Published online Sep 20, 2022. doi: 10.5662/wjm.v12.i5.438
Peer-review started: March 5, 2022
First decision: June 16, 2022
Revised: June 29, 2022
Accepted: August 30, 2022
Article in press: August 30, 2022
Published online: September 20, 2022
Processing time: 195 Days and 4.9 Hours
Growth differentiation factor (GDF)-15 is a member of a transforming growth factor-β cytokine superfamily that regulates metabolism and is released in response to inflammation, hypoxia and tissue injury. It has evolved as one of the most potent cytokines for predicting the severity of infections and inflammatory conditions, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
To investigate the utility of GDF-15 in predicting the severity of SARS-CoV-2 infection.
PubMed, Reference Citation Analysis, CNKI, and Goggle Scholar were explored by using related MeSH keywords and data such as the first author’s name, study duration, type and place of study, sample size and subgroups of participants if any, serum/plasma GDF- 15 level in pg/mL, area under the curve and cut-off value in receiver operating characteristic analysis, method of measurement of GDF-15, and the main conclusion were extracted.
In all studies, the baseline GDF-15 level was elevated in SARS-CoV-2-infected patients, and it was significantly associated with severity, hypoxemia, viral load, and worse clinical consequences. In addition, GDF-15 levels were correlated with C-reactive protein, D-dimer, ferritin and procalcitonin, and it had superior discriminatory ability to detect severity and in-hospital mortality of SARS-CoV-2 infection. Hence, GDF-15 might be used to predict the severity and prognosis of hospitalized patients with SARS-CoV-2.
Serial estimation of GDF-15 levels in hospitalized patients with SARS-CoV-2 infection appeared to have useful prognostic value and GDF-15 can be considered a clinically prominent sepsis biomarker for SARS-CoV-2 infection.
Core Tip: Growth differentiation factor (GDF)-15 levels are higher in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and higher levels are associated with disease severity, viremia and hypoxemia. The consistent increase in the concentration of GDF-15 during a hospital stay is associated with worse outcomes. Hence, serial monitoring of GDF-15 concentrations may provide useful prognostic value for hospitalized patients with SARS-CoV-2. GDF-15 appears to be involved in the underlying pathophysiology, laying the foundation for a novel therapeutic approach for SARS-CoV-2.
- Citation: Parchwani D, Dholariya S, Katoch C, Singh R. Growth differentiation factor 15 as an emerging novel biomarker in SARS-CoV-2 infection. World J Methodol 2022; 12(5): 438-447
- URL: https://www.wjgnet.com/2222-0682/full/v12/i5/438.htm
- DOI: https://dx.doi.org/10.5662/wjm.v12.i5.438
Coronavirus disease-2019 (COVID-19), an extremely contagious disease, caused by severe acute respi
SARS-CoV-2 is an enveloped virion with positive sense, single-stranded RNA with a genome size of 29.99 kb encoding for multiple nonstructural and structural proteins. The viral envelope contains four anchored structural proteins, spike protein (S), enveloped protein (E), nucleocapsid protein (N) and membrane protein (M)[6]. S glycoprotein (type 1 transmembrane protein) protrudes from the virus surface and embraces two functional components, S1 and S2. S1 helps the virus to binds with host cell through its receptor-binding domain (RBD) and S2 possesses an element essential for SARS-CoV-2 fusion with the host cell membrane[7].
SARS-CoV-2 enters type II pneumocytes in the lungs by binding with membrane-bound angiotensin-converting enzyme (ACE) 2 receptor through its RBD[6], primed by host cell surface transmembrane serine protease-2[8]. SARS-CoV-2 then starts replicating and migrating down to the airways and enters alveolar epithelial cells in the lungs, resulting in pre-eminent early viral loads and soluble ACE2 (sACE2) protein release into the bloodstream[9]. Cumulative viral load destroys type II alveolar epithelial cells and decreases the synthesis of pulmonary surfactants[10]. Simultaneously, infiltration of macrophages causes secretion of various cytokines namely tumor necrosis factor (TNF)-α, interleukin (IL)-1 and IL-6, instigating migration of lymphocytes and neutrophils and vasodilatation. This dysregulated host immune response plays a crucial role in the pathogenesis of the cytokine storm in SARS-CoV-2 infection[11].
Common clinical manifestations of SARS-CoV-2 infection are pyrexia, tussis, dyspnea, pharyngitis, myalgia, headache, and olfactory and taste dysfunction (hyposmia/anosmia or ageusia)[12]. However, severe consequences such as viral sepsis have been observed in approximately 20% of SARS-CoV-2-infected patients. Sepsis is a life-threatening systemic condition that graduates to cytokine storm followed by immune dysregulation, leading to systemic hyperinflammatory state, acute respiratory distress syndrome (ARDS), multiorgan failure, and development of sepsis-related complications with increased mortality[13].
Apart from inflammation and virulence, tissue tolerance and host response are also important factors for the pathogenesis and resultant consequences of SARS-CoV-2 infection[14]. A member of the transforming growth factor-β superfamily, growth and differentiation factor (GDF)-15, is a multifunctional anti-inflammatory cytokine that increases immunotolerance physiologically. It is an evolving modulator of immune responses and facilitates inflammation-induced tissue tolerance through metabolic adaptation[15,16]. Various pathways such as inflammation, hypoxia and oxidative stress tightly regulate expression of GDF-15[17]. In an animal model infected by human rhinovirus, GDF-15 promotes viral replication and virus-induced inflammation in the lungs[18]. Thus, GDF-15 may attenuate the antiviral immune response and affect the consequences of SARS-CoV-2 infection. Conversely, GDF-15 might increase in SARS-CoV-2 infection due to the altered balance of proinflammatory and anti-inflammatory cytokines[14].
Some biomarkers, such as C-reactive protein (CRP), D-dimer, ferritin[19] and presepsin[20], have been identified as biomarkers to assess the inflammation and consequences of SARS-CoV-2 infection. However, more than a year into the pandemic with little evidence of specific therapeutic regimens, front-line clinicians are still reliant on clinical presentation and basic imaging facilities for assessing risk stratification of SARS-CoV-2[21]. Since there are limited data on the accuracy of laboratory investigations for evaluating the severity of SARS-CoV-2 infection[22], identifying a novel biomarker such as GDF-15 offers the opportunity to triage patients for disease severity, allowing better care and timely management of critical patients. As GDF-15 predicts tissue tolerance in SARS-CoV-2-induced inflammation[14], it is worth reviewing the importance of GDF-15 for diagnosis and risk stratification of SARS-CoV-2. This systematic review emphasizes the importance of GDF-15 in SARS-CoV-2 infection by providing the most current evidence from studies that have examined GDF-15 in SARS-CoV-2 patients.
The highly sensitive systematic literature search was carried out in multiple electronic databases: PubMed, Reference Citation Analysis (RCA), China National Knowledge Infrastructure (CNKI), Web of Science and Google Scholar. The following MeSH keywords were used to search the literature: GDF-15 AND SARS-CoV-2 OR GDF-15 AND COVID-19 OR GDF-15 AND 2019-nCoV OR GDF-15 AND Coronavirus Disease 2019. The inclusion criteria were English language articles published between December 1, 2020 and February 15, 2022. The original research articles, case series, brief reports, and letters were accepted for review. All selected articles’ reference list was further screened to identify additional possible research literature. There was no exclusion based on the study outcome and stage or severity of SARS-CoV-2 infection. Finally, seven out of 24 articles were selected for the review after removing the duplicate research literature.
Using the above key terms, the first two authors independently searched for research literature following the inclusion and exclusion criteria, and both authors selected the final articles. The data were extracted in duplicate by standardized data extraction tables by two researchers. The following data were extracted: first author, place of study, sample size, disease severity/stage, intensive care unit (ICU) admission, survivors and nonsurvivors/death, GDF-15 level, and correlation with other inflammatory or sepsis biomarkers.
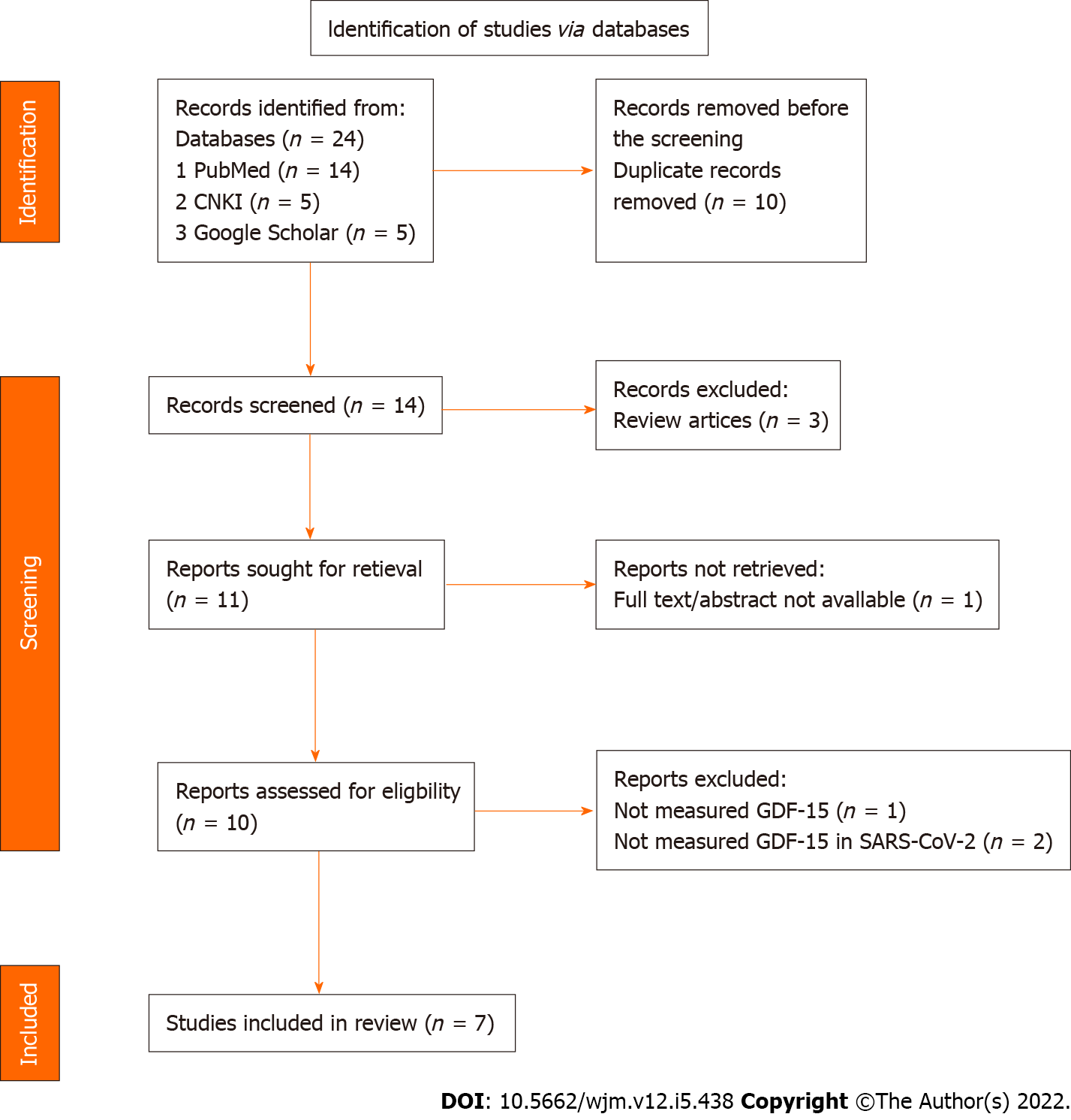
A total of 14 studies were retrieved after removing the duplicate or repeated publications; 13 of which were evaluated in full text. Among the included studies, seven were considered suitable for the qualitative synthesis. The process flow for the extraction of research literature (Figure 1) was conducted according to the guidelines defined in the PRISMA statement 2020 and was performed in accordance with a predetermined published protocol (PROSPERO ID: CRD42022311838).
The primary analysis of this systematic review revealed a high level of GDF-15 in SARS-CoV-2-infected patients and found a significant interaction with the severity of COVID-19. GDF-15 was also found to be positively correlated to predict the disease severity and to some degree is worthier than other inflammatory biomarkers as CRP, D-dimer, procalcitonin and ferritin. This conclusion came firstly from the study by Myhre and colleagues in 2020, which evaluated the utility of serum GDF-15 as a prognostic biomarker in hospitalized patients with SARS-CoV-2, and compared it with other known inflammatory biomarkers (CRP, D-dimer, IL-6, procalcitonin and ferritin) in the Norwegian population from March 18, 2020 to May 4, 2020. The baseline GDF-15 level was elevated in 78% of cases of SARS-CoV-2 infection and it was found to be associated with viral load and hypoxemia. The GDF-15 concentrations were higher in patients who met the primary endpoint of ICU admission or death [4225.0 (3197.0–5972.0) pg/mL vs 2187.0 (1344–3620.0) pg/mL, P < 0.001]. Patients who reached the primary endpoint had a significant rise in GDF-15 from baseline to day 3 [86.0 (322.0–491.0) vs 1208.0 (0–4305.0) pg/mL, P < 0.001]. The area under the receiver operating characteristic curve (ROC) was 0.78 (95% confidence interval = 0.70–0.86), indicating a better prognostic significance of GDF-15 than for recognized inflammatory biomarkers such as CRP, ferritin, procalcitonin and IL-6. They derived a cut-off value of 2252.0 pg/mL that differentiated non-ICU survivors from nonsurvivors or ICU admission with good accuracy[23].
Secondly, Notz et al[24] measured blood GDF-15 in patients with SARS-CoV-2-induced ARDS in the German population from March 14 to May 28, 2020 and reported an increased level of GDF-15 in patients during their ICU stay. In addition, they testified that comorbidities were unlikely to influence the blood GDF-15 levels, and GDF-15 was not correlated with age, BMI or other anthropometric variables of patients[24]. Subsequently, Luis García de Guadiana Romualdo et al[25] evaluated the effect of circulating GDF-15 levels to predict the mortality of hospitalized SARS-CoV-2-infected patients in the Swedish population from March 14 to April 12, 2020. They found a significantly elevated level of GDF-15 in nonsurvivors compared to survivors of SARS-CoV-2 infection [9448.0 (6462.0–11707.0) vs 2590.0 (1886.0–4811.0) pg/mL]; a superior discriminatory ability of GDF-15 to predict in-hospital mortality at the cut-off value ≥ 7789.0 pg/mL [AUC = 0.892 (0.792–0.955), P < 0.001]. GDF-15 levels were also positively correlated with CRP (r = 0.527; P < 0.001), ferritin (r = 0.334; P = 0.006) and D-dimer (r = 0.260; P = 0.035). They concluded that GDF-15 might be used to predict the prognosis of in-hospitalized patients with SARS-CoV-2[25].
Likewise, Teng et al[26] retrospectively evaluated the profile of inflammatory factors in SARS-CoV-2-infected patients and healthy controls in China from January 22 to May 13, 2020. They assessed GDF-15 by categorizing SARS-CoV-2 patients into asymptomatic, mild, moderate, severe and convalescent; GDF-15 at admission, remission and discharge to find the association between dynamic alteration in GDF-15 with the progression of SARS-CoV-2 infection, and found that GDF-15 concentration escalated consistently with disease severity. GDF-15 expression returned to normal in the convalescent group, as it did in the healthy participants. In continuance, study data revealed GDF-15 levels acutely upsurged with the worsening of symptoms before death, inferring that GDF-15 aptly monitors progression of SARS-CoV-2 infection. They reported an AUC value of 0.89 for GDF-15, which implied that the serum GDF-15 is an effective diagnostic biomarker to assess the severity of SARS-CoV-2 infection[26].
A prospective study conducted in the Swedish population[27] to evaluate the GDF-15 in SARS-CoV-2-infected patients and healthy controls reported a significantly (P < 0.001) higher level of GDF-15 in the severe [3562.0 pg/mL (2458.0–5880.0)] and moderate [3450.0 pg/mL (2337.0–4105.0)] type of SARS-CoV-2 infection compared to mild infection [748.0 pg/mL (586.0–1087.0)] and healthy participants [703.0 pg/mL (501.0–949.0)] throughout the acute phase. In the follow-up visit at 6 mo, severe and moderate SARS-CoV-2 infection was recorded with a high GDF-15 level compared to mild type and healthy controls (P < 0.05). Like the findings of Myhre et al[23], these authors also reported a significant association of GDF-15 with hypoxemia, viral load, and worse clinical consequences in SARS-CoV-2 infection[27].
Ebihara et al[28] conducted a prospective, multicenter observational study in the Japanese population to evaluate the role of cytokines in the pathogenesis of SARS-CoV-2 infection, through proteomics analysis. They found: an increased level of GDF-15 in patients with SARS-CoV-2 infection during ICU stay; an AUC of 0.764 and 0.740 for SARS-CoV-2 infection severity and prognosis, respectively; plasma level of GDF-15 was significantly associated with the time to wean off mechanical ventilation and delay recovery in ICU. Based on these results, the authors concluded that GDF-15 was positively related to the severity of SARS-CoV-2 infection and its concentration was significantly higher in patients with sepsis compared with SARS-CoV-2 infection[28].
Alserawan et al[29] evaluated serum GDF-15 level and correlated it with SARS-CoV-2 infection severity in the Spanish population. They reported a significantly (P < 0.0001) higher level of GDF-15 in SARS-CoV-2-infected patients [2051.0 (1474.0–2925.0) pg/mL] compared to healthy controls [582.0 (370.0–807.0) pg/mL] and in patients who were admitted to hospital for > 9 d. They categorized SARS-CoV-2 patients into SpO2/FiO2 ≤ 400 and > 400 to find an association of GDF-15 with lung involvement. They found high GDF-15 levels in SARS-CoV-2-infected patients with SpO2/FiO2 ≤ 400 or lung impairment. GDF-15 concentrations ≥ 1675.0 pg/mL were found to be a good predictor for impaired pulmonary function or SpO2/FiO2 ≤ 400 compared with CRP and D-dimer, according to ROC analysis (AUC = 0.729, P < 0.002)[29]. Wallentin et al[30] observed that GDF-15 was associated with sACE2 levels, increased risk of mortality, and cardiovascular disease, which could help identify those at risk for severe COVID-19 infection.
Table 1 abridges the findings of included studies in this systematic review and Table 2 gives an overview of the data pertaining to GDF-15 in the included studies. Gleaned from the included studies, we conclude that GDF-15 has both diagnostic and prognostic importance in SARS-CoV-2 infection. As SARS-CoV-2 invades the lungs, it causes leukocyte migration, endothelialitis, hypoxia and tissue destruction by enhanced innate immunity[31]. All these factors promote secretion of GDF-15 from infected alveolar epithelial cells. Migration of leukocytes releases proinflammatory cytokines such as TNF-α, IL-8, IL-6, IL-1β, interferon- and granulocyte–macrophage colony-stimulating factor, which in turn stimulates the Notch pathway. The Notch pathway may well activate the Wnt and Hippo pathways, which, in succession cause differentiation of IL-17- and GDF-15-mediated inhibition of the T regulatory suppressor cell activity, respectively, which individually and in conjunction with one another results into extreme activation of the immune system. Concurrently, syncytical development further hyperactivates the immune system and results in a cytokine storm. Thus, GDF-15 plays a pivotal role in the immunological context and may influence the pathogenesis of SARS-CoV-2[14,32].
| Refs | Study duration | Type of study | Region/place | Main findings |
| Myhre et al[23], 2020 | March 18, 2020 to May 4, 2020 | Prospective, observational study | Norway | GDF-15 has a better prognostic significance than recognized inflammatory biomarkers like CRP, ferritin, procalcitonin, and IL-6 |
| Notz et al[24], 2020 | March 14 to May 28, 2020 | A single-center retrospective study | Germany | There was no evident imbalance of pro-and anti-inflammatory pathways, with higher GDF-15 levels in patients with SARS-CoV-2 infection during ICU stay, implying elevated tissue resilience |
| Luis García de Guadiana Romualdo et al[25], 2021 | March 14 to April 12, 2020 | Case-series | Spain | The GDF level was significantly high in nonsurvivors compared to survivors of SARS-CoV-2 infection, and it may be useful to predict prognosis |
| Teng et al[26], 2021 | January 22, 2020, to May 13, 2020 | Retrospective study | China | GDF-15 could be used as a biomarker to predict the severity of SARS-CoV-2 infection. GDF-15 level increased consistently with increased severity of SARS-CoV-2 infection, and GDF-15 expression returned to normal level similarly in a convalescent group compared to the healthy control participants. Hence, it implies that the GDF-15 precisely monitors the progression of SARS-CoV-2 infection |
| Kanberg et al[27], 2021 | February 21 to November 5, 2020 | Prospective study | Sweden | Patients with severe and moderate SARS-CoV-2 infection exhibited significantly increased GDF-15 levels compared with participants with mild infection and controls throughout the acute phase. Even after 6 mo of infection, GDF-15 concentrations persisted considerably higher in the severe and moderate infections compared to patients with mild infection and controls |
| Ebihara et al[28], 2022 | August 2020 to December 2020 | Prospective multicenter observational study | Japan | GDF-15 may be beneficial to predict delayed recovery or mortality of SARS-CoV-2-infected patients during ICU treatment |
| Alserawan et al[29], 2021 | Not mentioned | Prospective study | Spain | GDF-15 may play a role in categorizing SARS-CoV-2-infected patients based on severityGDF-15 is an excellent biomarker to detect impaired respiratory function compared to CRP and D-dimer |
| Refs | Myhre et al[23], 2020 | Notz et al[24], 2020 | Luis García de Guadiana Romualdo et al[25], 2021 | Teng et al[26], 2021 | Kanberg et al[27], 2021 | Ebihara et al[28], 2022 | Alserawan et al[29], 2021 | |
| Sample size and subgroup of participants, if any | 123 confirmed cases of SARS-CoV-2 infection (non-ICU survivor = 88, ICU admission/ death = 28) | 13 cases of SARS-CoV-2 infection with ARDS | 66 confirmed cases of SARS-CoV-2 infection (non-survival = 58, survival = 6) | 111 confirmed cases of SARS-CoV-2 infection and 20 healthy controls (asymptomatic = 14, mild = 12, moderate = 34, severe = 18, and convalescent = 33) | 100 confirmed cases of SARS-CoV-2 infection (mild = 24, moderate = 28, severe = 48] and 51 healthy controls | 306 confirmed cases of SARS-CoV-2 infection | 84 confirmed cases of SARS-CoV-2 infection and 20 healthy controls | |
| GDF-15 level in pg/mL | Healthy controls | 13.5 (8.0–79.0) | 703.0 (501.0–949.0) | - | 582.0 (370.0-807.0) | |||
| Mild | 136.4 (44.7–321.4) | 748.0 (586.0–1087.0) | - | 2051.0 (1474.0-2925.0) | ||||
| Moderate | 12400.0 | 256.2 (76.1–341.0) | 3450.0 (2337.0–4105.0) | - | ||||
| Severe | - | 524.8 (405.1–831.1) | 3562.0 (2458.0–5880.0) | Increased during ICU stay | ||||
| Critical | 621.0 | - | - | - | ||||
| Non-ICU survivor | 2187.0 (1344.0-3620.0) | - | 2590.0 (1886.0-4811.0) | - | - | - | ||
| ICU admission or death | 4225.0 (3197.0-5972.0) | - | 9448.0 (6462.0-11707.0) | - | - | - | - | |
| AUC and 95% CI of GDF-15 in ROC analysis | 0.78 (0.70–0.86) P < 0.001 | Not mentioned | 0.89 (0.792–0.955) P < 0.001 | 0.89 | Not mentioned | For severity: 0.764; For prognosis: 0.740 | 0.729 (0.602-0.857) P = 0.002 | |
| The optimal cut-off value of GDF-15 | 2252.0 pg/mL, to differentiate non-ICU survivors and ICU admission or death | Not mentioned | 7789.00 pg/mL, to differentiate non-ICU survivors and ICU admission or death | Not mentioned | Not mentioned | Not mentioned | 1675.0 pg/mL, to recognize deprived respiratory function (SpO2/FiO2 ≤ 400) | |
| Method of GDF-15 measurement | ELISA | ELISA | Electro-chemiluminescent | ELISA | Electro-chemiluminescent | ELISA | ELISA | |
| Additional findings related to GDF-15 | It was associated with viral load and hypoxemia. Better prognostic significance compared to CRP, ferritin, IL-6, and procalcitonin | It was not correlated with age and BMI | Positively correlated with CRP, ferritin, and D-dimer | GDF-15 indicates the severity and closely monitor the progression of SARS-CoV-2 | Elevated GDF-15 was significantly related to hypoxemia, viral load, and worse clinical consequences | The plasma level of GDF-15 was significantly associated with the time to wean-off mechanical ventilation | Positively correlated with CRP, D-dimer, and neutrophil count and negatively correlated with lymphocyte count |
Impaired iron metabolism has also been hypothesized in the development of hyperinflammation and oxidative stress in patients with SARS-CoV-2 infection. GDF-15 has also been found to interact with iron metabolism, hepcidin and erythropoiesis during inflammation. More specifically, elevated GDF-15 during hypoxia and anemia has been found to suppress hepcidin expression, which boosts the iron level for hemoglobin production. As a result, GDF-15 has been considered as an immune modifier to regulate altered erythropoiesis and ferroptosis in patients with SARS-CoV-2 infection with anemia. During inflammation, GDF-15 overexpression has been associated with iron overload, which could increases ferritin, another key biomarker to assess severity of SARS-CoV-2 infection[33]. Hence, this hypothesis supports the association between high GDF-15 and anemia in inflammatory conditions such as SARS-CoV-2 infection, chronic kidney disease[34], diabetes, cardiovascular diseases[35], and cancer[36].
The iron chelation therapy improves innate immunity and endothelialitis in SARS-CoV-2 infection through its antifibrotic and antiviral properties[37] and is substantiated by the fact that the US FDA approved iron chelation therapy as an adjuvant treatment for the management of critical patients with SARS-CoV-2 infection[38]. Consequently, GDF-15 could be considered a crucial biomarker to indicate the prompt use of iron-chelating therapy in SARS-CoV-2 infection.
Metformin has recently been shown to elevate blood GDF15 levels, resulting in decreased satiety and body weight in clinical investigations. In animal studies, metformin was also associated with increased GDF-15 levels, along with increased GDF-15 expression in kidneys and intestines. In addition, metformin supplementation decreased weight in high-fat-fed mice, but not in GDF15-deficient mice and mice deficient for GFRAL (GDNF family receptor α-like, receptor for GDF-15)[39-42]. Thus, metformin supplementation has been associated with reduction in mortality in patients with SARS-CoV-2 infection with diabetes.
A few limitations of this analysis should be taken into consideration when interpreting the results for any potential clinical implications. Firstly, the sample size was small. Secondly, heterogeneity was a major issue in the included studies, especially in terms of methodology, type of ongoing treatment, time of sample collection after hospital admission, non-consideration of the disease-onset time and divergence in adjusting study variables (age, gender, and various comorbidities). Thirdly, variance in the quantification of GDF-15 and subclassification of patient populations in the included studies. Lastly, the literature search and coverage were limited to articles published in English; languages other than English were not considered for analysis, which is susceptible to a local literature bias. Nevertheless, the goal of this study was not to create a predictive model but to investigate the potential importance of GDF-15 as a novel biomarker[39-42]. Hence, despite these limitations, this systematic review offers vital information on the risk stratification of SARS-CoV-2, which could in the future become an important part of the clinical process.
GDF-15 appeared to be an important determinant in the etiopathogenesis of disease and might serve as a predictor for onset and severity of SARS-CoV-2 infection. Hence, GDF-15 can be considered a clinically prominent sepsis biomarker for screening, risk stratification, and monitoring SARS-CoV-2.
Growth differentiation factor (GDF)-15 is a modulator of immune responses and facilitates inflammation-induced tissue tolerance through metabolic adaptation. Experimental studies reveled that GDF-15 promotes virus replication and virus-induced inflammation in the lungs. Thus, GDF-15 may attenuate the antiviral immune response and affect the consequences of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
To identify a novel biomarker for the guidance of severity of disease, so as to provide better care and timely management of critical patients.
To investigate the utility of GDF-15 in predicting the risk stratification of SARS-CoV-2.
A systematic literature search was carried out in multiple electronic databases: PubMed, Reference Citation Analysis, China National Knowledge Infrastructure (CNKI), Web of Science and Google Scholar using MeSH keywords. The inclusion criteria were research articles of any type written in the English language and published between December 1, 2020 and February 15, 2022. There was no exclusion based on the study outcome and stage or severity of SARS-CoV-2 infection. Finally, seven of 24 articles were selected for the review after removing the duplicate research literature.
The primary analysis of this systematic review revealed a high level of GDF-15 in SARS-CoV-2-infected patients and found a significant interaction with the severity of COVID-19. GDF-15 was also found to be positively correlated to predict the disease severity and is superior to other inflammatory biomarkers such as C-reactive protein, D-dimer, procalcitonin and ferritin.
Serial estimation of GDF-15 levels in hospitalized patients with SARS-CoV-2 infection may have useful prognostic value and GDF-15 can be considered a clinically prominent sepsis biomarker for screening, risk stratification, and monitoring SARS-CoV-2.
Additional prospective studies are warranted in this regard to justify GDF-15 as an ideal biomarker which should provide optimization of disease status.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hashemi S, Iran; Munteanu C, Romania; Papadopoulos K, Thailand S-Editor: Liu JH L-Editor: Kerr C P-Editor: Liu JH
| 1. | Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4848] [Cited by in RCA: 4389] [Article Influence: 877.8] [Reference Citation Analysis (1)] |
| 2. | WHO Coronavirus Disease (COVID-19) Dashboard n.d. Accessed February 28 2022. Available from: https://covid19.who.int. |
| 3. | Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, Iosifidis C, Agha R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020;76:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3213] [Cited by in RCA: 2654] [Article Influence: 530.8] [Reference Citation Analysis (0)] |
| 4. | Witzenrath M, Kuebler WM. Pneumonia in the face of COVID-19. Am J Physiol Lung Cell Mol Physiol. 2020;319:L863-L866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, Hosein Z, Padda I, Mangat J, Altaf M. Comorbidity and its Impact on Patients with COVID-19. SN Compr Clin Med. 2020;2:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 973] [Cited by in RCA: 781] [Article Influence: 156.2] [Reference Citation Analysis (0)] |
| 6. | Mariano G, Farthing RJ, Lale-Farjat SLM, Bergeron JRC. Structural Characterization of SARS-CoV-2: Where We Are, and Where We Need to Be. Front Mol Biosci. 2020;7:605236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 7. | Tang T, Bidon M, Jaimes JA, Whittaker GR, Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antiviral Res. 2020;178:104792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 610] [Cited by in RCA: 547] [Article Influence: 109.4] [Reference Citation Analysis (0)] |
| 8. | Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ Res. 2020;126:1456-1474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1325] [Cited by in RCA: 1377] [Article Influence: 275.4] [Reference Citation Analysis (0)] |
| 9. | Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, Hou C, Wang H, Liu J, Xu Y, Cao Z, Gao Z. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 843] [Cited by in RCA: 703] [Article Influence: 140.6] [Reference Citation Analysis (1)] |
| 10. | Carcaterra M, Caruso C. Alveolar epithelial cell type II as main target of SARS-CoV-2 virus and COVID-19 development via NF-Kb pathway deregulation: A physio-pathological theory. Med Hypotheses. 2021;146:110412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 11. | Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 552] [Cited by in RCA: 776] [Article Influence: 155.2] [Reference Citation Analysis (0)] |
| 12. | Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J Gen Intern Med. 2020;35:1545-1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 869] [Cited by in RCA: 703] [Article Influence: 140.6] [Reference Citation Analysis (0)] |
| 13. | Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and Multiorgan Response. Curr Probl Cardiol. 2020;45:100618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 636] [Cited by in RCA: 642] [Article Influence: 128.4] [Reference Citation Analysis (0)] |
| 14. | Rochette L, Zeller M, Cottin Y, Vergely C. GDF15: an emerging modulator of immunity and a strategy in COVID-19 in association with iron metabolism. Trends Endocrinol Metab. 2021;32:875-889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Wischhusen J, Melero I, Fridman WH. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front Immunol. 2020;11:951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 325] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 16. | Luan HH, Wang A, Hilliard BK, Carvalho F, Rosen CE, Ahasic AM, Herzog EL, Kang I, Pisani MA, Yu S, Zhang C, Ring AM, Young LH, Medzhitov R. GDF15 Is an Inflammation-Induced Central Mediator of Tissue Tolerance. Cell. 2019;178:1231-1244.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 354] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 17. | Baek SJ, Eling T. Growth differentiation factor 15 (GDF15): A survival protein with therapeutic potential in metabolic diseases. Pharmacol Ther. 2019;198:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 18. | Wu Q, Jiang D, Schaefer NR, Harmacek L, O’Connor BP, Eling TE, Eickelberg O, Chu HW. Overproduction of growth differentiation factor 15 promotes human rhinovirus infection and virus-induced inflammation in the lung. Am J Physiol Lung Cell Mol Physiol. 2018;314:L514-L527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 330] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 20. | Dholariya S, Parchwani DN, Singh R, Radadiya M, Katoch CDS. Utility of P-SEP, sTREM-1 and suPAR as Novel Sepsis Biomarkers in SARS-CoV-2 Infection. Indian J Clin Biochem. 2022;37:131-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Lee JE, Hwang M, Kim YH, Chung MJ, Sim BH, Chae KJ, Yoo JY, Jeong YJ. Imaging and Clinical Features of COVID-19 Breakthrough Infections: A Multicenter Study. Radiology. 2022;303:682-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 22. | La Marca A, Capuzzo M, Paglia T, Roli L, Trenti T, Nelson SM. Testing for SARS-CoV-2 (COVID-19): a systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod Biomed Online. 2020;41:483-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 238] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 23. | Myhre PL, Prebensen C, Strand H, Røysland R, Jonassen CM, Rangberg A, Sørensen V, Søvik S, Røsjø H, Svensson M, Berdal JE, Omland T. Growth Differentiation Factor 15 Provides Prognostic Information Superior to Established Cardiovascular and Inflammatory Biomarkers in Unselected Patients Hospitalized With COVID-19. Circulation. 2020;142:2128-2137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 24. | Notz Q, Schmalzing M, Wedekink F, Schlesinger T, Gernert M, Herrmann J, Sorger L, Weismann D, Schmid B, Sitter M, Schlegel N, Kranke P, Wischhusen J, Meybohm P, Lotz C. Pro- and Anti-Inflammatory Responses in Severe COVID-19-Induced Acute Respiratory Distress Syndrome-An Observational Pilot Study. Front Immunol. 2020;11:581338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Luis García de Guadiana Romualdo, Mulero MDR, Olivo MH, Rojas CR, Arenas VR, Morales MG, Abellán AB, Conesa-Zamora P, García-García J, Hernández AC, Morell-García D, Dolores Albaladejo-Otón M, Consuegra-Sánchez L. Circulating levels of GDF-15 and calprotectin for prediction of in-hospital mortality in COVID-19 patients: A case series. J Infect. 2021;82:e40-e42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 26. | Teng X, Zhang J, Shi Y, Liu Y, Yang Y, He J, Luo S, Huang Y, Liu D, Li Y, Zhang S, Huang RP, Wang D, Xu J. Comprehensive Profiling of Inflammatory Factors Revealed That Growth Differentiation Factor-15 Is an Indicator of Disease Severity in COVID-19 Patients. Front Immunol. 2021;12:662465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Kanberg N, Simrén J, Edén A, Andersson LM, Nilsson S, Ashton NJ, Sundvall PD, Nellgård B, Blennow K, Zetterberg H, Gisslén M. Neurochemical signs of astrocytic and neuronal injury in acute COVID-19 normalizes during long-term follow-up. EbioMedicine. 2021;70:103512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 28. | Ebihara T, Matsumoto H, Matsubara T, Togami Y, Nakao S, Matsuura H, Kojima T, Sugihara F, Okuzaki D, Hirata H, Yamamura H, Ogura H. Cytokine Elevation in Severe COVID-19 From Longitudinal Proteomics Analysis: Comparison With Sepsis. Front Immunol. 2021;12:798338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 29. | Alserawan L, Peñacoba P, Orozco Echevarría SE, Castillo D, Ortiz E, Martínez-Martínez L, Moga Naranjo E, Domingo P, Castellví I, Juárez C, Mariscal A. Growth Differentiation Factor 15 (GDF-15): A Novel Biomarker Associated with Poorer Respiratory Function in COVID-19. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Wallentin L, Lindbäck J, Eriksson N, Hijazi Z, Eikelboom JW, Ezekowitz MD, Granger CB, Lopes RD, Yusuf S, Oldgren J, Siegbahn A. Angiotensin-converting enzyme 2 (ACE2) levels in relation to risk factors for COVID-19 in two large cohorts of patients with atrial fibrillation. Eur Heart J. 2020;41:4037-4046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 31. | Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front Immunol. 2020;11:1708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 768] [Cited by in RCA: 726] [Article Influence: 145.2] [Reference Citation Analysis (0)] |
| 32. | Al-Mudares F, Reddick S, Ren J, Venkatesh A, Zhao C, Lingappan K. Role of Growth Differentiation Factor 15 in Lung Disease and Senescence: Potential Role Across the Lifespan. Front Med (Lausanne). 2020;7:594137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Cavezzi A, Troiani E, Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10:1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 282] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 34. | Gisby J, Clarke CL, Medjeral-Thomas N, Malik TH, Papadaki A, Mortimer PM, Buang NB, Lewis S, Pereira M, Toulza F, Fagnano E, Mawhin MA, Dutton EE, Tapeng L, Richard AC, Kirk PD, Behmoaras J, Sandhu E, McAdoo SP, Prendecki MF, Pickering MC, Botto M, Willicombe M, Thomas DC, Peters JE. Longitudinal proteomic profiling of dialysis patients with COVID-19 reveals markers of severity and predictors of death. Elife. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 35. | Eddy AC, Trask AJ. Growth differentiation factor-15 and its role in diabetes and cardiovascular disease. Cytokine Growth Factor Rev. 2021;57:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 36. | Jiang F, Yu WJ, Wang XH, Tang YT, Guo L, Jiao XY. Regulation of hepcidin through GDF-15 in cancer-related anemia. Clin Chim Acta. 2014;428:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Dalamaga M, Karampela I, Mantzoros CS. Commentary: Could iron chelators prove to be useful as an adjunct to COVID-19 Treatment Regimens? Metabolism. 2020;108:154260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 38. | Poonkuzhi Naseef P, Elayadeth-Meethal M, Mohammed Salim KT, Anjana A, Muhas C, Abdul Vajid K, Saheer Kuruniyan M. Therapeutic potential of induced iron depletion using iron chelators in Covid-19. Saudi J Biol Sci. 2022;29:1947-1956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Ouyang J, Isnard S, Lin J, Fombuena B, Peng X, Chen Y, Routy JP. GDF-15 as a Weight Watcher for Diabetic and Non-Diabetic People Treated With Metformin. Front Endocrinol (Lausanne). 2020;11:581839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Apolzan JW, Venditti EM, Edelstein SL, Knowler WC, Dabelea D, Boyko EJ, Pi-Sunyer X, Kalyani RR, Franks PW, Srikanthan P, Gadde KM; Diabetes Prevention Program Research Group. Long-Term Weight Loss With Metformin or Lifestyle Intervention in the Diabetes Prevention Program Outcomes Study. Ann Intern Med. 2019;170:682-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 41. | Kaneto H, Kimura T, Obata A, Shimoda M, Kaku K. Multifaceted Mechanisms of Action of Metformin Which Have Been Unraveled One after Another in the Long History. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 42. | Patel S, Alvarez-Guaita A, Melvin A, Rimmington D, Dattilo A, Miedzybrodzka EL, Cimino I, Maurin AC, Roberts GP, Meek CL, Virtue S, Sparks LM, Parsons SA, Redman LM, Bray GA, Liou AP, Woods RM, Parry SA, Jeppesen PB, Kolnes AJ, Harding HP, Ron D, Vidal-Puig A, Reimann F, Gribble FM, Hulston CJ, Farooqi IS, Fafournoux P, Smith SR, Jensen J, Breen D, Wu Z, Zhang BB, Coll AP, Savage DB, O’Rahilly S. GDF15 Provides an Endocrine Signal of Nutritional Stress in Mice and Humans. Cell Metab. 2019;29:707-718.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 336] [Article Influence: 56.0] [Reference Citation Analysis (0)] |