Published online Mar 20, 2024. doi: 10.5662/wjm.v14.i1.88619
Peer-review started: October 1, 2023
First decision: December 11, 2023
Revised: December 16, 2023
Accepted: January 18, 2024
Article in press: January 18, 2024
Published online: March 20, 2024
Processing time: 157 Days and 12.4 Hours
Inflammatory bowel disease, including ulcerative colitis, microscopic colitis, and Crohn’s disease (CD), has a global impact. This review focuses on duodenal CD (DCD), a rare subtype affecting the duodenum. DCD’s rarity and asymptomatic nature create diagnostic challenges, impacting prognosis and patient well-being. Delayed diagnosis can worsen DCD outcomes.
To report a rare case of DCD and to discuss the diagnostic challenges and its implications on prognosis.
A systematic literature search, following the PRISMA statement, was conducted. Relevant studies were identified and analysed using specific Medical Subject Terms (MeSH) from PubMed/MEDLINE, American Journal of Gastroenterology, and the University of South Wales database. Data collection included information from radiology scans, endoscopy procedures, biopsies, and histopathology results.
The review considered 8 case reports and 1 observational study, involving 44 participants diagnosed with DCD, some of whom developed complications due to delayed diagnosis. Various diagnostic methods were employed, as there is no gold standard workup for DCD. Radiology scans [magnetic resonance imaging (MRI), computed tomography (CT), and upper gastrointestinal X-ray], endoscopy procedures (colonoscopy and esophagogastroduodenoscopy), biopsies, and clinical suspicions were utilized.
This review discusses DCD diagnosis challenges and the roles of CT, MRI, and fluoroscopy. It notes their limitations and compares findings with endoscopy and histopathology studies. Further research is needed to improve diagnosis, emphasizing scan interpretation, endoscopy procedures, and biopsies, especially in high-risk patients during routine endoscopy.
Core Tip: This systematic review explores the diagnostic challenges and implications of duodenal Crohn’s disease (DCD), a rare subtype. Delayed diagnosis can worsen DCD outcomes, emphasizing the need for improved diagnostic methods. The study considers various diagnostic approaches, including radiology scans and endoscopy procedures, highlighting their limitations and the importance of biopsy and histopathology. Further research is essential to enhance DCD diagnosis, particularly in high-risk patients during routine endoscopy.
- Citation: Amadu M, Soldera J. Duodenal Crohn’s disease: Case report and systematic review. World J Methodol 2024; 14(1): 88619
- URL: https://www.wjgnet.com/2222-0682/full/v14/i1/88619.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i1.88619
Inflammatory bowel disease (IBD) comprises various chronic inflammatory gut conditions, including Crohn’s disease (CD), Microscopic Colitis, and ulcerative colitis, imposing a longstanding challenge for those affected. Among these, CD and ulcerative colitis have emerged as global health concerns, impacting millions, with significant prevalence in Europe (3.2 million) and North America (2 million)[1,2].
CD is an autoimmune inflammatory condition affecting the gastrointestinal tract, presenting inflammation from the mouth to the anus, with the ileum and colon being commonly affected[3]. Distinct types of CD include ileocolitis, ileitis (ileum inflammation), gastroduodenal Crohn’s (stomach and duodenum inflammation), jejunoileitis (jejunum inflammation), and Crohn’s colitis (granulomatous). It can manifest from childhood to adulthood, affecting both genders equally[4]. The pathogenesis involves gene susceptibility, immune system vulnerability, environmental factors, and microbiome alterations, disrupting intestinal mucosa[5].
Despite variations in reported figures, the exact prevalence and incidence of DCD worldwide remain uncertain[6]. Estimates reach millions globally, with the United Kingdom showing a prevalence of 10.6 per 100000[7]. In the United States and Europe, prevalence ranges from 1.6 million to 3 million, predominantly affecting younger individuals[8]. Incidence patterns vary across regions and age groups. China reports a peak incidence at 32.3 years, while Western studies identify peaks between 20-39 years and 50-79 years, with Asian studies displaying different patterns[9,10]. Factors contributing to these disparities, such as smoking initiation age and infection sensitivity, are still unclear[10,11].
The duodenum, situated between the stomach and the jejunum, marks the initial section of the small intestine. Duodenal Crohn’s disease (DCD), though rare, carries the potential for significant complications if not promptly identified. First documented by Gottlieb et al[12] in 1937[13], DCD’s prevalence is estimated at 0.5% to 4% among DCD patients[14], constituting less than 0.07% of all CD cases[15,16]. However, these figures may underestimate the actual occurrence due to the asymptomatic nature of many cases and the absence of routine endoscopy in initial evaluations[17].
While some DCD cases remain asymptomatic, symptomatic presentations also occur, either concurrently or subsequent to related bowel symptoms[17]. Symptoms of DCD encompass weight loss, early satiation, nausea, occasional vomiting, dyspepsia, and anorexia. Epigastric pain, typically postprandial and non-radiating, often responds to antacids and specific foods and is the most frequently reported symptom[16,18]. Rarely, melena and haematemesis are observed, and chronic anaemia may result from upper gastrointestinal bleeding[19]. Lossing et al[20] study noted abdominal cramp pain and diarrhoea as common presenting complaints among DCD patients, with some displaying additional symptoms like haematemesis, postprandial vomiting, epigastric pain, and upper intestinal bloating, especially in those with pre-existing intestinal disease.
DCD is associated with various complications discussed in multiple articles, impacting patients’ health. These complications include strictures causing gastric outlet obstruction, acute or chronic pancreatitis, and stenosis leading to obstruction. Fistulas, such as duodenopancreatic, duodenobiliary, duodenocolic, and duodenocutaneous, may develop in active or inactive DCD regions[21]. The diverse complications contribute to variations in patient symptoms, posing challenges for diagnosis. Differential diagnoses for DCD encompass peptic ulcer, pancreatic cancer, lymphoma, pancreatitis, and carcinoma. The complexity of diagnosing DCD can be attributed to its variable presentation, subtle nature, lack of a definitive diagnostic standard, and propensity to remain asymptomatic.
Additionally, DCD poses multifaceted challenges affecting various aspects of patients’ lives, including dietary, financial, physical, psychological, sexual, and social dimensions, ultimately impacting their overall quality of life[22]. Physically, patients grapple with unattractiveness, debilitating cramp-like pain, urgency, increased bowel movements, fatigue, and sleep disruptions. Excessive flatulence is also a distressing symptom reported by patients[23].
Furthermore, DCD can trigger feelings of isolation and depression, stemming from a lack of understanding and support from others, making it challenging for patients to discuss their condition, especially with those less familiar or misunderstood by their family and friends[24]. Socially, some patients may withdraw from social gatherings, offering excuses to avoid specific foods and frequent restroom trips. Their interests and activities may need adjustment due to the disease’s limitations[23]. Moreover, DCD-related complications significantly worsen patients’ quality of life, imposing a substantial financial burden on both patients and the healthcare system[25].
Patients often modify their diets post-diagnosis in an attempt to extend remission periods and alleviate symptoms, but this can inadvertently result in adopting restrictive diets, leading to malnutrition and diminished quality of life. Foods commonly avoided include spicy items, alcoholic beverages, dairy products, and fried foods. Some of these restricted foods contain essential nutrients like calcium, protein, vitamins, and minerals necessary for bodily functions. Historically, patients have regarded food as playing a pivotal role in managing IBD symptoms, akin to medication[26,27]. Fur
Research indicates that IBD conditions, including DCD, decrease sexual activity frequency in patients experiencing inadequate disease control, thereby affecting their overall quality of life. Some patients may perceive themselves as less attractive, impacting their desirability. Rates of sexual dysfunction in this chronic disease surpass those in the general population[29]. This issue may manifest before diagnosis and worsen as the disease progresses, with multiple factors contributing, including disease activity, surgery due to complications, and psychosocial and biological factors[30]. Some authors argue that patients with this chronic condition and the general population exhibit similar sexual activities but with lower sexual satisfaction.
Diagnosing DCD is a complex process due to variations in presentation, subtle symptoms, lack of a definitive diagnostic standard, and its asymptomatic nature. The asymptomatic nature can cause delays in diagnosis and low clinical suspicion[31]. Diagnostic delays are also linked to vague symptoms and diagnostic challenges[32]. A comprehensive approach involving various diagnostic methods is necessary for accurate diagnosis[33]. The European Crohn’s and Colitis Organisation [ECCO] recommends endoscopy, radiology evaluation, clinical suspicion, and histology for diagnosing DCD, even without colonoscopy findings[17]. Biopsy often reveals granulomas, mucosal erosion, and active inflammation[34]. The choice of imaging modality varies, with some preferring computer tomography initially and others upper gastrointestinal X-rays. Computer tomography enterography (CTE) and Magnetic resonance enterography (MRE) provide valuable information, with MRE being advantageous due to its radiation-free nature[35]. Endoscopy is a relevant standard for a definite diagnosis[35].
Timely and accurate diagnosis is crucial. DCD diagnosis can be delayed for weeks, months, or even years, leading to a poor prognosis. However, DCD generally has a good prognosis[24]. Further research and awareness are needed to improve understanding and support for DCD patients.
This systematic review aims to report a rare case of DCD and to discuss the diagnostic challenges and its implications on prognosis.
A 53-year-old female patient presented with upper abdominal pain. She had a previous diagnosis of long-term serum-negative peripheral symmetric arthritis and was undergoing daily treatment with leflunomide, along with dipirone on an as-needed basis, effectively controlling the disease. The patient denied the use of non-steroidal anti-inflammatory drugs. An upper digestive endoscopy revealed small ulcers in the second portion of the duodenum. Biopsies yielded non-specific results, and testing for Helicobacter pylori was negative. Immunohistochemistry for cytomegalovirus and herpes virus, as well as special colorations for fungi, acid-resistant bacilli, and PAS-positive pathogens, all returned negative results. Laboratory data showed an elevated C-reactive protein level of 2.7 mg/L, gastrin at 10 pg/mL, erythrocyte sedimentation rate at 18 mm/h, and negative results for ASCA, ANCA, and syphilis.
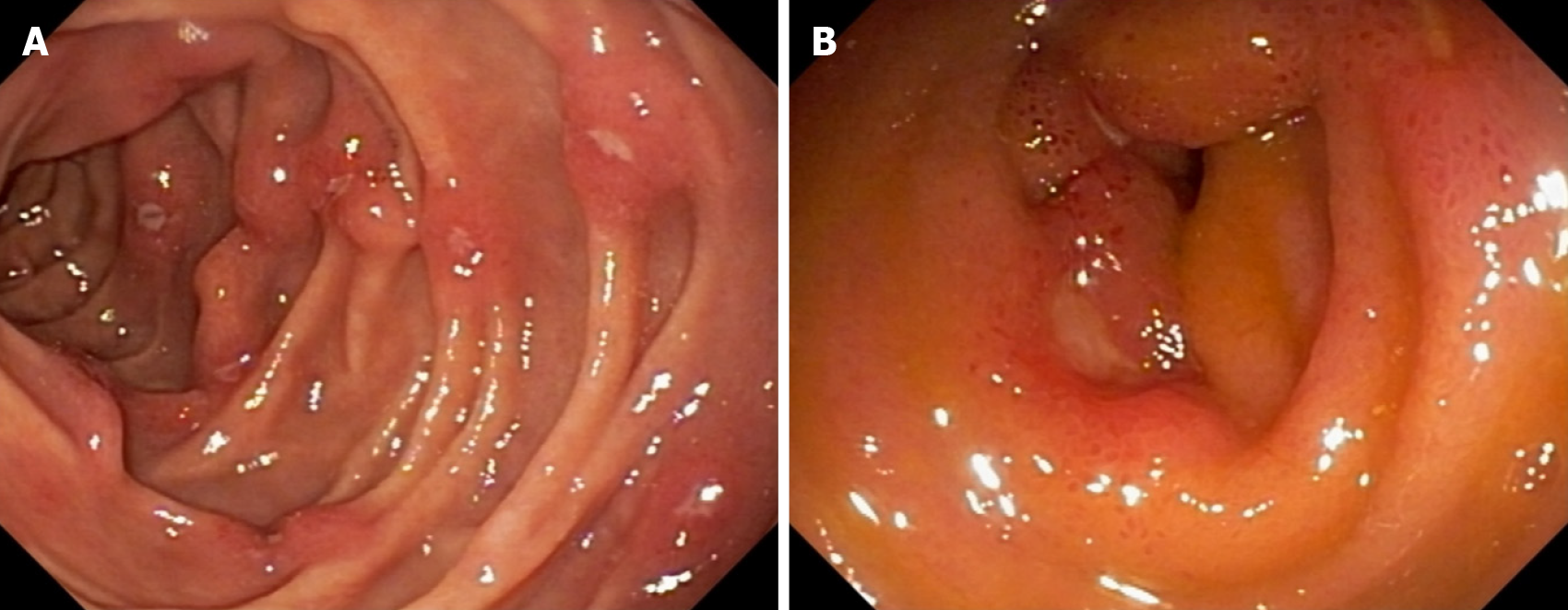
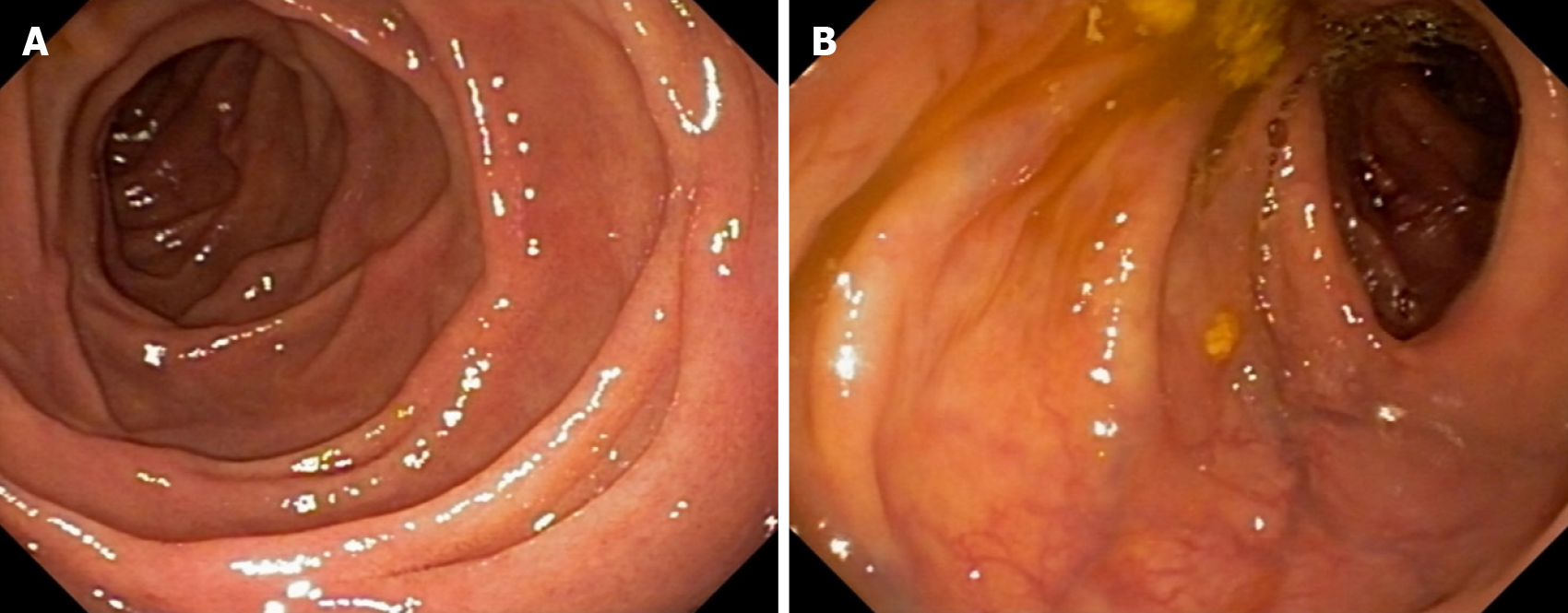
Despite initiating proton pump inhibitors, there was no improvement in symptoms, and a subsequent upper digestive endoscopy confirmed the persistence of the duodenal lesions (Figure 1). On the same day, a colonoscopy was performed, revealing aphthous ulcers in the terminal ileum (Figure 1). Enteric magnetic resonance imaging (MRI) indicated mild enteritis in the terminal ileum. With a diagnosis of DCD, azathioprine treatment was initiated but did not lead to endoscopic improvement within 6 months. Due to worsening arthritis, azathioprine was replaced with methotrexate, and adalimumab was introduced, resulting in complete healing of the ulcers (Figure 2). After 24 months of continuous adalimumab and methotrexate use, the patient remains in remission for both arthritis and DCD.
This systematic review strictly adhered to the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 checklist[36].
Included in this review were studies that met the following criteria: participants diagnosed with DCD within healthcare settings and published in English. The study designs considered encompassed observational and retrospective studies that specifically focused on the diagnosis and prognosis of DCD. Studies falling under systematic reviews, randomized control trials, editorials, and meta-analyses were excluded.
The initial search was performed on Google, followed by subsequent searches on PubMed/MEDLINE, American Journal of Gastroenterology, and the University of South Wales database using the search terms “duodenal crohn’s disease”[tiab:~0]. The study selection process involved three phases. Phase 1 involved searching titles and screening abstracts. Phase 2 entailed obtaining full-text articles for meticulous examination based on the selected abstracts. In phase 3, relevant papers were chosen for further review and data collection.
All selected studies included adult patients diagnosed with DCD who had undergone investigations within a healthcare setting. There were no restrictions on the publication timeframe or year, considering the limited research available on this specific area of review. Papers that matched the search terms and criteria were included, while those related to complications, surgery, treatment, management, and other types of DCD were excluded. The geographic location of the research and publication was not a limiting factor, as DCD is a global concern.
This review can provide valuable insights into selecting the appropriate investigation process or imaging for DCD cases. However, discrepancies in the results of these investigations may lead to challenges in definitively confirming whether a presented case is indeed DCD or another differential disease. As with any systematic review, the limitations of available data and potential bias in the included studies must also be considered.
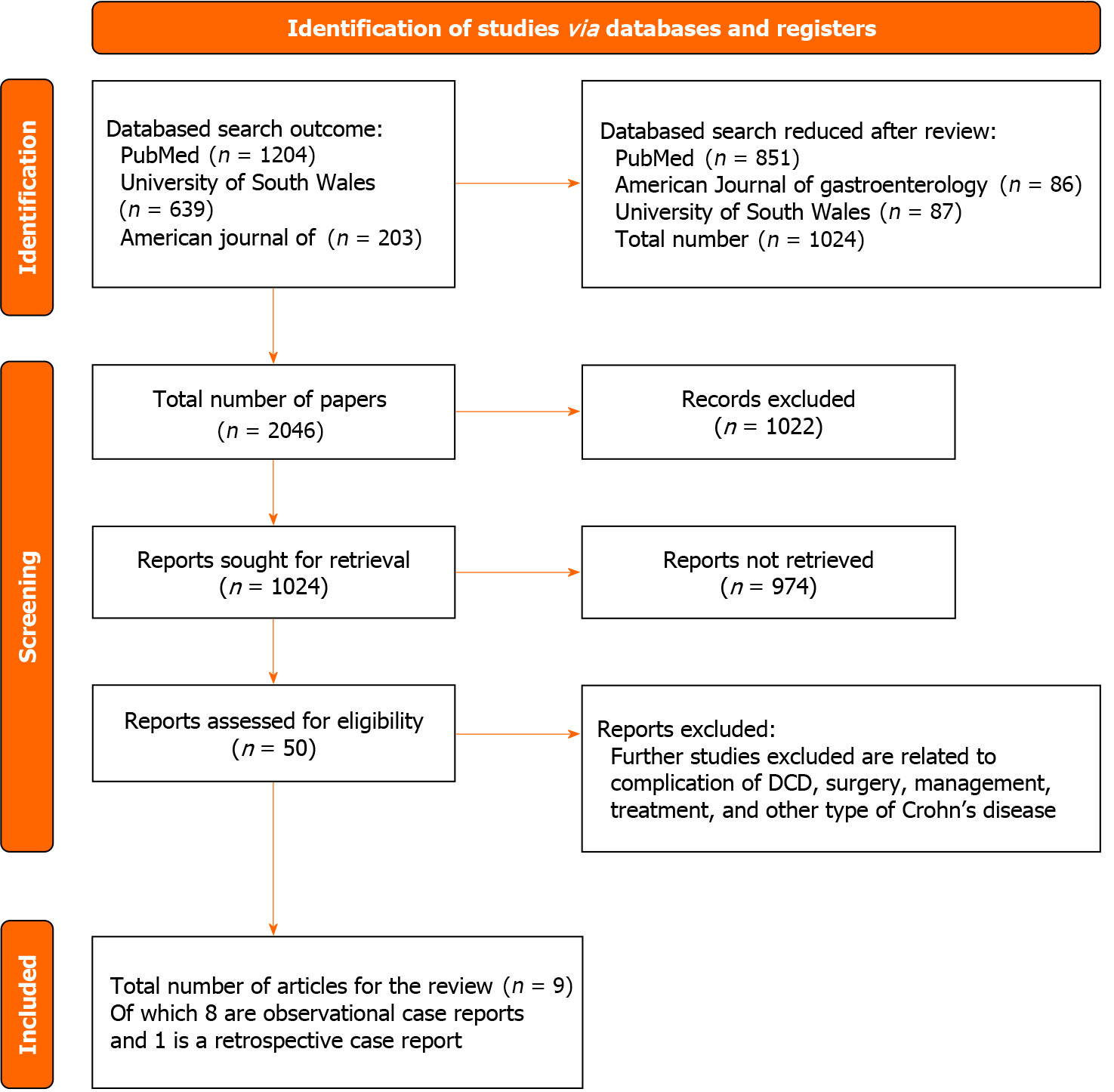
A total of 2046 studies were retrieved, and following a review of titles and abstracts, 1022 studies were excluded. Among the remaining studies related to DCD, 1024 were identified for further analysis. Subsequent examination led to the selection of 50 full-length papers. After careful scrutiny, 9 relevant studies were identified, with a combined sample size of 44 participants diagnosed with DCD. These chosen studies were included in the review for comprehensive reading and analysis. The search strategy is illustrated in Figure 3, and detailed information on the selected studies is presented in Table 1.
| Ref. | Study population | Study design | Presentation/investigation | Result | Treatment |
| Song et al[38], 2016 | A single case | Observational study (case report) | Presentation: Constant epigastric soreness with a month of dyspepsia. Investigations: EGD, colonoscopy, physical examination, biopsy, histology, chest X-ray, blood works and vital signs checks | Vital signs were normal. Physical examination showed no epigastric tenderness. Chest X-ray was unremarkable. Blood works showed no alarming result. Colonoscopy showed unremarkable terminal ileum and entire colon. EGD showed multiple progressive ulcers and erosion in the duodenal bulb and second portion of the duodenum with mucosal oedematous. Biopsy and histology showed ulceration of the duodenal bulb indicating erosion of the infiltration of inflammatory cell | 20 mg of prednisolone was given for two week and reduced to 10 mg for 1 wk and reduced to 5 mg after a week to take for a week |
| Lightner et al[68], 2018 | A single patient | Observational study (case report) | Presentation: Early satiety, distention, recent weight loss, nausea, microcytic anaemia, and vomiting. Investigations are: MRE, biopsy, EGD and histology | MRE showed high grade stricture in the distal portion of the abnormal segment resulting in dilatation of both the stomach and the second part of the duodenum. EGD showed ulceration with stenosis in D2 and stricture in D3 and oedema in the surrounding the area which confirmed the finding of MRE to be highly suspicious of duodenal Crohn’s disease. Histology confirmed the suspicion of EGD | Laparoscopic gastroduodenal bypass with ongoing proton pump inhibitor |
| Ashraf et al[56], 2022 | A single case | Observational study (case reports) | Presentation: 6 mo of 60 pounds weight loss, intractable nausea, mild epigastric pain, and vomiting. Investigations: CT, biopsies and EGD | CT result showed distention of the stomach with tapering into the proximal duodenum followed by a marked diffuse distention of the rest of the duodenum and narrowing of the duodenojejunal junction. Biopsy showed duodenitis consistence with duodenal Crohn’s disease. EGD showed stricture at proximal of the duodenum, distended duodenum, and stricture at the duodenojejunal junction coupled with severe stenosis and inflamed mucosa | PEG J tube was inserted for feeding but the patient did not tolerate. A duodenal resection was done |
| Plerhoples et al[37], 2012 | A single case | Observation (case reports) | Presentation: 25 yr of intermittent nausea, precipitous with loss of 50 pound in last 7 mo, bloating and vomiting. Investigations: EGD, colonoscopy, biopsy, fluoroscopy, and exploratory laparotomy | EGD showed ulcerating inflammation at the distal duodenum associated with stricture, dilation in the oesophagus and pyloric lumina and retained food in the proximal megaduodenum and antrum. Biopsy showed active acute inflammation. Fluoroscopy showed mild inflammation and delayed proximal duodenal and gastric emptying | Exploratory laparotomy with mobilization and duodenal bypass using antecolic roux-en-Y duodeno-jejunostomy bypas |
| Helms et al[55], 2016 | A single case | Observational study (case report) | Presentation: Unintentional weight loss, abdominal pain, bright red blood in stool, vomiting and diarrhoea. Investigations: CT, colonoscopy, biopsy, EGD, and upper endoscopy | EDG showed persistence duodenal Crohn’s. Unremarkable CT. Colonoscopy showed ileocecal valve stenosis preventing intubation. Upper endoscopy showed partially gastric outlet obstruction, duodenitis couple with edematous stenosis. Biopsy showed duodinitis with granulating tissue and ulceration | Protonix, Amoxil and Biaxin were used to treat H. pylori. Asacol was used to treat Crohn’s flare up |
| Ehwarieme et al[39], 2015 | A single case | Observational study (case report) | Presentation: Intermittent epigastric pain, post prandial nausea and vomiting for more than 9 yr and unintentional weight loss. Investigation: Chest X-ray, colonoscopy, endoscopic ultrasound, abdominal examination, biopsy, CT and upper endoscopy | CT showed antral mass which suggest malignancy. Initial endoscopy showed atypical cell. Chest X-ray is normal. Colonoscopy is unremarkable. Endoscopic ultrasound. Showed diffused thickening of the antral wall. Upper endoscopy showed oedematous, abnormal granular, ulcerated mucosa, friable affecting the duodenal bulb, pylorus, second part of the duodenum, antrum, pre pyloric area accompanied by a mild gastric outlet obstruction. Biopsy showed multinucleated cell and granulating tissue in addition to chronic inflammation | Patient was treated with proton pump inhibitor, prednisolone and sucralfate |
| Odashima et al[41], 2006 | A single case | Observational study (case report) | Presentation: Abdominal pain, abdominal distention, vomiting and nausea. Investigation: CT, upper GI X-ray, endoscopy and biopsy | CT showed thickness of the duodenum wall. Upper GI X-ray showed duodenal stricture. Endoscopy revealed mucosal oedema, large ulceration and stricture in the duodenal bulb | Patient was treated with inflixamab |
| Nugent et al[14], 1977 | 36 patients | Retrospective study (case report) | Presentation: Abdominal pain, abdominal distention, vomiting and nausea significant weight loss. Investigation: Endoscopy, pathology features, radilogy features and abdominal examination | Endoscopy features showed superficial ulceration in duodenum and antrum with granular appearance in addition to stenosis. Radiology feature showed irregular thickness, oedema and cobblestone pattern of the mucosa. Pathology findings showed mesentric lymph node enlargement with oedema and thickness. Duodenal wall fibrosis with chronic inflammation was noted | Vagotomy, gastroenterostomy amd Billroth |
| Karateke et al[54], 2013 | A single case | Observational study (case report) | Presentation: 6 mo of abdominal pain with progressive nausea, weight loss and bilious emesis. Investigation: Physical examination, CT routine blood work, biopsy, EGD and colonoscopy | Blood works showed mild normocytic anaemia. CT and colonoscopy are normal. Physical examination shows fulness and slight tenderness in the epigastric region. EGD showed tight stricture and oedema in the mucosal, long ulceration of the duodenal bulb and nearly complete obstruction. Biopsy showed cryptitis and severe inflammation, mixed chronic inflammatory infiltration of the laminal propria evidencing duodenal Crohn’s disease | Gastrojejunostomy without vagotmoy and subsequent proton pump inhibitor |
Concerning the participants, their ages ranged from 25 to late 70s, with the majority consisting of 30 males and 14 females. Among these participants, 8 were observed individually at various healthcare facilities in different countries and time periods, while retrospective data extraction from medical files was conducted for 36 participants.
All 9 studies included in this review exclusively focused on DCD. Notably, the work conducted by Plerhoples et al[37] stood out. The participant in this study exhibited symptoms persisting for over 25 years, leading to diverse diagnoses during each visit to healthcare facilities. Initially diagnosed with celiac sprue, the participant was subsequently diagnosed with follicular hyperplasia, despite presenting with consistent symptoms throughout the duration.
Among the 44 participants, 11 had a prior history of DCD affecting other parts of the digestive system, occurring between 4 to 40 years before the involvement of the duodenum. While one of the studies specifically reported dyspepsia as a symptom of DCD, none of the studies employed a standardized scale, such as the Crohn’s Disease Activity Index, to assess the severity of DCD.
The studies employed various investigative methods to diagnose DCD, with esophagogastroduodenoscopy (EGD) and biopsy commonly utilized. Furthermore, X-ray, MRI, blood tests, and computer tomography (CT) were used as diagnostic tools to facilitate the identification of DCD. The following paragraphs provide detailed findings obtained through these investigative approaches.
Among the studies reviewed, five included X-ray examinations as part of the initial assessment. Song et al[38] and Ehwarieme et al[39] reported normal chest X-ray findings in their patients. In contrast, Nugent et al[40] identified a duodenal stricture in the upper gastrointestinal (GI) X-ray, similar to Odashima et al[41]. Plerhoples et al[37] reported findings of mild inflammation and delayed proximal duodenal and gastric emptying during upper GI fluoroscopy. A summary of these results is provided in Table 2.
| Type of X-ray | Number of studies | Results |
| Chest X-ray | 2 | Unremarkable |
| Upper GI X-ray | 2 | Duodenal stricture |
| Upper GI fluoroscopy | 1 | Delayed emptying in both proximal duodenal and gastric |
Although chest X-rays are not typically employed for diagnosing DCD, research indicates that nearly half of DCD patients may exhibit subclinical alterations, suggesting underlying bronchial inflammation[42,43]. Importantly, this subclinical inflammation in DCD appears unrelated to its asymptomatic nature[44]. During DCD exacerbations, pulmonary function abnormalities and reduced diffusing capacity may lead to pulmonary inflammation, possibly correlated with small bowel inflammation[45].
Fluoroscopy plays a crucial role in assessing mucosal disease, peristalsis abnormalities, and postoperative complications like extraluminal leaks and obstructions. It allows real-time visualization of duodenal motility and mucosa[46]. Fluoroscopy can be conducted via small bowel follow-through (SBFT), which involves oral ingestion of a barium solution, with the progression monitored during the examination to record multiple images. The presence of intestinal strictures and peristalsis can influence the examination’s success[47]. Alternatively, enteroclysis administers a water contrast solution with or without methylcellulose through a nasoduodenal tube.
Historically, SBFT and small bowel enteroclysis (SBE) have been the standard investigative methods for suspected or confirmed DCD. However, controversies exist regarding the appropriateness and accuracy of these procedures in diagnosing DCD. Ott et al’s prospective study indicated that patients prefer fluoroscopy due to its safety compared to SBE, which is also less likely to miss gastroduodenal disease[48]. Conversely, Bernstein et al[49] suggested that SBE is more effective in detecting early mucosal lesions than fluoroscopy. Notably, SBE demonstrates high accuracy in diagnosing small bowel diseases, with reported sensitivity of 100% and specificity of 98.3%[49]. This was reaffirmed by Panes et al[51] with a sensitivity of 95% and specificity of 96.5%, whereas SBFT reported sensitivity ranging from 67% to 72%[51,52]. However, Wills et al[53] and Maglinte et al[50] argue that both SBE and fluoroscopy provide limited and varied information regarding the bowel wall and extraluminal extension of DCD.
In conclusion, upper GI X-rays can visualize duodenal abnormalities like strictures and inflammation, and existing literature has explored their utility in diagnosing DCD with positive outcomes. Nevertheless, further research is necessary to determine the effectiveness of chest X-rays in diagnosing DCD and its complications. Additionally, SBFT may have limitations in visualizing the intestine and its structures, while SBE’s drawback lies in providing limited information at the disease onset and regarding extraintestinal involvement[50,52].
CT scans are crucial in diagnosing DCD. They reveal various findings, such as normal results[54,55], a distended stomach leading to the proximal duodenum, diffuse duodenal distention, and duodenojejunal junction narrowing[56], an antral mass suggesting malignancy[40], and marked duodenal wall thickness[41]. Conventional CT, with or without contrast, is used for previously unknown Crohn’s cases or acute complications like perforation and abscess[57,58]. Table 3 contains the CT findings from the aforementioned studies. Accurate evaluation of DCD on CT scan requires the administration of enteric contrast before the examination and a fasting period of 4-6 h[57,58]. Enteric contrast can be administered orally or via a nasoenteric tube. On the other hand, CTE enhances visualization by distending the small bowel, making it increasingly preferred as the first-line imaging modality for patients with existing DCD and those with suspected DCD[59].
| Features | Number of studies |
| Distention of stomach and duodenum including narrowing of the duodenojejunal junction | 1 |
| Antral mass | 1 |
| Wall thickness | 1 |
| Nothing unusual found | 2 |
In a study of non-neoplastic duodenal diseases, CT revealed findings such as perforation of the duodenum, thickening, hyperemia of the wall, and fibrotic bulbar stenosis[60]. CT can also detect bowel wall thickening, strictures, free fluid, fistulas, bowel obstruction, and abscesses, consistent with magnetic resonance enterography[61]. CT images can be reconstructed three-dimensionally, facilitating the assessment of mucosal abnormalities, extraintestinal complications, bowel wall thickening, and intestinal loops. Multidetector CT is now a preferred and readily available modality for evaluating peri-duodenal and duodenal abnormalities, offering less invasiveness than traditional barium studies[60].
CTE accurately assesses small bowel damage, including the duodenum, with a sensitivity of 77.8% and specificity of 86.8% for detecting fistulas compared to surgery findings[62]. It has identified penetrating disease in Crohn’s patients, highlighting the limitation of relying solely on clinical symptoms[63,64]. CTE is also useful in detecting asymptomatic stenosis associated with small bowel Crohn’s, with a specificity of 38.9% and sensitivity of 92.3%[62]. However, CTE may struggle to differentiate intramural conditions and duodenal wall layers[60].
While multidetector CT is highly sensitive and specific in detecting DCD, it may face challenges in differentiating intramural conditions and duodenal wall layers[60]. Inflammatory processes in the duodenum are rarely diagnosed using CT due to nonspecific findings like luminal dilation, peri-duodenal fat stranding, and duodenal wall thickening.
CT and CTE have the disadvantage of exposing patients to radiation, which can be harmful, especially in younger and elderly patients. This risk is amplified when patients require frequent CT or CTE for follow-up examinations, potentially increasing the risk of GI cancer[65,66]. The ECCO consensus suggests considering radiation exposure when choosing scanning techniques for Crohn’s patients and recommends MRI as the preferable scan during follow-up[67]. The ECCO consensus also highlights the value of CT in diagnosing acute DCD complications, such as abscesses and obstructions, making it a definitive choice for managing such complications.
In conclusion, CT and CTE are essential in diagnosing DCD and assessing duodenal and small bowel abnormalities. However, their limitations in differentiating intramural conditions and potential radiation exposure risk should guide personalized imaging choices for Crohn’s patients. Further research is necessary to enhance the accuracy and specificity of CT-based DCD diagnosis.
MRI has been investigated for its utility in diagnosing DCD in a limited number of studies. Lightner et al[68] conducted MRE as part of their investigations and reported that MRE revealed a high-grade stricture in the distal portion of the abnormal segment, resulting in duodenal and stomach dilation. MRE is valued for its impartial and comprehensive evaluation of the intestine[63,69]. These findings align with a study by Tsai et al[70], which identified features like dilation, strictures, and abnormal segments in their MRE, consistent with standard DCD features.
While many of the selected studies did not utilize MRE/MRI, other relevant research by Ram et al[71], Gourtsoyiannis et al[72], and Sinha et al[73] explored the relevance of MRE in DCD investigations. Ram et al[71] observed small aphthous ulcers and deep transmural ulcers in the bowel wall, corresponding with Sinha et al[73], who linked deep ulceration to cobblestone-like mucosa and the potential for fistula and disease penetration. Additionally, Sinha et al[73] and Gourtsoyiannis et al[72] reported findings such as intestinal ulcers, bowel wall thickness, and lymph node enhancement, indicating active DCD.
MRE is a radiation-free technique gaining popularity as the preferred choice for evaluating inflammation in DCD, especially in younger and older patients[67]. It excels in detecting penetrating DCD complications, with a reported sensitivity and specificity exceeding 93% for small bowel CD diagnosis[74,75].
However, MRE’s sensitivity and specificity in detecting strictures are reported to be suboptimal. While it may identify strictures in symptomatic patients, it can miss partial or incipient strictures[76]. This limitation may stem from enterography techniques that provide insufficient bowel distention to highlight partial or early-stage strictures. In terms of penetrating and fistulizing disease, MRE is reported to have high specificity (100%) and varying sensitivity (83.3% to 84.4%)[77]. Nevertheless, partial volume averaging effects can lead to the overlooking of small interloop fistulas on MRE. Patients with DCD often face challenges in drinking and retaining contrast for MRE scans, potentially resulting in inadequate bowel distention and false appearances of bowel wall thickening and superficial enhancement[71].
In conclusion, further research is necessary to establish the role and effectiveness of MRI, particularly MRE, in assessing duodenal abnormalities associated with DCD.
Endoscopy, encompassing colonoscopy and EGD, is integral in the diagnosis and management of duodenal DCD and other IBD. The studies under analysis underscore the efficacy of combining endoscopy and biopsy as a diagnostic approach for DCD[4,19].
Song et al[38] reported unremarkable findings in the terminal ileum and entire colon during colonoscopy. Similarly, Karateke et al[54] and Plerhoples et al[37] documented normal colonoscopy outcomes in their patients. Conversely, Helms et al[55] encountered ileocecal valve stenosis hindering colonoscopy intubation. Odashima et al[41] noted a stricture with extensive ulceration and mucosal edema in the duodenal bulb during endoscopy. Nugent et al[40] observed diffuse granularity with nodularity, varying stenosis degrees, and superficial ulceration in the duodenum and antrum, obstructing duodenal and pyloric canal traversal in 17 out of 36 participants. Helms et al[55] described inflammation and ulceration with stricture in the distal duodenum during endoscopy. Ehwarieme et al[39] reported oedematous, granular, ulcerated mucosa with mild gastric outlet obstruction in upper endoscopy.
Despite varied findings, these studies collectively underscore the diagnostic complexity of DCD. The recommendation is to employ endoscopy in conjunction with biopsy as an effective diagnostic approach[4,19]. Endoscopic features of DCD encompass friable mucosa, irregular erythema, aphthous ulcers, gastric outlet narrowing, and mucosal thickness[19,78]. Duodenal manifestations may include polypoid lesions, a cobblestone appearance, and Kerckring’s folds, considered pathognomonic[79]. Serpiginous or linear ulcers distinguish DCD ulcers from peptic ulcers[80]. Graca-Pakulsa et al[81] reported a higher likelihood of duodenal abnormalities in DCD patients through endoscopy, including aphthous lesions, duodenal bulb deformation, duodenal ulceration, and mucosal swelling.
In summary, colonoscopy, coupled with biopsy, remains a valuable diagnostic tool for identifying and managing ileal CD. Nonetheless, the variability in endoscopic findings across studies underscores the need for further research and standardization in DCD diagnosis.
EGD, featured in 5 of 9 reviewed studies, proved valuable in diagnosing DCD. Lightner et al[68] identified ulceration with stenosis in D2, stricture in D3, and surrounding edema, aligning with MRE findings. Song et al[38] reported multiple duodenal bulb ulcers, gastric erosion, and fundal hemorrhage during initial EGD, with progressive ulcers and erosions in follow-up. Karateke et al[54] noted a tight duodenal stricture, mucosal edema, and extensive ulceration. Ashraf et al[56] found duodenal distention, strictures, mucosal inflammation, and severe stenosis. Plerhoples et al[37] documented ulcerating inflammation, strictures, dilation, and food retention. Helms et al[55] confirmed persistent DCD during EGD.
A two-decade-old prospective study found abnormalities like mucosal thickening, ulcers, and aphthoid erosion in 56% of Crohn’s patients undergoing EGD[82]. Kefalas et al[18] associated granuloma presence on EGD with existing endoscopic abnormalities. Other Crohn’s EGD findings include erythematous mucosa, fistulas, thickened folds, erosions, bamboo joint-like stomach appearance, cobblestone appearance, villous patterns, nodular lymphoid hyperplasia, and notch-like or longitudinal protrusions in the second part of the duodenum[21,83-85]. Notably, the mucosal DCD feature on EGD is non-specific, but a notch-like or longitudinal protrusion in the second part of the duodenum may serve as a reliable inflammatory marker[86].
In conclusion, endoscopy, encompassing colonoscopy and EGD, is pivotal for diagnosing, monitoring, and managing DCD. It facilitates visual assessment, treatment confirmation, disease activity evaluation, and complication detection. Regular endoscopic evaluation using the Simple Endoscopic Score for Crohn’s Disease (SES-CD) is recommended. However, expertise is essential for interpreting DCD endoscopic findings due to potential overlap with other gastrointestinal conditions.
Biopsy and histology serve as vital components in diagnosing and understanding DCD. Biopsy procedures aim to acquire tissue samples for pathological examination, often playing a role in surveillance or disease detection in the duodenum[87]. However, certain contraindications, including perforation, varices, bleeding, and coagulation disorders, can limit the use of duodenal biopsies[87].
Histological findings in DCD encompass various markers, including transmural inflammation, granulomas, mucosal erosion, and more. Studies reviewed revealed a range of histological observations. These findings included erosion and inflammatory cell infiltration, especially plasma cells, in the ulcerated duodenal bulb[38]. Lightner et al[68] identified foveolar and pyloric metaplasia, crypt abscess, and inceptive granulomas. Karateke et al[54] noted severe inflammation, villous blunting, mixed chronic inflammation, and cryptitis. Ashraf et al[56] reported duodenitis consistent with DCD in their biopsy samples. Plerhoples et al[37] found active acute inflammation in the biopsy from the stricture and mild inflammation in colonoscopy biopsy, along with an increase in intraepithelial lymphocytes and other histological changes. Helms et al[55] observed duodenitis with granulating tissue and ulceration. Ehwarieme et al[39] found giant Polynuclear cells and granulating tissue. Nugent et al[40] reported fibrosis and chronic inflammation in some participants, with a few showing granulomas in capsule biopsies. Odashima et al[41] reported active chronic inflammation in the biopsy and histology of the duodenal mucosa.
Histological findings in DCD demonstrated a variety of markers, with fibrosis, chronic inflammation, and duodenitis emerging as prominent features. There is some debate about which histological features are the most reliable for diagnosing DCD. While some studies emphasize the significance of granulomas, others suggest the presence of granuloma and another feature, such as architectural abnormalities or focal inflammation, for confirming the diagnosis of DCD[88]. Discrepancies in the significance of granulomas in diagnosing DCD highlight the challenges faced by clinicians in reaching a conclusive diagnosis.
Fibrosis in DCD remains poorly understood, with emerging evidence suggesting it may result from adaptive immune responses regulated by noncoding RNA molecules[89]. Early diagnosis of fibrosis is crucial, as it currently lacks pharmaceutical treatment, making surgery or endoscopic balloon dilatation the primary options. Potential biomarkers for fibrosis require further research to develop reliable and cost-effective diagnostic tools.
Villous blunting or atrophy in the duodenum can take various forms, including fused or branched villi. Diagnosis of duodenal bulb villous atrophy can be challenging due to the short and thick villi in this region, often leading to interpretation difficulties[90].
Duodenitis is characterized by inflammatory cell infiltration, changes in crypt epithelium, and villous atrophy[91]. Other diagnostic features for DCD include focal chronic inflammation without crypt atrophy, increased intraepithelial lymphocytes, submucosal inflammation, focal cryptitis, proximal ulceration, hyperplasia, and aphthoid ulcers[92,93]. Table 4 provides a summary of the histology and biopsy findings from the reviewed studies.
| Duodenal biopsy and histology | Number of cases |
| Chronic inflammation | 18 |
| Erosion | 1 |
| Infiltration of inflammatory cells. | 1 |
| Granulomas | 4 |
| Foveolar metaplasia | 2 |
| Duodenitis | 12 |
| Increased intraepithelial lymphocytes | 1 |
| Fibrosis | 17 |
| Villous blunting | 1 |
| Ulceration | 2 |
| Acute active inflammation | 2 |
| Polynuclear cells | 1 |
Common presentations of DCD were reported in various studies. Epigastric pain and unintentional weight loss were observed by several studies[38-41,54-56,68]. Additionally, vomiting was reported by various studies[38-41,54-56,68]. Abdominal distention was also noted by another few studies[37,41,68]. Upper gastrointestinal bleeding was observed by two studies[40,55], while microcytic anemia[68], dyspepsia[38] and diarrhea[55] was identified by only one study[68].
These symptoms, including nausea, vomiting, epigastric pain, weight loss, gastrointestinal bleeding, and microcytic anemia, are common in DCD. They are associated with the disease’s severity, particularly obstruction[15]. Some symptoms, such as gastrointestinal bleeding and microcytic anemia, are interconnected and result from chronic blood loss and impaired iron absorption due to inflammation related to DCD[94]. However, the similarity of these symptoms with those of other conditions poses challenges in diagnosing DCD. Table 5 presents the count of patients experiencing the aforementioned symptoms in the reviewed studies.
| Symptoms | Cases |
| Epigastric pain | 7 |
| Weight loss | 7 |
| Nausea and vomiting | 6 |
| Vomiting | 1 |
| Dyspepsia | 1 |
| Abdominal distention | 3 |
| Upper GI bleeding | 2 |
| Microcytic anaemia | 1 |
| Diarrhoea | 1 |
In the reviewed studies, only 2 out of the 9 investigations included blood tests as part of their initial assessment. Karateke et al[54] conducted blood work, revealing mild normocytic anemia in their subjects, while Song et al[38] reported blood work with no alarming results. Historically, serology, particularly blood tests, has been undervalued in the diagnosis of DCD due to its low specificity. However, recent developments in the field, especially the introduction of biological drugs, have prompted a reevaluation of the role of serological markers.
One of the key serological biomarkers used in assessing responses to drugs in DCD patients is C-reactive protein (CRP). Recent biologics trials often include raised CRP levels as an inclusion criterion to determine the efficacy of drugs. An increasing CRP level after drug administration is interpreted as a sign of treatment failure, whereas a decrease in CRP indicates the drug’s effectiveness. Furthermore, CRP levels are used to monitor disease activity, particularly in patients with severe disease, where elevated CRP levels are more common compared to patients in mild or remission states.
A prospective study conducted by Brignola et al[95] investigated inflammation markers in the blood results of 41 DCD patients who were in remission for 6 months. This study found that CRP levels remained elevated after 2 years in remission patients who initially had high CRP levels. Despite efforts to include serology in the criteria for diagnosing IBD, the diagnostic benefits of serology remain limited and lack sensitivity. However, certain antibodies tested for DCD, such as perinuclear antineutrophil cytoplasmic antibody negative and anti-Saccharomyces cerevisiae antibody immunoglobulin G, or a positive immunoglobulin A, have shown a sensitivity of 55% and specificity of 93%[96].
In conclusion, blood tests, particularly serological markers like CRP, have gained importance in assessing drug responses and monitoring disease activity in DCD. However, their role in the diagnosis of IBD remains limited and less sensitive, with the use of specific antibodies showing varying levels of diagnostic accuracy.
The presentation of DCD is highly variable, presenting a considerable challenge to physicians. Studies have indicated that this diversity in symptoms can lead to delayed diagnoses, ranging from a minimum of 5 months to several years[97]. Such delays can result in additional complications and irreversible damage to the small bowel over time[98], which may occur rapidly[99]. Diseases with more common symptoms are typically easier to identify and diagnose, enabling early intervention and improved prognoses. Research has shown that the risk of complications in DCD increases with delayed diagnosis, ranging from 18.2% within 90 d to 22% within a year[100].
Furthermore, observations from studies comparing DCD in the Chinese population to Western countries have revealed significant differences in disease characteristics, such as location, age of onset, extraintestinal manifestations, disease behavior, treatment approaches, and gender distribution[101].
In addition to the variability in symptoms, challenges arise from the endoscopic techniques used for DCD diagnosis. Standard upper gastrointestinal endoscopy may encounter difficulties in reaching the distal duodenum, necessitating a greater reliance on imaging techniques. MRE and CTE are currently employed to visualize the small bowel and detect complications like fistulas and strictures[102]. However, capsule endoscopy is favored over CTE, despite the potential risk of capsule retention in patients with strictures.
Performing biopsy tissue sampling during endoscopy can also be challenging when attempting to intubate an inflamed site, further complicating the diagnostic process. Moreover, endoscopy tends to be primarily conducted in symptomatic patients, potentially overlooking a significant number of asymptomatic DCD cases.
The variations in symptoms and subtle presentation of DCD underscore the need to address these diagnostic challenges. Physicians must navigate the complexities of reaching the distal duodenum during endoscopy, with imaging modalities playing a critical role in achieving accurate diagnoses. Addressing these challenges is crucial for achieving early diagnosis and effective management of DCD, ultimately improving patient outcomes. Further research into the clinical behavior of the disease in different populations can contribute to more tailored and effective management strategies.
It is paramount to consider many differentials when DCD is suspected. For example, celiac disease[103,104], enteric neoplasms and metastasis[105,106], foreign body ingestion[107], pellagra[108], enteric infections[109-111], sprue-like enteropathy associated with the angiotensin II receptor blocker[112], extra-intestinal manifestations[113] and hemophagocytic lymphohistiocytosis[114,115].
Generally, the prognosis for DCD is favorable, even in patients who require surgery due to medical refractoriness. The ability to manage DCD symptoms is also generally good. However, DCD can lead to complications that may necessitate surgical or medical intervention. These complications include pancreatitis, stenosis, fistulas, and strictures. Pancreatitis can occur due to inflammation damaging the duodenal bulb or compression of the pancreatic head, leading to fibrosis[116]. Stenosis, which occurs in about 1 in 10 DCD patients within ten years of diagnosis, results from chronic inflammation, tissue remodeling, and mesenchymal cell hypertrophy[117,118]. DCD strictures develop due to repeated submucosal injury and chronic inflammation, leading to the accumulation of extracellular components like smooth muscle cells and collagen, causing scar tissue formation and narrowing[119,120]. Strictures can put patients at risk of abscesses and fistulas, which may require surgery[121].
In cases of suspected stricture, radiologic investigation, such as CT scans, is essential to assess the severity and nature of the obstruction[122]. Another severe complication is the development of duodenal fistulas, which can lead to the leakage of duodenal contents into other body parts or organs. Duodenal fistulas are challenging to treat and have a high mortality rate, often requiring complex surgical interventions[123].
Initial management of DCD typically involves medications like 6-mercaptopurine or sulfasalazine[124]. However, the management of DCD-related ulceration frequently involves proton pump inhibitors, corticosteroids, or histamine 2 receptor antagonists. It’s worth noting that previous studies[40,125] have indicated that these pharmacological treatments may be ineffective for some patients, leading to surgery due to controllable pain and complications. In fact, one-third of patients with refractory DCD end up requiring surgery[125].
Various pharmacological treatments, including Infliximab and Adalimumab, have been effective in managing complications of DCD. Infliximab has shown positive outcomes in treating fistulas and refractory DCD[126]. Studies have also indicated that Infliximab is beneficial in addressing issues like duodenal stenosis, fistulas, and ulcers[127,128]. The ACCENT I study reported a 56% success rate within a week of Infliximab therapy for gastroduodenal DCD[129]. Adalimumab is another pharmacological option, particularly effective in severe cases of gastroduodenal DCD[130,131]. A prospective study by Annunziata et al[132] found that 72.7% of patients treated with Adalimumab or Infliximab achieved mucosal healing within 12 wk, compared to only 12.5% with conventional therapy.
Anti-inflammatory therapy, often involving tumor necrosis factor (TNF) inhibitors, is increasingly used in acute settings to manage fibrotic strictures in DCD patients. TNF inhibitors are used alone or in combination with steroids as a first-line treatment and for maintaining DCD[119,133]. Effective therapy can help prevent or delay long-term complications associated with strictures[134,135]. Surgical procedures, including strictureplasty, resection, bypass, and endoscopic balloon dilation, are available for DCD complications. However, their efficacy varies, and further research is needed to refine their management[136].
The treatment of DCD-induced pancreatitis involves analgesia, intravenous fluid resuscitation, and nutritional support in acute stages. In chronic or severe cases, treatment may include antibiotic therapy, pancreatic necrosis drainage, and necrosectomy. Despite available management options, treating DCD remains challenging due to its heterogeneous presentations. Therapy aims for deep remission and mucosal healing, encompassing symptom relief, endoscopic improvements, and histological changes. There is limited evidence regarding patient assessments of medication tolerability before treatment initiation[137].
However, managing DCD remains intricate due to its heterogeneous presentations. Recent studies, such as one investigating the efficacy of rapamycin in Crohn’s-related strictures, provide insights into potential therapeutic avenues. Rapamycin’s effectiveness in upper gastrointestinal strictures but not in lower gastrointestinal tract lesions has been reported[138]. As we navigate the challenges of DCD treatment, it becomes imperative to explore emerging strategies that go beyond conventional approaches, considering location-specific treatments and tailoring interventions based on the anatomical site of involvement[139].
In conclusion, this review has focused on the challenges associated with diagnosing DCD and its profound impact on prognosis. The diagnosis of DCD necessitates a comprehensive approach that involves histopathology, endoscopy, clinical evaluation, and radiological imaging. The prognosis of DCD is intricately linked to early diagnosis and is contingent on the specific type of the disease and the extent of its complications.
Imaging, notably radiological techniques, plays a pivotal role in the identification and management of DCD by providing critical insights into the disease’s location and severity. Radiologists and endoscopists should familiarize themselves with the common sites of DCD and potential areas of complications. This knowledge, coupled with histopathological and clinical findings, enhances the ability to diagnose symptomatic patients accurately. Moreover, conducting endoscopic examinations in individuals at risk of developing DCD can facilitate early detection in asymptomatic cases, ultimately leading to more favorable prognoses.
Significant delays in diagnosis can have detrimental effects on patients, affecting their well-being, quality of life, and overall disease outcomes. While there is no universally accepted gold standard for DCD workup, EGD stands out as the preferred endoscopic method for investigation. Other radiological modalities, such as CT and MRI, may be employed initially to assess small bowel damage, with considerations for radiation exposure and the adaptability of subsequent scans. Endoscopy with biopsy aids in excluding alternative diagnoses and associated complications, thereby reducing the risks of comorbidities and mortality linked to a poor prognosis.
Nonetheless, the challenges inherent in diagnosing DCD necessitate further research to comprehensively understand their implications on prognosis. Additionally, the complications, prevalence, therapeutic implications, and the interpretation of histological findings related to DCD remain subjects of ongoing investigation. A more profound comprehension of DCD’s characteristics is essential for gastroenterologists to effectively differentiate it from conditions that mimic its presentation and enable timely and accurate diagnoses. As such, further research is imperative to unravel the full significance of diagnosing and managing DCD.
Inflammatory bowel disease (IBD), comprising Crohn’s disease (CD) and ulcerative colitis, presents a global health challenge affecting millions. While duodenal CD (DCD) manifests throughout the gastrointestinal tract, duodenal DCD specifically involves the initial section of the small intestine. Despite its rarity (0.5%-4% prevalence among Crohn’s patients), DCD poses significant complications, including strictures, fistulas, and varied symptoms. Diagnostic challenges arise from its asymptomatic nature, subtle presentation, and lack of a definitive standard, leading to delayed diagnosis and potential health consequences. The prevalence and incidence of DCD vary globally, with factors contributing to these disparities still unclear. Additionally, the multifaceted impact of DCD on patients’ lives extends beyond physical symptoms, affecting dietary, financial, psychological, and social dimensions, highlighting the need for comprehensive research and awareness to enhance patient support.
This study is motivated by the need to address critical gaps in understanding and managing DCD. The rare yet impactful nature of DCD necessitates a focused exploration of its complications, varied presentations, and their implications on patients’ quality of life. The diagnostic challenges associated with DCD demand attention to improve clinical suspicion, reduce delays in diagnosis, and enhance prognosis. By investigating the multifaceted challenges patients face, including the physical, psychological, and social dimensions, this research seeks to contribute valuable insights for future studies in IBD research. Understanding the significance of dietary modifications and their impact on malnutrition, as well as the complexities of sexual dysfunction in IBD patients, can inform tailored interventions and improve overall patient care.
The primary objectives of this systematic review include reporting a rare case of DCD, elucidating the diagnostic challenges associated with the condition, and exploring its implications on prognosis. The study aims to provide a comprehensive overview of DCD’s clinical manifestations, emphasizing the necessity for timely and accurate diagnosis. Realizing these objectives will contribute to enhancing awareness and support for DCD patients, paving the way for future research addressing critical gaps in understanding the global prevalence, diagnostic standards, and comprehensive management strategies for this rare but impactful facet of IBD.
The research employed a rigorous methodology, adhering to the PRISMA 2020 guidelines, ensuring transparency in its systematic review. Utilizing Google, PubMed/MEDLINE, American Journal of Gastroenterology, and the University of South Wales database, a focused search with the term “duodenal Crohn’s disease” emphasized the specificity of investigation. The three-phase study selection involved meticulous screening of titles and abstracts, followed by a thorough examination of full-text articles based on selected abstracts. Eligible studies, comprising observational and retrospective research in healthcare settings, addressed the diagnosis and prognosis of DCD in adults. Exclusion criteria targeted systematic reviews, randomized control trials, editorials, and meta-analyses, along with studies on complications, surgery, treatment, management, and other DCD types. No restrictions on publication timeframe or geographic location were imposed, acknowledging DCD as a global concern. The review recognizes limitations, including potential discrepancies in investigation results impacting definitive DCD confirmation and inherent biases within available data.
The systematic review identified and scrutinized 9 relevant studies encompassing 44 participants diagnosed with DCD. From an initial pool of 2046 studies, careful screening led to the exclusion of 1022, with 50 full-length papers selected for further analysis. The participant ages ranged from 25 to late 70s, predominantly comprising 30 males and 14 females. Remarkably, Plerhoples et al’s work highlighted a case with persistent symptoms spanning over 25 years, undergoing diverse diagnoses, initially as celiac sprue and later as follicular hyperplasia. Of the 44 participants, 11 had a history of DCD affecting other digestive system parts, occurring 4 to 40 years prior to duodenal involvement. Notably, none of the studies utilized standardized scales, like the Crohn’s Disease Activity Index, to assess DCD severity, indicating a gap in consistent measurement. While shedding light on the clinical aspects of DCD, these findings underscore the need for standardized assessment tools and further exploration of the condition’s long-term impact and diagnostic challenges.
This study proposes novel insights into the diagnostic approaches for DCD. The research underscores the effectiveness of upper gastrointestinal X-rays, computed tomography, magnetic resonance imaging, endoscopy, and histology in diagnosing DCD. The study critically evaluates the strengths and limitations of each method, highlighting the need for personalized imaging choices. The significance of endoscopic techniques, particularly EGD, is underscored, emphasizing its combination with biopsy for effective DCD diagnosis and acknowledges the evolving diagnostic landscape, considering the influence of biological drugs on serology’s reevaluation in DCD diagnosis.
The direction of future research should focus on enhancing the diagnostic accuracy and specificity of investigative methods for DCD. Prospective research could delve deeper into histological features, unraveling the significance of granulomas, fibrosis, and villous blunting. Further research should explore emerging strategies beyond conventional approaches, considering anatomical site-specific interventions and the potential role of rapamycin in managing strictures. Overall, future research should aim for a comprehensive understanding of DCD characteristics, encompassing prevalence, complications, therapeutic implications, and histological interpretations. This knowledge is vital for gastroenterologists to accurately diagnose and manage DCD, ultimately improving patient outcomes.
We would like to extend our sincere appreciation to the Acute Medicine MSc program at the University of South Wales for their invaluable assistance in our work. We acknowledge and commend the University of South Wales for their commitment to providing advanced problem-solving skills and life-long learning opportunities for healthcare professionals.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Federação Brasileira De Gastroenterologia; Grupo de Estudos da Doença Inflamatória Intestinal do Brasil; Sociedade Brasileira de Endoscopia Digestiva.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hariyanto TI, Indonesia; Nasa P, United Arab Emirates; Zhang F, China S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY
| 1. | Dall'Oglio VM, Balbinot RS, Muscope ALF, Castel MD, Souza TR, Macedo RS, Oliveira TB, Balbinot RA, Balbinot SS, Brambilla E, Soldera J. Epidemiological profile of inflammatory bowel disease in Caxias do Sul, Brazil: a cross-sectional study. Sao Paulo Med J. 2020;138:530-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 2. | Ananthakrishnan AN, Kaplan GG, Ng SC. Changing Global Epidemiology of Inflammatory Bowel Diseases: Sustaining Health Care Delivery Into the 21st Century. Clin Gastroenterol Hepatol. 2020;18:1252-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 3. | Sands BE. Crohn's disease. In: Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger & Fordtran's Gastrointestingal and Liver Disease: Pathophysiology, Diagnosis, Management. 7th ed. Philadelphia: Saunders, 2002. |
| 4. | Reynolds HL Jr, Stellato TA. Crohn's disease of the foregut. Surg Clin North Am. 2001;81:117-135, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Petagna L, Antonelli A, Ganini C, Bellato V, Campanelli M, Divizia A, Efrati C, Franceschilli M, Guida AM, Ingallinella S, Montagnese F, Sensi B, Siragusa L, Sica GS. Pathophysiology of Crohn's disease inflammation and recurrence. Biol Direct. 2020;15:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 6. | Kalla R, Ventham NT, Satsangi J, Arnott ID. Crohn's disease. BMJ. 2014;349:g6670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |
| 8. | Feuerstein JD, Cheifetz AS. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin Proc. 2017;92:1088-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 312] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 9. | Mocanu D, Catuneanu AM, Diculescu M, Gologan S, Sporea I. Current epidemiologic trends in Crohn's disease: data from a tertiary referral centre in Bucharest: (Fundeni Institute, Center of Gastroenterology and Hepatology). Maedica (Bucur). 2010;5:95-101. [PubMed] |
| 10. | Park SJ, Kim WH, Cheon JH. Clinical characteristics and treatment of inflammatory bowel disease: a comparison of Eastern and Western perspectives. World J Gastroenterol. 2014;20:11525-11537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Wang YF, Zhang H, Ouyang Q. Clinical manifestations of inflammatory bowel disease: East and West differences. J Dig Dis. 2007;8:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Kwon, Lee, Park, Chi. Isolated duodenal Crohn's disease. Ann Surg Treat Res. 1999;56:602-607. |
| 14. | Nugent FW, Roy MA. Duodenal Crohn's disease: an analysis of 89 cases. Am J Gastroenterol. 1989;84:249-254. [PubMed] |
| 15. | Mottet C, Juillerat P, Pittet V, Gonvers JJ, Michetti P, Vader JP, Felley C, Froehlich F. Upper gastrointestinal Crohn's disease. Digestion. 2007;76:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Ingle SB, Adgaonkar BD, Jamadar NP, Siddiqui S, Hinge CR. Crohn's disease with gastroduodenal involvement: Diagnostic approach. World J Clin Cases. 2015;3:479-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Pimentel AM, Rocha R, Santana GO. Crohn's disease of esophagus, stomach and duodenum. World J Gastrointest Pharmacol Ther. 2019;10:35-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 18. | Kefalas CH. Gastroduodenal Crohn's disease. Proc (Bayl Univ Med Cent). 2003;16:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Loftus EV Jr. Upper gastrointestinal tract Crohn's disease. Gastroenterology: ClinicalPerspective. 2002; 5: 188-191. Available from: https://scholar.google.com/scholar?cluster=11718709885779257132&hl=pt-BR&as_sdt=0,5. |
| 20. | Lossing A, Langer B, Jeejeebhoy KN. Gastroduodenal Crohn's disease: diagnosis and selection of treatment. Can J Surg. 1983;26:358-360. [PubMed] |
| 21. | van Hogezand RA, Witte AM, Veenendaal RA, Wagtmans MJ, Lamers CB. Proximal Crohn's disease: review of the clinicopathologic features and therapy. Inflamm Bowel Dis. 2001;7:328-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Whittemore R, Dixon J. Chronic illness: the process of integration. J Clin Nurs. 2008;17:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Norton BA, Thomas R, Lomax KG, Dudley-Brown S. Patient perspectives on the impact of Crohn's disease: results from group interviews. Patient Prefer Adherence. 2012;6:509-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn's and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis. 2007;1:10-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 239] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 25. | Bassi A, Dodd S, Williamson P, Bodger K. Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut. 2004;53:1471-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 241] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | de Vries DA, Vossen HGM, van der Kolk-van der Boom P. Social Media and Body Dissatisfaction: Investigating the Attenuating Role of Positive Parent-Adolescent Relationships. J Youth Adolesc. 2019;48:527-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Crooks MG, Elkes J, Storrar W, Roy K, North M, Blythin A, Watson A, Cornelius V, Wilkinson TMA. Evidence generation for the clinical impact of myCOPD in patients with mild, moderate and newly diagnosed COPD: a randomised controlled trial. ERJ Open Res. 2020;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Limdi JK, Aggarwal D, McLaughlin JT. Dietary Practices and Beliefs in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 29. | Perez de Arce E, Quera R, Ribeiro Barros J, Yukie Sassaki L. Sexual Dysfunction in Inflammatory Bowel Disease: What the Specialist Should Know and Ask. Int J Gen Med. 2021;14:2003-2015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Marín L, Mañosa M, Garcia-Planella E, Gordillo J, Zabana Y, Cabré E, Domènech E. Sexual function and patients' perceptions in inflammatory bowel disease: a case-control survey. J Gastroenterol. 2013;48:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Aggarwal SN, Cavanagh Y, Wang L, Akmal A, Grossman MA. Upper Gastrointestinal Crohn's Disease: Literature Review and Case Presentation. Case Rep Gastrointest Med. 2019;2019:2708909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Moon CM, Park DI, Kim ER, Kim YH, Lee CK, Lee SH, Kim JH, Huh KC, Jung SA, Yoon SM, Song HJ, Jang HJ, Kim YS, Lee KM, Shin JE. Clinical features and predictors of clinical outcomes in Korean patients with Crohn's disease: a Korean association for the study of intestinal diseases multicenter study. J Gastroenterol Hepatol. 2014;29:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1164] [Article Influence: 194.0] [Reference Citation Analysis (0)] |
| 34. | AbdullGaffar B, Quraishi H. Histopathologic Manifestations of Crohn Disease in Duodenal Endoscopy Biopsy: The Value of Different Patterns of Involvement of Brunner Glands. Int J Surg Pathol. 2021;29:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Cicero G, Mazziotti S. Crohn's disease at radiological imaging: focus on techniques and intestinal tract. Intest Res. 2021;19:365-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40536] [Article Influence: 10134.0] [Reference Citation Analysis (2)] |
| 37. | Plerhoples TA, Norton JA. Recurrent duodenal stricture secondary to untreated Crohn's disease. Dig Dis Sci. 2012;57:2516-2518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Song DJ, Whang IS, Choi HW, Jeong CY, Jung SH. Crohn's disease confined to the duodenum: A case report. World J Clin Cases. 2016;4:146-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Ehwarieme R, Patel S, Ehrinpreis M, Jinjuvadia K. Isolated Gastroduodenal Crohn's Disease: Masked as a Malignancy: 651. Am J Gastroenterol. 2015;110:S287. [DOI] [Full Text] |
| 40. | Nugent FW, Richmond M, Park SK. Crohn's disease of the duodenum. Gut. 1977;18:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Odashima M, Otaka M, Jin M, Horikawa Y, Matsuhashi T, Ohba R, Koizumi S, Kinoshita N, Takahashi T, Watanabe S. Successful treatment of refractory duodenal Crohn's disease with infliximab. Dig Dis Sci. 2007;52:31-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Kuzela L, Vavrecka A, Prikazska M, Drugda B, Hronec J, Senkova A, Drugdova M, Oltman M, Novotna T, Brezina M, Kratky A, Kristufek P. Pulmonary complications in patients with inflammatory bowel disease. Hepatogastroenterology. 1999;46:1714-1719. [PubMed] |
| 43. | Fireman Z, Osipov A, Kivity S, Kopelman Y, Sternberg A, Lazarov E, Fireman E. The use of induced sputum in the assessment of pulmonary involvement in Crohn's disease. Am J Gastroenterol. 2000;95:730-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Mikhaĭlova ZF, Levchenko SV, Karagodina IuIa, Barinov VV. Pulmonary disorders in patients with chronic inflammatory bowel disease. Eksp Klin Gastroenterol. 2011;54-59. [PubMed] |
| 45. | Songür N, Songür Y, Tüzün M, Doğan I, Tüzün D, Ensari A, Hekimoglu B. Pulmonary function tests and high-resolution CT in the detection of pulmonary involvement in inflammatory bowel disease. J Clin Gastroenterol. 2003;37:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Johnson LN, Moran SK, Bhargava P, Revels JW, Moshiri M, Rohrmann CA, Mansoori B. Fluoroscopic Evaluation of Duodenal Diseases. Radiographics. 2022;42:397-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Fichera A, Krane MK. Crohn's disease: basic principles. Cham: Springer International Publishing 2015. [DOI] [Full Text] |
| 48. | Ott DJ, Chen YM, Gelfand DW, Van Swearingen F, Munitz HA. Detailed per-oral small bowel examination vs. enteroclysis. Part I: Expenditures and radiation exposure. Radiology. 1985;155:29-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 52] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Bernstein CN, Boult IF, Greenberg HM, van der Putten W, Duffy G, Grahame GR. A prospective randomized comparison between small bowel enteroclysis and small bowel follow-through in Crohn's disease. Gastroenterology. 1997;113:390-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 70] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Maglinte DD, Chernish SM, Kelvin FM, O'Connor KW, Hage JP. Crohn disease of the small intestine: accuracy and relevance of enteroclysis. Radiology. 1992;184:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Panes J, Bouhnik Y, Reinisch W, Stoker J, Taylor SA, Baumgart DC, Danese S, Halligan S, Marincek B, Matos C, Peyrin-Biroulet L, Rimola J, Rogler G, van Assche G, Ardizzone S, Ba-Ssalamah A, Bali MA, Bellini D, Biancone L, Castiglione F, Ehehalt R, Grassi R, Kucharzik T, Maccioni F, Maconi G, Magro F, Martín-Comín J, Morana G, Pendsé D, Sebastian S, Signore A, Tolan D, Tielbeek JA, Weishaupt D, Wiarda B, Laghi A. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 478] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 52. | Lee SS, Kim AY, Yang SK, Chung JW, Kim SY, Park SH, Ha HK. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 303] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 53. | Wills JS, Lobis IF, Denstman FJ. Crohn disease: state of the art. Radiology. 1997;202:597-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Karateke F, Menekşe E, Das K, Ozyazici S, Demirtürk P. Isolated duodenal Crohn's disease: a case report and a review of the surgical management. Case Rep Surg. 2013;2013:421961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 55. | Helms D, Skrove J, Shobani V, Patel N, Sharma S, Sobrado J, Sarol J. Crohn's disease: A case of rare, isolated duodenal involvement. Am J Gastroenterol. 2016;111 S824 [DOI:10.14309/00000434-201610001. |
| 56. | Ashraf MF, Gul E, Malik UE, Hasak S. A Rare Case of Duodenal Crohn’s Disease Presenting as Megaduodenum. Am J Gastroenterol. 2022;117:e1825. [DOI] [Full Text] |
| 57. | Baker ME, Hara AK, Platt JF, Maglinte DD, Fletcher JG. CT enterography for Crohn's disease: optimal technique and imaging issues. Abdom Imaging. 2015;40:938-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Baumgart DC. Crohn's disease and ulcerative colitis. Cham: Springer International Publishing AG, 2017. Available from: https://link.springer.com/book/10.1007/978-3-319-33703-6. |
| 59. | Dave-Verma H, Moore S, Singh A, Martins N, Zawacki J. Computed tomographic enterography and enteroclysis: pearls and pitfalls. Curr Probl Diagn Radiol. 2008;37:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Juanpere S, Valls L, Serra I, Osorio M, Gelabert A, Maroto A, Pedraza S. Imaging of non-neoplastic duodenal diseases. A pictorial review with emphasis on MDCT. Insights Imaging. 2018;9:121-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Chang CW, Wong JM, Tung CC, Shih IL, Wang HY, Wei SC. Intestinal stricture in Crohn's disease. Intest Res. 2015;13:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 62. | Chiorean MV, Sandrasegaran K, Saxena R, Maglinte DD, Nakeeb A, Johnson CS. Correlation of CT enteroclysis with surgical pathology in Crohn's disease. Am J Gastroenterol. 2007;102:2541-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 63. | Bruining DH, Siddiki HA, Fletcher JG, Tremaine WJ, Sandborn WJ, Loftus EV Jr. Prevalence of penetrating disease and extraintestinal manifestations of Crohn's disease detected with CT enterography. Inflamm Bowel Dis. 2008;14:1701-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 64. | Booya F, Akram S, Fletcher JG, Huprich JE, Johnson CD, Fidler JL, Barlow JM, Solem CA, Sandborn WJ, Loftus EV Jr. CT enterography and fistulizing Crohn's disease: clinical benefit and radiographic findings. Abdom Imaging. 2009;34:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Desmond AN, O'Regan K, Curran C, McWilliams S, Fitzgerald T, Maher MM, Shanahan F. Crohn's disease: factors associated with exposure to high levels of diagnostic radiation. Gut. 2008;57:1524-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 265] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 66. | Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6212] [Cited by in RCA: 5800] [Article Influence: 322.2] [Reference Citation Analysis (3)] |
| 67. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E, Kupcinskas L, Mantzaris G, Travis S, Stange E; European Crohn's and Colitis Organisation (ECCO). The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 792] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 68. | Lightner AL, Fletcher JG. Duodenal Crohn's Disease-a Diagnostic Conundrum. J Gastrointest Surg. 2018;22:761-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Nehra AK, Sheedy SP, Wells ML, VanBuren WM, Hansel SL, Deepak P, Lee YS, Bruining DH, Fletcher JG. Imaging Findings of Ileal Inflammation at Computed Tomography and Magnetic Resonance Enterography: What do They Mean When Ileoscopy and Biopsy are Negative? J Crohns Colitis. 2020;14:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | Tsai R, Mintz A, Lin M, Mhlanga J, Chiplunker A, Salter A, Ciorba M, Deepak P, Fowler K. Magnetic resonance enterography features of small bowel Crohn's disease activity: an inter-rater reliability study of small bowel active inflammation in clinical practice setting. Br J Radiol. 2019;92:20180930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Ram R, Sarver D, Pandey T, Guidry CL, Jambhekar KR. Magnetic resonance enterography: A stepwise interpretation approach and role of imaging in management of adult Crohn's disease. Indian J Radiol Imaging. 2016;26:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Gourtsoyiannis NC, Grammatikakis J, Papamastorakis G, Koutroumbakis J, Prassopoulos P, Rousomoustakaki M, Papanikolaou N. Imaging of small intestinal Crohn's disease: comparison between MR enteroclysis and conventional enteroclysis. Eur Radiol. 2006;16:1915-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 73. | Sinha R, Murphy P, Sanders S, Ramachandran I, Hawker P, Rawat S, Roberts S. Diagnostic accuracy of high-resolution MR enterography in Crohn's disease: comparison with surgical and pathological specimen. Clin Radiol. 2013;68:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 74. | Siddiki HA, Fidler JL, Fletcher JG, Burton SS, Huprich JE, Hough DM, Johnson CD, Bruining DH, Loftus EV Jr, Sandborn WJ, Pardi DS, Mandrekar JN. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn's disease. AJR Am J Roentgenol. 2009;193:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 75. | Horsthuis K, Bipat S, Bennink RJ, Stoker J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology. 2008;247:64-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 432] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 76. | Negaard A, Paulsen V, Sandvik L, Berstad AE, Borthne A, Try K, Lygren I, Storaas T, Klow NE. A prospective randomized comparison between two MRI studies of the small bowel in Crohn's disease, the oral contrast method and MR enteroclysis. Eur Radiol. 2007;17:2294-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 77. | O'Malley RB, Al-Hawary MM, Kaza RK, Wasnik AP, Liu PS, Hussain HK. Rectal imaging: part 2, Perianal fistula evaluation on pelvic MRI--what the radiologist needs to know. AJR Am J Roentgenol. 2012;199:W43-W53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | Wagtmans MJ, Verspaget HW, Lamers CB, van Hogezand RA. Clinical aspects of Crohn's disease of the upper gastrointestinal tract: a comparison with distal Crohn's disease. Am J Gastroenterol. 1997;92:1467-1471. [PubMed] |
| 79. | Maeda K, Okada M, Seo M, Aoyagi K, Yamaguchi M, and Sakisaka S. Evaluation of gastroduodenal mucosal lesions in patients with Crohn's disease and ulcerative colitis. Digestive Endoscopy. 2004;16:199-203. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 80. | Rutgeerts P, Onette E, Vantrappen G, Geboes K, Broeckaert L, Talloen L. Crohn's disease of the stomach and duodenum: A clinical study with emphasis on the value of endoscopy and endoscopic biopsies. Endoscopy. 1980;12:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Graca-Pakulska K, Błogowski W, Zawada I, Deskur A, Dąbkowski K, Urasińska E, Starzyńska T. Endoscopic findings in the upper gastrointestinal tract in patients with Crohn's disease are common, highly specific, and associated with chronic gastritis. Sci Rep. 2023;13:703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 82. | Horjus Talabur Horje CS, Meijer J, Rovers L, van Lochem EG, Groenen MJ, Wahab PJ. Prevalence of Upper Gastrointestinal Lesions at Primary Diagnosis in Adults with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:1896-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 83. | Sakuraba A, Iwao Y, Matsuoka K, Naganuma M, Ogata H, Kanai T, Hibi T. Endoscopic and pathologic changes of the upper gastrointestinal tract in Crohn's disease. Biomed Res Int. 2014;2014:610767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 84. | Fujiya M, Sakatani A, Dokoshi T, Tanaka K, Ando K, Ueno N, Gotoh T, Kashima S, Tominaga M, Inaba Y, Ito T, Moriichi K, Tanabe H, Ikuta K, Ohtake T, Yokota K, Watari J, Saitoh Y, Kohgo Y. A Bamboo Joint-Like Appearance is a Characteristic Finding in the Upper Gastrointestinal Tract of Crohn's Disease Patients: A Case-Control Study. Medicine (Baltimore). 2015;94:e1500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Carbo AI, Reddy T, Gates T, Vesa T, Thomas J, Gonzalez E. The most characteristic lesions and radiologic signs of Crohn disease of the small bowel: air enteroclysis, MDCT, endoscopy, and pathology. Abdom Imaging. 2014;39:215-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Tremaine WJ. Diagnosis and treatment of indeterminate colitis. Gastroenterol Hepatol (N Y). 2011;7:826-828. [PubMed] |
| 87. | Friedel D, Sharma J. Duodenal Biopsy. [Updated 2023 Jul 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560637/. |
| 88. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1445] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 89. | Dalal SR, Kwon JH. The Role of MicroRNA in Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2010;6:714-722. [PubMed] |
| 90. | Ravelli A, Bolognini S, Gambarotti M, Villanacci V. Variability of histologic lesions in relation to biopsy site in gluten-sensitive enteropathy. Am J Gastroenterol. 2005;100:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 91. | Serra S, Jani PA. An approach to duodenal biopsies. J Clin Pathol. 2006;59:1133-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 92. | Bentley E, Jenkins D, Campbell F, Warren B. How could pathologists improve the initial diagnosis of colitis? Evidence from an international workshop. J Clin Pathol. 2002;55:955-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Tanaka M, Riddell RH. The pathological diagnosis and differential diagnosis of Crohn's disease. Hepatogastroenterology. 1990;37:18-31. [PubMed] |
| 94. | Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6:62-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 95. | Brignola C, Campieri M, Bazzocchi G, Farruggia P, Tragnone A, Lanfranchi GA. A laboratory index for predicting relapse in asymptomatic patients with Crohn's disease. Gastroenterology. 1986;91:1490-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 104] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 96. | Cappello M, Morreale GC. The Role of Laboratory Tests in Crohn's Disease. Clin Med Insights Gastroenterol. 2016;9:51-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Nahon S, Lahmek P, Lesgourgues B, Poupardin C, Chaussade S, Peyrin-Biroulet L, Abitbol V. Diagnostic delay in a French cohort of Crohn's disease patients. J Crohns Colitis. 2014;8:964-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 98. | Schoepfer AM, Dehlavi MA, Fournier N, Safroneeva E, Straumann A, Pittet V, Peyrin-Biroulet L, Michetti P, Rogler G, Vavricka SR; IBD Cohort Study Group. Diagnostic delay in Crohn's disease is associated with a complicated disease course and increased operation rate. Am J Gastroenterol. 2013;108:1744-53; quiz 1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 99. | Pariente B, Mary JY, Danese S, Chowers Y, De Cruz P, D'Haens G, Loftus EV Jr, Louis E, Panés J, Schölmerich J, Schreiber S, Vecchi M, Branche J, Bruining D, Fiorino G, Herzog M, Kamm MA, Klein A, Lewin M, Meunier P, Ordas I, Strauch U, Tontini GE, Zagdanski AM, Bonifacio C, Rimola J, Nachury M, Leroy C, Sandborn W, Colombel JF, Cosnes J. Development of the Lémann index to assess digestive tract damage in patients with Crohn's disease. Gastroenterology. 2015;148:52-63.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 100. | Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. 2010;139:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 577] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 101. | Li Y, Chen B, Gao X, Hu N, Huang M, Ran Z, Liu Z, Zhong J, Zou D, Wu X, Ren J, Sheng J, Zheng P, Wang H, Chen M, Chen J, Xi P, Lu J, Handel M, Liu Y, Fan H, Qian J. Current diagnosis and management of Crohn's disease in China: results from a multicenter prospective disease registry. BMC Gastroenterol. 2019;19:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 102. | Cohen BL. Gastroduodenal and Jejunoileal Crohn's Disease. Gastroenterol Hepatol (N Y). 2022;18:418-421. [PubMed] |
| 103. | Soldera J, Coelho GP, Heinrich CF. Life-Threatening Diarrhea in an Elderly Patient. Gastroenterology. 2021;160:26-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 104. | Soldera J, Salgado K, Pêgas KL. Refractory celiac disease type 2: how to diagnose and treat? Rev Assoc Med Bras (1992). 2021;67:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 105. | Rech MB, da-Cruz ER, Salgado K, Balbinot RA, Balbinot SS, Soldera J. Metastatic gastric cancer from breast carcinoma presenting with paraneoplastic rheumatic syndrome: A case report. World J Clin Cases. 2023;11:3282-3287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Fistarol CHDB, da Silva FR, Passarin TL, Schmitz RF, Salgado K, Soldera J. Obscure gastrointestinal bleeding due to gastrointestinal stromal tumour of duodenum. GastroHep. 2021;3:169-171. [DOI] [Full Text] |
| 107. | Pante L, Brito LG, Franciscatto M, Brambilla E, Soldera J. A rare cause of acute abdomen after a Good Friday. World J Clin Cases. 2022;10:9539-9541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (0)] |
| 108. | Moro C, Nunes C, Onzi G, Terres AZ, Balbinot RA, Balbinot SS, Soldera J. Gastrointestinal: Life-threatening diarrhea due to pellagra in an elderly patient. J Gastroenterol Hepatol. 2020;35:1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 109. | Soldera J. Disseminated histoplasmosis with duodenal involvement. Gastroenterol Hepatol. 2020;43:453-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 110. | da Cruz ER, Forno AD, Pacheco SA, Bigarella LG, Ballotin VR, Salgado K, Freisbelen D, Michelin L, Soldera J. Intestinal Paracoccidioidomycosis: Case report and systematic review. Braz J Infect Dis. 2021;25:101605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 111. | Kanika A, Soldera J. Pulmonary cytomegalovirus infection: A case report and systematic review. WJMA. 2023;11:151-166. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Reference Citation Analysis (5)] |
| 112. | Soldera J, Salgado K. Gastrointerestinal: Valsartan induced sprue-like enteropathy. J Gastroenterol Hepatol. 2020;35:1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 113. | Ballotin VR, Bigarella LG, Riva F, Onzi G, Balbinot RA, Balbinot SS, Soldera J. Primary sclerosing cholangitis and autoimmune hepatitis overlap syndrome associated with inflammatory bowel disease: A case report and systematic review. World J Clin Cases. 2020;8:4075-4093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 114. | Brambilla B, Barbosa AM, Scholze CDS, Riva F, Freitas L, Balbinot RA, Balbinot S, Soldera J. Hemophagocytic Lymphohistiocytosis and Inflammatory Bowel Disease: Case Report and Systematic Review. Inflamm Intest Dis. 2020;5:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 115. | Soldera J, Bosi GR. Haemophagocytic lymphohistiocytosis following a COVID-19 infection: case report. J Infect Dev Ctries. 2023;17:302-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 116. | Fukunaga N, Ishikawa M, Yamamura Y, Ichimori T, Sakata A. Spontaneous intramural duodenal hematoma complicating acute pancreatitis. Surgery. 2011;149:143-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 117. | Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 979] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 118. | Loras Alastruey C, Andújar Murcia X, Esteve Comas M. The role of stents in the treatment of Crohn's disease strictures. Endosc Int Open. 2016;4:E301-E308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 119. | Rieder F, Fiocchi C, Rogler G. Mechanisms, Management, and Treatment of Fibrosis in Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152:340-350.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 366] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 120. | Chen YJ, Mao R, Li XH, Cao QH, Chen ZH, Liu BX, Chen SL, Chen BL, He Y, Zeng ZR, Ben-Horin S, Rimola J, Rieder F, Xie XY, Chen MH. Real-Time Shear Wave Ultrasound Elastography Differentiates Fibrotic from Inflammatory Strictures in Patients with Crohn's Disease. Inflamm Bowel Dis. 2018;24:2183-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 121. | Pittet V, Rogler G, Michetti P, Fournier N, Vader JP, Schoepfer A, Mottet C, Burnand B, Froehlich F; Swiss Inflammatory Bowel Disease Cohort Study Group. Penetrating or stricturing diseases are the major determinants of time to first and repeat resection surgery in Crohn's disease. Digestion. 2013;87:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 122. | Spiceland CM, Lodhia N. Endoscopy in inflammatory bowel disease: Role in diagnosis, management, and treatment. World J Gastroenterol. 2018;24:4014-4020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 123. | Gong K, Guo S, Wang K. Diagnosis and treatment of duodenal injury and fistula. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:266-269. [PubMed] |
| 124. | Ando T, Nobata K, Watanabe O, Kusugami K, Maeda O, Ishiguro K, Ohmiya N, Niwa Y, Goto H. Abnormalities in the upper gastrointestinal tract in inflammatory bowel disease. Inflammopharmacology. 2007;15:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 125. | Gonzalez M, Collaud S, Gervaz P, Frossard JL, Morel P. Surgical treatment of duodenal stenosis in Crohn's disease. Ann Chir. 2006;131:636-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 126. | Ferrante D, Varini S, Macchia A, Soifer S, Badra R, Nul D, Grancelli H, Doval H; GESICA Investigators. Long-term results after a telephone intervention in chronic heart failure: DIAL (Randomized Trial of Phone Intervention in Chronic Heart Failure) follow-up. J Am Coll Cardiol. 2010;56:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 127. | Rodríguez-Grau Mdel C, Chaparro M, Díaz R, Gisbert JP. Infliximab in the treatment of refractory gastroduodenal Crohn's disease. Gastroenterol Hepatol. 2014;37:21-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 128. | Kim YL, Park YS, Park EK, Park DR, Choi GS, Ahn SB, Kim SH, Jo YJ. Refractory duodenal Crohn's disease successfully treated with infliximab. Intest Res. 2014;12:66-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 129. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P; ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3055] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 130. | Gaggar S, Scott J, Thompson N. Pyloric stenosis associated Crohn's disease responding to adalimumab therapy. World J Gastrointest Pharmacol Ther. 2012;3:97-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 131. | Tursi A. Duodenal Crohn's disease successfully treated with adalimumab. Endoscopy. 2011;43 Suppl 2 UCTN:E22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 132. | Annunziata ML, Caviglia R, Papparella LG, Cicala M. Upper gastrointestinal involvement of Crohn's disease: a prospective study on the role of upper endoscopy in the diagnostic work-up. Dig Dis Sci. 2012;57:1618-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 133. | Sousa M, Proença L, Carlos Silva J, Ribeiro Gomes AC, Afeto E, Carvalho J. Duodenal Crohn's Disease Complicated by Pancreatitis and Common Bile Duct Obstruction. GE Port J Gastroenterol. 2020;27:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 134. | Pallotta N, Barberani F, Hassan NA, Guagnozzi D, Vincoli G, Corazziari E. Effect of infliximab on small bowel stenoses in patients with Crohn's disease. World J Gastroenterol. 2008;14:1885-1890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (9)] |
| 135. | Bouhnik Y, Carbonnel F, Laharie D, Stefanescu C, Hébuterne X, Abitbol V, Nachury M, Brixi H, Bourreille A, Picon L, Bourrier A, Allez M, Peyrin-Biroulet L, Moreau J, Savoye G, Fumery M, Nancey S, Roblin X, Altwegg R, Bouguen G, Bommelaer G, Danese S, Louis E, Zappa M, Mary JY; GETAID CREOLE Study Group. Efficacy of adalimumab in patients with Crohn's disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut. 2018;67:53-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 136. | Kochhar G, Shen B. Endoscopic fistulotomy in inflammatory bowel disease (with video). Gastrointest Endosc. 2018;88:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 137. | Shen B. Exploring endoscopic therapy for the treatment of Crohn's disease-related fistula and abscess. Gastrointest Endosc. 2017;85:1133-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 138. | Zhong M, Cui B, Xiang J, Wu X, Wen Q, Li Q, Zhang F. Rapamycin is Effective for Upper but not for Lower Gastrointestinal Crohn's Disease-Related Stricture: A Pilot Study. Front Pharmacol. 2020;11:617535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 139. | Xu L, He B, Sun Y, Li J, Shen P, Hu L, Liu G, Wang J, Duan L, Zhan S, Wang S. Incidence of Inflammatory Bowel Disease in Urban China: A Nationwide Population-based Study. Clin Gastroenterol Hepatol. 2023;21:3379-3386.e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |