.09 |
| Carbamazepine 4 mg/kg | 14.37 | 0.68 |
| Carbamazepine 6 mg/kg | 11.63 | 0.39 |
| Carbamazepine 8 mg/kg | 10.33a | 0.58 |
| Rosuvastatin 10 mg/kg | 23.80 | 1.14 |
| Rosuvastatin 20 mg/kg | 21.13 | 0.74 |
| Rosuvastatin 30 mg/kg | 22.53 | 0.61 |
| Rosuvastatin 10 mg/kg + carbamazepine 4 mg/kg | 10.20a | 0.60 |
Mortality in the MES model: No mortality was observed in the mice subjected to MES in any of the groups, including the control group.
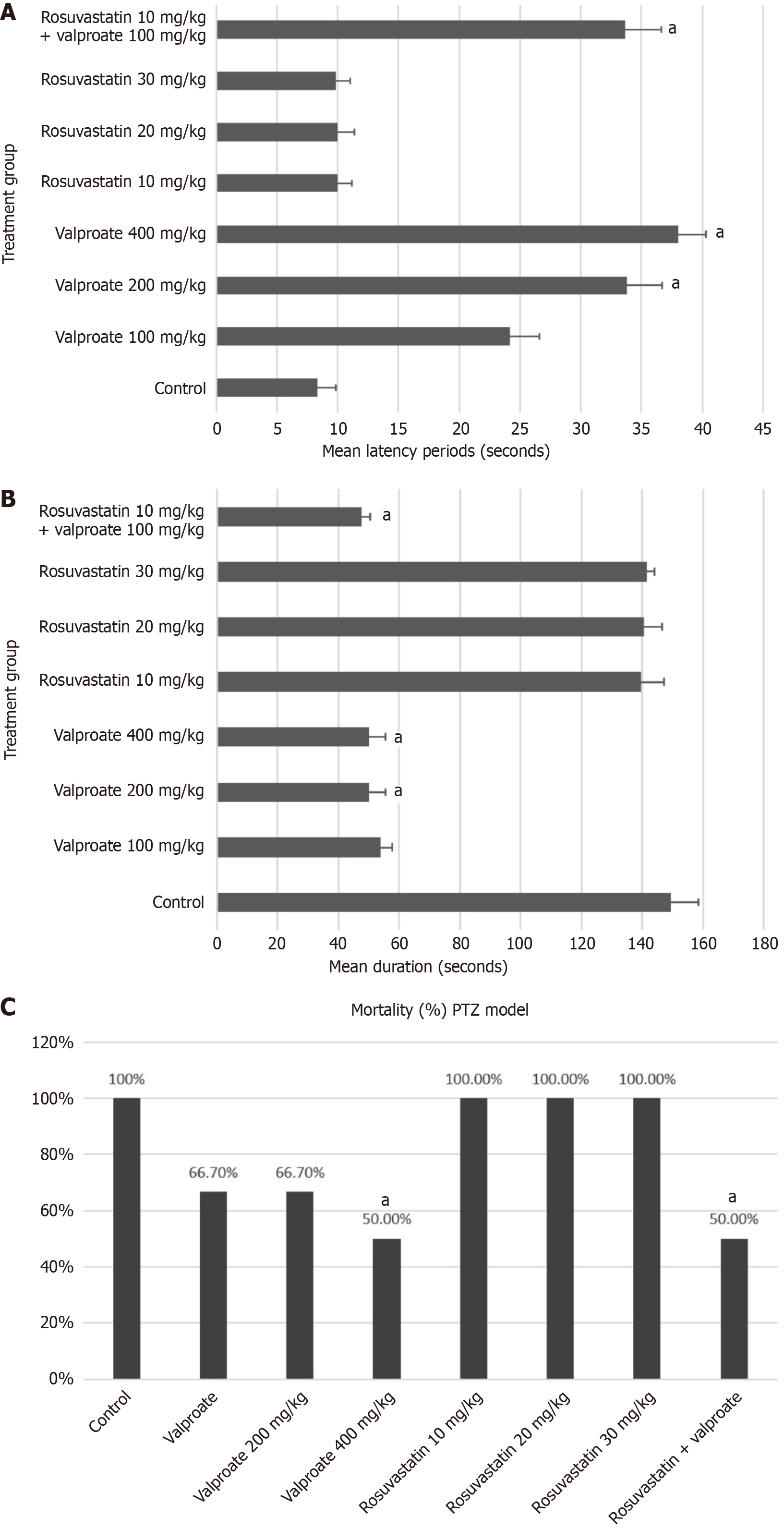
Latency period: The mean latency to seizure onset observed in the control group was 8.80 seconds ± 0.53 seconds (Figure 1A). In mice pretreated with 200 mg/kg or 400 mg/kg valproate, the mean latency was significantly greater (34-38 seconds). We also observed a longer mean latency in mice pretreated with 10 mg/kg, 20 mg/kg, and 30 mg/kg rosuvastatin (10.43 seconds ± 0.60 seconds, 10.50 seconds ± 0.59 seconds, and 10.23 seconds ± 0.43 seconds, respectively). Although slightly longer latency was observed in the rosuvastatin groups than in the control group, this difference was not statistically significant. In mice pretreated with rosuvastatin 10 mg/kg + valproate 100 mg/kg, the mean latency time was 33.70 seconds ± 0.87 seconds and was significantly longer compared to the control group. Moreover, the latency period in the valproate 100 mg/kg group was not statistically different from that in the control group (Figure 1A).
Duration of tonic-clonic convulsions: The tonic-clonic convulsions lasted an average of 149.33 seconds ± 3.58 seconds in the control group (Figure 1B). In the mice pretreated with 100 mg/kg, 200 mg/kg, and 400 mg/kg valproate, the mean durations of tonic-clonic convulsions were 54.47 seconds ± 1.41 seconds, 50.93 seconds ± 2.15 seconds, and 50.47 seconds ± 0.97 seconds, respectively. The shorter seizure duration observed at doses of 200 mg/kg and 400 mg/kg valproate was statistically significant compared with that of the control group.
We observed that in the mice pretreated with 10 mg/kg, 20 mg/kg, and 30 mg/kg rosuvastatin, the mean durations of tonic-clonic convulsions were 140.13 seconds ± 2.91 seconds, 141.20 seconds ± 2.40 seconds, and 142.00 seconds ± 1.05 seconds, respectively. Although a slightly shorter duration of tonic-clonic convulsions was observed in the rosuvastatin groups than in the control group, the differences were not statistically significant. In mice pretreated with 10 mg/kg rosuvastatin + 100 mg/kg valproate, the mean duration of tonic-clonic convulsions was 48.17 seconds ± 1.19 seconds, which was significantly shorter than that in the control group. We also noted a slightly shorter duration of tonic-clonic convulsions in the rosuvastatin 10 mg/kg + valproate 100 mg/kg combination group than in the valproate (100 mg/kg) alone group. However, this difference was not statistically significant (Figure 1B).
Mortality in PTZ model: We recorded 100% mortality in the control group and in the groups administered rosuvastatin at dosages of 10 mg/kg, 20 mg/kg, and 30 mg/kg (Figure 1C). In the 100 mg/kg and 200 mg/kg valproate groups, four out of six mice (66.7%) died, whereas the 400 mg/kg valproate group exhibited a mortality rate of 50%. We also observed a mortality of 50% in the group receiving a combination of rosuvastatin (10 mg/kg) and valproate (100 mg/kg). When mortality was compared with that of the control group, only valproate 400 mg/kg and rosuvastatin (10 mg/kg) + valproate (100 mg/kg) provided statistically significant protection (Figure 1C).
The aim of the present study was to evaluate and compare the anticonvulsant potential of rosuvastatin alone and in combination with standard antiepileptic drugs in albino mice. Two different seizure models were used for evaluation, namely the MES model for inducing generalized tonic-clonic seizures (GTCS) and the PTZ model for inducing absence seizures. Carbamazepine, indicated in the GTCS was used as the comparison standard for the MES model, whereas sodium valproate was used as the comparison standard for the PTZ model. The results of the present study revealed a dose-dependent decrease in seizure activity with both carbamazepine and valproate in the MES and PTZ seizure models, respectively. These observations are consistent with previous studies that have demonstrated the effectiveness of carbamazepine and valproate in reducing seizure activity in various animal models of seizures[13].
In the MES model, only a small proportion of mice treated with rosuvastatin at different doses exhibited protection against THLE. We also found that increasing rosuvastatin doses resulted in a shorter duration of THLE than did the control. However, these findings were not statistically significant compared with that in control mice. We were unable to ascertain whether rosuvastatin has a synergistic effect because both mice treated with carbamazepine alone and mice treated with the combination of carbamazepine-rosuvastatin showed 100% protection against THLE. This finding is in line with earlier studies using atorvastatin, which, when given as a single acute dose in MES animal models, did not demonstrate any protective effect[14-16]. However, in a study administration of acute dose of fluvastatin (80 mg/kg) enhanced the anticonvulsant potential of carbamazepine and valproate by decreasing their half effective dose values[14]. The interaction of fluvastatin with carbamazepine was found to be pharmacokinetic in nature as it resulted in a 61% significant increase in total brain concentration of carbamazepine. Similarly, in another study, acute lovastatin dose (10 mg/kg) was observed to significantly enhance the anticonvulsant effect of valproate in MES model, which was accompanied with a 34% significant increase in total brain concentration of valproate[17]. Their findings also revealed that chronic administration of lovastatin (10 mg/kg, once daily for 7 consecutive days) potentiated the antiseizure properties of phenytoin in the MES test in mice but this was without any impact on total brain level of phenytoin in mice[17].
In our study, the mean seizure duration was not shorter in the rosuvastatin alone group than in the control group at any of the tested doses. However, the combination of rosuvastatin 10 mg/kg with carbamazepine 4 mg/kg resulted in a significant reduction in seizure duration compared with that to the control group. It should be noted that seizure duration was not significantly shortened when carbamazepine 4 mg/kg was administered alone. Previous studies did not mention the total duration of seizure activity in MES models[17].
Based on the above findings, we assume that rosuvastatin has the potential to potentiate the antiepileptic effects of carbamazepine through either pharmacokinetic or pharmacodynamic interactions. We could not ascertain from the existing literature the precise mechanism through which rosuvastatin may have enhanced carbamazepine, which thus needs to be explored in further studies.
In the PTZ model, rosuvastatin 10 mg/kg in combination with valproate 100 mg/kg significantly delayed seizure onset in mice. Compared with that in the control group and the 100 mg/kg valproate group, the mean latency period was significantly longer in the group pretreated with the rosuvastatin-valproate combination. However, in mice pretreated with rosuvastatin 10 mg/kg, 20 mg/kg, or 30 mg/kg, the latency period was similar to that in the control group. In a previous study which investigated the protective role of intranasal rosuvastatin liquid crystal nanoparticles against PTZ-induced seizures, it was observed that intranasal rosuvastatin had significantly longer latency and was more effective than oral and intraperitoneal rosuvastatin groups[11]. The study was based on the fact that rosuvastatin is more hydrophilic and the BBB permeability is comparatively poor compared to atorvastatin, simvastatin, etc. The same limitation of rosuvastatin may have undermined the antiepileptic potential of rosuvastatin, as shown in our study[9]. Likewise, no effect on the mean duration of PTZ-induced tonic-clonic convulsions was observed after administration of rosuvastatin at any dose.
In one study, a single oral dose of atorvastatin (10 mg/kg) did not alter the latency of PTZ-induced seizures, whereas chronic treatment (over 7 days) delayed PTZ-induced generalized seizures[9]. In another study, a single oral dose of atorvastatin in rats significantly prolonged the time to seizure onset[15]. However, in the PTZ model, simvastatin was found to be ineffective, although it had an anticonvulsant effect on kainic acid-induced, picrotoxin-induced, and audiogenic seizure models[17]. In another study, lovastatin at higher doses administered intraperitoneally four days before testing significantly increased the threshold for PTZ-induced convulsions in mice[18].
We observed a 50% significant reduction in mortality in mice pretreated with the combination of 10 mg/kg rosuvastatin and 100 mg/kg valproate. Similar protection was found in mice administered a higher dose of valproate (400 mg/kg). We assume that rosuvastatin might have enhanced the protective anticonvulsant effect of valproate here. Even in the mice given rosuvastatin alone, 33% of mice were protected, but this difference was not statistically significant. In a previous study, intraperitoneal rosuvastatin prevented mortality in PTZ-induced status epilepticus by only 17%, while intranasal rosuvastatin protected 66% of the mice from mortality[11]. In a previously published study with atorvastatin, mortality protection from PTZ-induced seizures was only up to 33%[19].
Some inconsistencies between the present study results and those of previous studies with different statins may be attributed to several factors, such as differences in animal species, dose, duration, dosage form/route of administration, and seizure models. The hydrophilic nature of rosuvastatin and poor BBB permeability may be a major limiting factor for its potential antiepileptic effect[20].
The present results showed that acute single-dose treatment with rosuvastatin, when administered alone, had no anticonvulsant effect in either the maximal electroshock-induced seizure model or the pentylene-tetrazole-induced seizure model. However, a combination of rosuvastatin and standard anticonvulsants provided protection in the MES and PTZ models; therefore, rosuvastatin may have the potential to enhance the antiepileptic effects of carbamazepine and valproate. Therefore, further studies are needed to explore the potential of rosuvastatin with longer durations, different dosage forms, and routes of administration in different seizure models and to further clarify the anticonvulsant potential of rosuvastatin in epilepsy.
The authors acknowledge all the supporting staffs in the Central Animal House Facility and CNS Laboratory in Maulana Azad Medical College, New Delhi.
| 1. | World Health Organization. Epilepsy. Available from: https://www.who.int/news-room/fact-sheets/detail/epilepsy. |
| 2. | Sultana B, Panzini MA, Veilleux Carpentier A, Comtois J, Rioux B, Gore G, Bauer PR, Kwon CS, Jetté N, Josephson CB, Keezer MR. Incidence and Prevalence of Drug-Resistant Epilepsy: A Systematic Review and Meta-analysis. Neurology. 2021;96:805-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 216] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 3. | Terrone G, Salamone A, Vezzani A. Inflammation and Epilepsy: Preclinical Findings and Potential Clinical Translation. Curr Pharm Des. 2017;23:5569-5576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 1374] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 5. | Kavalipati N, Shah J, Ramakrishan A, Vasnawala H. Pleiotropic effects of statins. Indian J Endocrinol Metab. 2015;19:554-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | Reddy DS. Role of hormones and neurosteroids in epileptogenesis. Front Cell Neurosci. 2013;7:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Scicchitano F, Constanti A, Citraro R, De Sarro G, Russo E. Statins and epilepsy: preclinical studies, clinical trials and statin-anticonvulsant drug interactions. Curr Drug Targets. 2015;16:747-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Quintana-Pájaro LJ, Ramos-Villegas Y, Cortecero-Sabalza E, Joaquim AF, Agrawal A, Narvaez-Rojas AR, Moscote-Salazar LR. The Effect of Statins in Epilepsy: A Systematic Review. J Neurosci Rural Pract. 2018;9:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Funck VR, de Oliveira CV, Pereira LM, Rambo LM, Ribeiro LR, Royes LF, Ferreira J, Guerra GP, Furian AF, Oliveira MS, Mallmann CA, de Mello CF, Oliveira MS. Differential effects of atorvastatin treatment and withdrawal on pentylenetetrazol-induced seizures. Epilepsia. 2011;52:2094-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Husain I, Khan S, Khan S, Madaan T, Kumar S, Najmi AK. Unfolding the pleiotropic facades of rosuvastatin in therapeutic intervention of myriads of neurodegenerative disorders. Clin Exp Pharmacol Physiol. 2019;46:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Ahmed MZ, Khan UA, Haye A, Agarwal NB, Alhakamy NA, Alhadrami HA, Warsi MH, Jain GK. Liquid Crystalline Nanoparticles for Nasal Delivery of Rosuvastatin: Implications on Therapeutic Efficacy in Management of Epilepsy. Pharmaceuticals (Basel). 2020;13:356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4:303-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 717] [Cited by in RCA: 1218] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 13. | Yuen ES, Trocóniz IF. Can pentylenetetrazole and maximal electroshock rodent seizure models quantitatively predict antiepileptic efficacy in humans? Seizure. 2015;24:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Stepien KM, Tomaszewski M, Luszczki JJ, Czuczwar SJ. The interactions of atorvastatin and fluvastatin with carbamazepine, phenytoin and valproate in the mouse maximal electroshock seizure model. Eur J Pharmacol. 2012;674:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Singh A, S. R. Potential anti-seizure activity of atorvastatin in rat models of seizure. Int J Basic Clin Pharmacol. 2017;. [DOI] [Full Text] |
| 16. | Rajangam J, Manogna K, Mounica K, Padma C, Swathi A. Behavioural Influence of Atorvastatin alone and in Combination with Antiepileptics against Electroconvulsions in Mice. Malays J Med Biol Res. 2017;4:63-70. [DOI] [Full Text] |
| 17. | Banach M, Czuczwar SJ, Borowicz KK. Statins - are they anticonvulsant? Pharmacol Rep. 2014;66:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Mirhadi K, Dariush M, Reza A. Effect of Lovastatin on PTZ-Induced Seizure Threshold in Mice. Global Veterinaria. 2011;7:386-390. |
| 19. | Shafaroodi H, Moezi L, Fakhrzad A, Hassanipour M, Rezayat M, Dehpour AR. The involvement of nitric oxide in the anti-seizure effect of acute atorvastatin treatment in mice. Neurol Res. 2012;34:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Climent E, Benaiges D, Pedro-Botet J. Hydrophilic or Lipophilic Statins? Front Cardiovasc Med. 2021;8:687585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |