Published online Sep 20, 2022. doi: 10.5662/wjm.v12.i5.331
Peer-review started: December 5, 2021
First decision: January 25, 2022
Revised: March 17, 2022
Accepted: July 19, 2022
Article in press: July 19, 2022
Published online: September 20, 2022
Processing time: 284 Days and 12.7 Hours
Since the discovery of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its resultant coronavirus disease 2019 (COVID-19) pandemic, respiratory manifestations have been the mainstay of clinical diagnosis, laboratory evaluations, and radiological investigations. As time passed, other pathological aspects of SARS-CoV-2 have been revealed. Various hemostatic abnormalities have been reported since the rise of the pandemic, which was sometimes super
Core Tip: The pathogenesis of hypercoagulable state and thrombosis related to coronavirus disease 2019 (COVID-19) is unclear. Evidence on endothelial cell injury by direct infection of severe acute respiratory syndrome coronavirus 2 is increasing. Histologic and immunohistochemistry examination of lung autopsies and/or the skin of patients who have died of severe COVID-19 has shown microvascular injury and thrombosis, consistent with intensive and generalized activation of both alternative and lectin-based pathways of complement.
- Citation: Wifi MN, Morad MA, El Sheemy R, Abdeen N, Afify S, Abdalgaber M, Abdellatef A, Zaghloul M, Alboraie M, El-Kassas M. Hemostatic system and COVID-19 crosstalk: A review of the available evidence. World J Methodol 2022; 12(5): 331-349
- URL: https://www.wjgnet.com/2222-0682/full/v12/i5/331.htm
- DOI: https://dx.doi.org/10.5662/wjm.v12.i5.331
One of the frequently encountered complications of systemic infections is activation of the coagulation cascade, which can present with a broad spectrum of clinical manifestations varying from subclinical activation, which is expressed by elevated laboratory markers for thrombin and fibrin products, to disseminated intravascular coagulation (DIC) and resultant formation of microvascular thrombi in various body tissues and organs[1]. Inflammation affects all phases of blood coagulation, which in turn, leads to both thrombotic as well as hemorrhagic complications[2]. Various viral infections, such as the human immunodeficiency virus, Dengue virus, and Ebola virus, occur by activation of the coagulation cascade[3-5]. Either direct or indirect activation of endothelial cells by viral infection can affect the balance between the coagulation and fibrinolytic systems[6,7]. The clinical presentation of this altered coagulation appears in hemorrhage, thrombosis, or both. An exaggerated response may even lead to DIC with the formation of microvascular thrombi in various organs[8]. Tissue factor (TF) expression is increased in herpes simplex virus and Dengue virus-infected endothelial cells[9].
The Ebola virus induces TF expression in circulating blood cells, especially macrophages, a condition known as Ebola hemorrhagic fever[4,9]. Stimulation by the poly I:C toll-like receptor 3 (TLR3) agonist induces activation of many proinflammatory cytokines as an antiviral chemokine, which is a selective chemoattractant for both activated type 1 T lymphocytes and natural killer cells. Thus, poly I:C increases TF expression in cultured endothelial cells and activates the coagulation system in mice [4]. On the other hand, inhibition of the TF/factor VIIa (FVIIa) complex was shown to decrease the cytokine storm and mortality in a rhesus monkey model of Ebola hemorrhagic fever[10]. Other hematological disorders that frequently occur with viral infections are hemolytic uremic syndrome, idiopathic thrombocytopenic purpura, and thrombotic thrombocytopenic purpura[7]. However, it is not clear why some viruses cause hemorrhage while others are associated with thrombosis as cytomegalovirus or both complications such as varicella-zoster virus[10,11].
Viral respiratory tract infections carry a higher risk for deep venous thrombosis and possibly pulmonary embolism (PE)[12]. Influenza A virus is associated with DIC and 18 pulmonary microembolism[13,14]. In the influenza A virus subtype H1N1, both thrombotic and hemorrhagic complications have been reported such as deep vein thrombosis (DVT), PE, and pulmonary hemorrhage with hemoptysis, hematemesis, petechial rash, and one case of disseminated petechial brain hemorrhage[15]. Another example of viral infection associated with coagulopathy is H5N1, the highly pathogenic avian influenza that results in DIC, pulmonary hemorrhage, and thrombocytopenia in many cases[16]. The outbreak of severe acute respiratory syndrome (SARS) has been associated with significant morbidity and mortality caused by a broad spectrum of clinical presentation, e.g., DIC, deep venous thrombosis, and pulmonary thromboembolic disasters resulting in pulmonary infarction, due to activated coagulation and vascular endothelial damage in both small and mid-sized pulmonary vessels[17].
Due to the ambiguity of the pathogenesis of the hypercoagulable state related to coronavirus disease 2019 (COVID-19) and the evidence of endothelial cell injury by direct infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, histologic and immunohistochemistry examination of lung autopsies and/or skin of patients who died of severe COVID-19 showed microvascular injury and thrombosis. This review discusses the evidence of coagulation disorders, management strategies, outcome, and prognosis associated with COVID-19 coagulopathy to guard against possible thromboembolic events.
SARS-CoV or SARS-CoV-1 emerged in China in 2003 and spread to another 26 countries and is associated with thrombotic complications and hematologic disorders. Histopathological examination of pulmonary vasculature has revealed fibrin thrombi in pulmonary, bronchial, and small lung veins. Many studies of postmortem autopsies identified PE, DVT, and widespread multi-organ infarcts due to thrombi associated with polyangiitis and microcirculation disturbance as ischemic stroke (IS). SARS-CoV-1 causes placental circulation dysfunction through fibrin deposition, avascular and fibrotic villi formation, and prothrombotic tendency resulting in many intrauterine fetal complications such as oligohydramnios, intrauterine growth delay, and small fetal size[18,19]. Laboratory parameters of SARS-CoV-1-infected patients show prolonged prothrombin time (PT), prolonged activated partial thromboplastin time (especially over the first 2 wk), elevated D-dimer, and worsening thrombocytopenia. Increased thrombopoietin level has been reported in SARS-CoV-1 patients in the convalescent phase compared to normal controls with a concomitant increase in platelet count. Anticardiolipin antibodies have been detected in patients with post-SARS osteonecrosis and those with positive lupus anticoagulant tests in children[20,21]. In vitro studies have revealed that some genes have procoagulant effects when expressed in SARS-CoV-1-infected mononuclear cells. TLR9 and thromboxane A synthase genes are the targets of the SARS-CoV-1, where the TLR9 receptor is expressed in platelets to increase platelet activation, degranulation, and aggregation while increased thromboxane production promotes vasoconstriction, platelet aggregation, and endothelial dysfunction[22-24]. Upregulation of the five genes is associated with changes in the coagulation pathway in human hepatoma cells. These genes are: (1) The TF pathway inhibitor 2, which usually inactivates the tissue factor-VIIa complex and thrombin generation, and upon upregulation, it counteracts the mechanism that inhibits overt coagulation cascade activation in response to inflammation; (2) Early growth response 1; (3) Plasminogen activator inhibitor 1, which causes inhibition of fibrinolysis and promotes fibrin deposition during inflammatory states; (4) Phospholipid scramblase 1; and (5) Thrombospondin 1[25-27]. Urokinase pathway dysregulation is involved in the pathogenesis of SARS-CoV-1-related coagulation disorders leading to fatal disease in mice. The nucleocapsid protein of SARS-CoV-1 is one of the determinants of the prothrombotic state caused by SARS-CoV-1 as it induces the human fibrinogen-like protein-2 prothrombinase gene with activation of the C/EBP-α transcription factor[28-31]. The Middle East respiratory syndrome (MERS-CoV) that occurred in Saudi Arabia in 2012 is also associated with thrombotic complications and hematologic manifestations. Histopathologic examination of MERS-CoV-infected patients revealed microthrombi on day 4 of infection in the pulmonary vessels associated with parenchymal consolidation, alveolar edema, and cellular infiltrates. Thrombocytopenia was identified in the 1st week of laboratory-confirmed MERS-CoV cases with relatively lower platelet count in MERS-CoV patients than negative controls. DIC was one of the major complications reported in fatal MERS-CoV infections[32-34].
The pathogenesis of hypercoagulable state and thrombosis related to COVID-19 is unclear. Evidence on endothelial cell injury by direct infection of the SARS-CoV-2 virus is increasing. Histologic and immunohistochemistry examination of lung autopsies and/or skin of patients who died of severe COVID-19 showed microvascular injury and thrombosis, consistent with intensive and generalized activation of both alternative and lectin-based pathways of complement[35]. Subsequent activation of the clotting pathway, causing fibrin deposition, might also be implicated[36]. The hypercoagulable state due to profound derangement of hemostasis is another contributor to venous thromboembolism (VTE), PE, and/or DVT of the lower limbs, which has been observed in patients with COVID-19. There is controversy about the pattern of hypercoagulability associated with COVID-19. Viral, bacterial, or fungal infection elicits a complex systemic inflammatory response as a part of innate immunity. Activation of the host defense mechanism induces subsequent coagulation and thrombin formation as a critical interaction between humoral and cellular mechanisms, a term called thromboinflammation or immunothrombosis[37]. Severe inflammation in patients with COVID-19, proved by elevated levels of interleukin 6 (IL-6), increased erythrocyte sedimentation rate, increased C-reactive protein (CRP), and elevated fibrinogen at presentation[38], results in subsequent activation of coagulation and may cause elevation of D-dimer levels[39]. Some experts have postulated that the predominant hypercoagulability in patients with COVID-19 suggests a unique hypercoagulable multifactorial state termed thromboinflammation or COVID-19-associated coagulopathy (CAC), which seems to be inconsistent with DIC, even though DIC has been reported in severely ill patients[40,41]. Other potential pathogenesis for coagulation abnormalities in patients with COVID-19 includes antiphospholipid antibodies, anticardiolipin and anti–β2-glycoprotein I immunoglobulin G (IgG) and IgA[42]. Another explanation for coagulation abnormalities in the presence of lupus anticoagulant has been observed in a high percentage (88%-91%) of COVID-19 patients[43,44].
Although COVID-19 pathogenesis is associated with pulmonary intravascular coagulopathy (PIC) and thrombosis, it differs from sepsis-associated DIC. The first explanation of the pathogenesis of PIC and thrombosis in COVID-19 was directed to binding of SARS-CoV-2 to angiotensin converting enzyme-2 receptors that are located on type II pneumocytes (and possibly on vascular endothelial cells). This binding results in lysis of the cells immediately causing activation of the endothelium and procoagulant activity with the activation of fibrin deposits and accumulation in pulmonary microcapillary venous vessels, finally ending in PIC and thrombosis[45]. The second opinion is that the immune-mediated mechanism results in marked microvascular thrombosis and hemorrhage linked to extensive alveolar and interstitial inflammation, sharing features with macrophage activation syndrome in terms of lung-restricted vascular immunopathology associated with COVID-19[46].
In this context, infection with COVID-19 presumably induces a process of immune system hyperactivation known as immunothrombosis, in which activated neutrophils and monocytes interact with platelets and the coagulation cascade, leading to intravascular clot formation in small and larger vessels. It is presumed that the exaggerated immunothrombosis that occurs within lung microvessels is the main driver of COVID-19 manifestations[47,48].
Endothelial dysfunction is thought to be the most striking pathophysiological event in COVID-19 that infects vascular endothelial cells leading to cellular damage and apoptosis, decreasing the antithrombotic activity of the normal endothelium[49-51].
Similar to other respiratory infections, leukocyte recruitment to the lungs, a higher percentage of macrophages and neutrophils together with higher levels of proinflammatory cytokines (e.g., IL-6, IL-8, and IL-1β) and chemokines (e.g., CCL2, CCL3, CCL4, and CCL7) found in the bronchoalveolar fluid are major contributors to inflammatory responses in COVID-19 infection[52].
Until recently, the association between COVID-19 and VTE, including PE and DVT, has been published as case reports. The prevalence of VTE in COVID-19 patients appears to be higher than that reported for patients admitted to intensive care units (ICUs) for other disease conditions[53]. Diagnosis of VTE was 12.7% in COVID-19 patients, as shown in a meta-analysis of multiple studies including 1783 ICU patients[54]. Patients with COVID-19 had some laboratory abnormalities, including a marked increase in D-dimer and, in some cases, mild thrombocytopenia, similar to DIC. However, other coagulation parameters in COVID-19, including high fibrinogen and high factor VIII activity, suggest that coagulation factors' consumption is not evident are inconsistent with DIC. Studies based on biochemical markers such as a marked increase of fibrin degradation products (FDP) (e.g., D-dimer), prolonged PT/activated partial thromboplastin time (aPTT), and low platelet counts were compatible with the state of DIC. However, prolonged PT/aPTT is not confirmed in some studies[55]. Other studies using thromboelastography (TEG), a method of testing the efficiency of blood coagulation, together with biochemical parameters, demonstrated that results observed in patients with COVID-19 are not compatible with DIC[55]. In this context, careful monitoring of PT, platelet count, and D-dimer concentrations may help predict the clinical improvement and the expected complications.
Despite the plethora of publications regarding SARS-CoV-2, there are no available solid epidemiologic data on the actual prevalence and incidence of hemostatic derangements among patients. Most available data to date are retrospective observational data and can be classified as case series from a single-center experience that cannot be considered a true reflection of the prevalence and incidence of hemostatic derangements associated with SARS-CoV-2. However, there is some light at the end of the tunnel as the World Health Organization registry has several ongoing prospective studies aimed towards accurately determining the incidence. For example, a French study located in Centre Hospitalier Universitaire de Nice, started February 28, 2020[56], aims to screen prospectively any cardiovascular complication in COVID-19 patients including PE, DVT, and VTE. Another study initiated in Shandong Provincial Hospital[57], where patients are recruited with novel coronavirus pneumonia (NCP), aims to calculate the rates of venous thrombosis among those patients and determine the risk factors. The Centre Hospitalier Universitaire de Nīmes registered in April 2020 is conducting a more dedicated study[58] to analyze coagulopathy. They observed any abnormality resulting from sepsis, including coagulopathy and DIC, and excluded all factors that would alter or influence outcomes such as pregnancy and lactation, anticoagulants, or antiplatelet therapy before recruitment or those with hypercoagulable states. In a study on 81 ICU hospitalized patients with NCP in Wuhan, 25% (20/81) had VTE with a significant increase in their D-dimer levels[59]. Dutch published data from three hospitals (184 patients) found that the cumulative incidence of thrombotic complications was 31%, most commonly PE (in 25 patients), VTE in 27%, and arterial thrombosis in 2.7% of all thrombotic events, despite receiving standard thromboprophylaxis[60]. In Italy, 22.2% of 54 ICU-admitted patients developed VTE despite prophylactic low molecular weight heparin (LMWH)[61].
Thrombocytopenia is one of the earliest observations in COVID-19 patients. A meta-analysis of nine studies suggested that thrombocytopenia was significantly associated with the severity of COVID-19, with more platelets found in non-survivors. Alhazzani et al[62] presented the data of 1099 patients from 522 hospitals and found that 36.2% of those patients had thrombocytopenia, which was even more evident in more severe cases (57.7%) vs 31.6% in non-severe cases[62]. However, in another case study performed on 150 COVID-19 patients in ICU, PE was reported in 43% of cases, besides extracorporeal circuit clotting, which was detected in 28 of 29 patients on renal dialysis. This research compared a group of patients with COVID-19 related acute respiratory distress syndrome (ARDS) vs non-COVID-19 ones and demonstrated a higher incidence of thrombotic events among COVID-19 patients[43]. In another series of 107 ICU-admitted COVID-19 cases, PE was found in 22% of cases despite receiving prophylactic anticoagulation[61]. VTE was noted in 39% of COVID-19 ICU cases in a case series composed of 74 patients, yet it was demonstrated in 25% of severe COVID-19 pneumonia patients in an earlier case series done on a cohort of 81 patients[59,63].
In a screening study done on 26 COVID-19 severely infected patients using Doppler lower limb ultrasound, VTE was detected in around 69% of patients; besides, bilateral DVT was demonstrated in 38% of cases though they were all on prophylactic anticoagulation therapy[64].
One of the earliest alarming laboratory findings observed in COVID-19 patients requiring hospitalization was marked elevation of the D-dimer. Elevated D-dimer levels are correlated with disease intensity and with high levels of proinflammatory cytokines, suggesting a possible relation between hypercoagulability and inflammation[65].
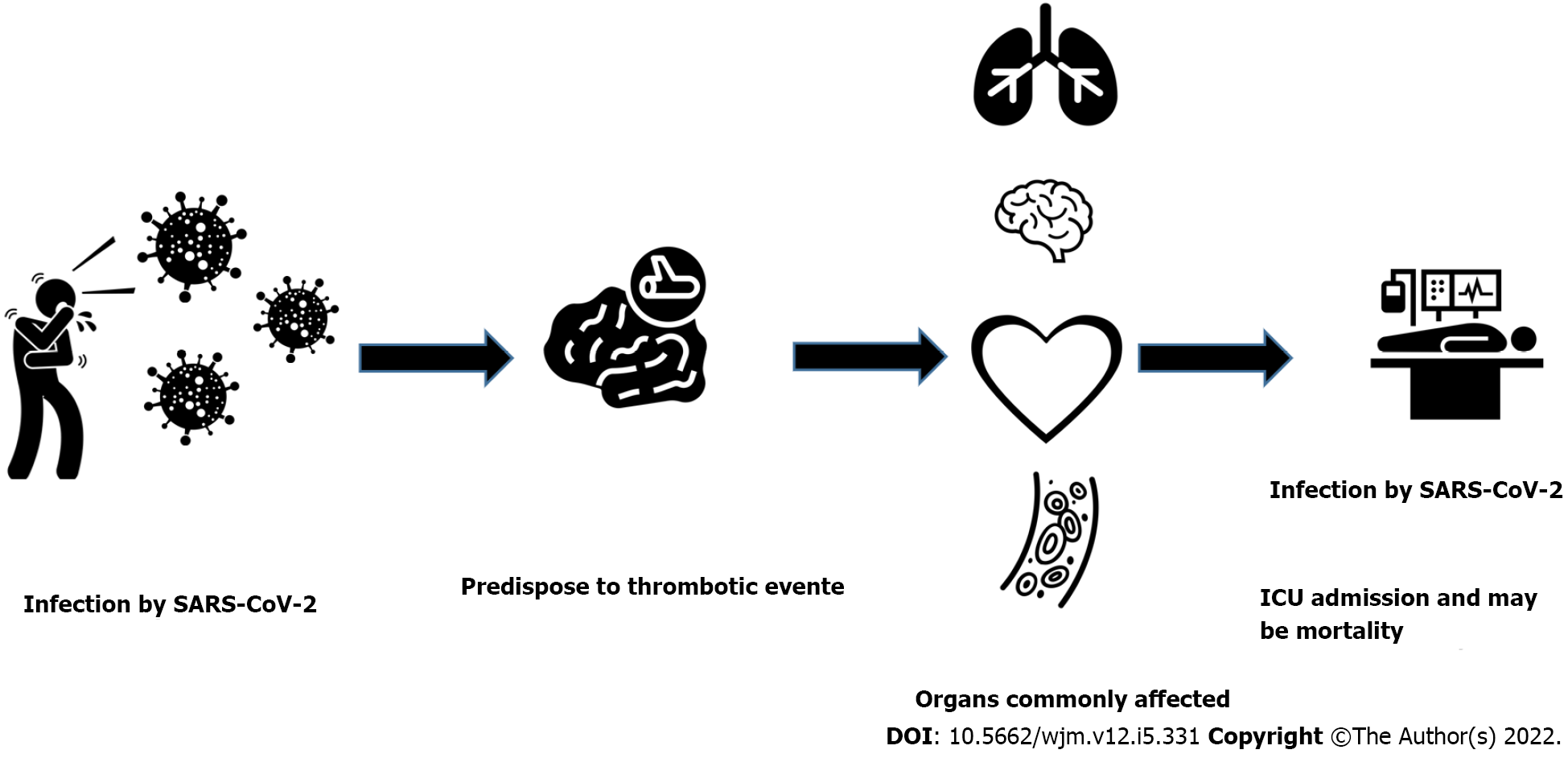
Different arterial thrombotic events have also been described in COVID-19 patients, and at the top of the list are ischemic central nervous system events. In a study performed in New York, 5 COVID-19 patients demonstrated large vessel occlusion and IS, astonishingly all these patients were young (under 50 years)[66]. Moreover, IS was noticed in 3.7% of patients in another case series composed of 184 COVID-19 patients[60]. Acute limb ischemia is the second most common arterial thrombotic event observed in COVID-19 patients. A recent study demonstrated acute lower limb arterial thrombosis in 20 COVID-19 patients; most were men with an average age above 75 years[67]. Another study reported acute lower limb ischemia in 4 patients, but they were young and did not suffer comorbidities[68]. Myocardial infarction was also described in COVID-19 patients and was reported in 2 Chinese studies[69,70]. Figure 1 demonstrates the hemostatic system and COVID-19 interplay, possible complications, organs affected and outcomes.
COVID-19 patients may have many hemostatic abnormalities (which may result in a hypercoagulable state as illustrated in Table 1[71-74]), so appropriate evaluation is mandatory for the correct diagnosis and management of COVID-19-associated thrombosis. Thromboinflammation or CAC is the predominant coagulation abnormality in COVID-19 patients, which will lead to a hypercoagulable state; it seems to be distinct from DIC, although DIC has been reported in severely affected patients[75]. A unique coagulopathy and procoagulant endothelial phenotype associated with a proinflammatory state with COVID-19 infection have a prominent effect on elevation of fibrinogen and D-dimer/fibrin(ogen) degradation products, which in turn results in systemic hypercoagulation and frequent venous thromboembolic events[76].
| Hematological parameter | Significant relation to COVID-19 | Mechanism |
| High RDW (greater than 14.5%) | Increase in mortality risk (from 11% to 31%)[86] | Not completely understood however reports suggested elevated RDW was attributed to affection of RBC production kinetics[86] |
| Leucopenia or lymphopenia (ALC < 1.0 × 109/L) | Observed in most of COVID cases especially hospitalized patients and associated with poor prognosis[86] | (1) Defective immune response; and (2) Drug induced as with steroids[87] |
| Normal or increased platelet count | Found in some cases of COVID-19 | May be caused by to the large amounts of platelets produced in response to increased thrombopoietin formation from liver stimulation and megakaryocytes in the lung[88] |
| Prolonged PT and aPTT, elevations of D dimer, fibrinogen and FDP and decreased levels of antithrombin III | Direct relationship was observed between severity of COVID and affection of coagulation profile, Overt DIC (ISTH score of 5 and higher) is seen more frequently in non-survivors[89] | aPTT prolongation is caused by increased Factor VIII level and Factor XII deficiency secondary to the presence of factor XII inhibitors. Von Willebrand factor is quantitatively increased. LA is positive in 91% of those with prolonged aPTT. The presence of both LA and Factor XII deficiency are not associated with bleeding tendency |
It is well known that the high level of D-dimer in COVID-19 is triggered by excessive clots and hypoxemia, which is likely reflecting pulmonary vascular bed thrombosis and fibrinolysis and correlates significantly with mortality. In many retrospective studies conducted in COVID-19 pneumonia patients, elevated baseline D-dimer levels are observed with inflammation. However, they cannot be accurately correlated with VTE score, which could help determine whether this is possible anticoagulation is needed or not based on levels of D-dimer[76,77].
The most common hemostatic abnormalities with COVID-19 include mild thrombocytopenia[78]; as reported in the literature, the incidence of thrombocytopenia ranges between 5%–41.7% of COVID-19 infected patients, and it varies according to the disease severity. Moreover, rebound thrombocytosis was also reported in some cases[79,80]. Several mechanisms of COVID-19-associated thrombocytopenia have been reported, such as direct viral-platelet interaction activation, platelet autoantibody formation, subsequent platelet clearance, splenic/hepatic sequestration, and/or marrow/megakaryocyte suppression owing to inflammatory response, direct viral infection, or reduced thrombopoietin level[81].
One study suggested that patients with COVID-19 have higher platelet counts than patients with other coronavirus infections[82] and elevation of D-dimer[83], which were related to increased risk of requiring mechanical ventilation, and death[65]. However, high D-dimer levels are common in acutely ill individuals with various infectious and inflammatory diseases. Disease severity is variably related to PT prolongation[84], thrombin time[85] and shortened aPTT[86]. The retrospective analysis of 99 COVID-19 patients conducted by Wuhan Jinyintan Hospital showed that 36% of patients had elevated D-dimer, 16% showed a reduced aPTT, 6% showed an extended aPTT, 30% showed a shortened PT, and 30% showed an extended PT[87]. In a large meta-analysis of 7613 COVID-19 patients, it was found that in severe infection and non-survivors, the platelet count was lower, indicating that platelet counts may be a predictor of COVID-19 mortality[88,89]. COVID-19-associated thrombocytopenia primarily affects clot formation kinetics and clot strength on Quantra viscoelastic analysis; however, the details of in vivo fibrinolysis in COVID-19 have not yet been thoroughly investigated[89].
A retrospective analysis of the routine coagulation parameters of 183 patients with COVID-19 revealed that plasma FDP and D-dimer in non-survivors were significantly above those in survivors; PT and aPTT were also significantly prolonged[39]. A retrospective analysis of 138 COVID-19 patients confirmed that D-dimers increased after admission[90]. Previous studies have shown that elevated D-dimer is an independent risk factor for ARDS and mortality in COVID-19 patients[91].
COVID-19 infection has a significantly elevated vWF level together with increased FVIII clotting activity; this likely reflects the combined effect of the more significant release of Weibel-Palade bodies from endothelial cells and the acute-phase reaction. Meanwhile, ADAMTS13 activity was found to be mildly to moderately reduced in COVID-19 patients[75,92,93]. Fibrinogen level is increased to 5.0–7.0 g/dL on average for COVID-19-infected patients, CRP is also increased as an acute-phase reactant associated with elevated IL-6[94,95]. Meanwhile, antithrombin is consumed during coagulation, and the mild antithrombin deficiency occurs in COVID-19 infection whereas protein C has not been decreased in any of the patients assessed[96]. Mildly prolonged aPTT clotting times have been reported in some COVID-19 patients, indicating a prothrombotic state[96].
In a series of 24 intubated patients with severe COVID-19 pneumonia, PT and aPTT were either normal or slightly prolonged, platelet counts were normal or increased (mean, 348000/mL), fibrinogen increased (mean, 680 mg/dL; range 234 to 1344), D-dimer increased (mean, 4877 ng/mL; range, 1197 to 16954), factor VIII activity increased (mean, 297 units/dL), vWF antigen significantly increased (mean, 529; range 210 to 863), per endothelial injury. A slight decline in antithrombin and free protein S, with a slight increase in protein C, were also reported. Regarding TEG, there was shortening in reaction time (R) in 50% of patients and in clot formation time (K) in 83% of patients. There was an increase in maximum amplitude in 83% of patients, and also a reduction in clot lysis (LY30) in 100% of patients. Other studies have reported similar hypercoagulable states, including very high D-dimer, vWF antigen and activity, and factor VIII activity[43,97]. Two studies showed a high rate of lupus anticoagulant in patients with prolonged aPTT [50 of 57 tested individuals (88%) and 31 of 34 tested individuals (91%)][42]. Another study reported 3 cases with severe COVID-19 and cerebral infarction, one with bilateral limb ischemia, within the setting of elevated antiphospholipid antibodies. Whether antiphospholipid antibodies play a significant role in the pathophysiology of thrombosis related to COVID-19 requires further investigation[41]. DIC manifests as coagulation failure and an intermediate phase within the development of multiple organ failure, which is common in many critically ill patients[98]. Tang et al[25] recently assessed 183 patients with COVID-19, of whom 21 (11.5%) died. The primary common differences between those who died and survivors were the increased levels of D-dimer and FDPs (an approximate 3.5- and approximately 1.9-fold increase, respectively) and PT prolongation (by 14%, P < 0.001), 71% of these patients who died fulfilled the International Society on Thrombosis and Hemostasis (ISTH) criteria for DIC compared with only 0.6% among survivors[40,99]. The COVID-19-related hypercoagulable state has been described as a DIC-like state, especially because many affected individuals are acutely ill and meet the criteria for probable DIC in the ISTH scoring system[99]. However, the main clinical finding in COVID-19 is thrombosis, whereas the main finding in acute decompensated DIC is bleeding. COVID-19 has similar laboratory findings of DIC, including elevated D-dimer and thrombocytopenia in some patients. However, in COVID-19, there is high fibrinogen and high factor VIII activity which are not found in DIC[40,99]. According to the recommendations from ISTH, the American Society of Hematology (ASH), and also the American College of Cardiology, routine testing for inpatients should include complete blood count, coagulation studies (PT and aPTT), fibrinogen, and D-dimer, and it will be repeated according to the clinical situation[100]. According to the American Society of Hematology recommendations regarding the diagnosis of PE, a normal D-dimer is sufficient to exclude the diagnosis of PE. In patients with suspected PE because of unexplained hypotension, tachycardia, worsening respiratory status, or other risk factors for thrombosis, computed tomography with pulmonary angiography (CTPA) is the preferred test. Ventilation/perfusion (V/Q) scan is an alternative if CTPA cannot be performed or is inconclusive, although the V/Q scan is also unhelpful in individuals with significant pulmonary involvement from COVID-19[101]. To date, whether these hemostatic changes are characteristic for SARS-CoV-2 or are an element of cytokine storm, as observed in other viral diseases, is unknown[102,103].
Regarding COVID-19 induced coagulopathy, we conclude that it meets the criteria of sepsis-induced coagulopathy (SIC), defined as a reduced platelet count, increased INR, and higher organ dysfunction score[40,104]. Table 2 shows the various laboratory parameters altered in SARS-CoV-2 and their implications in COVID-19 severity[105,106].
| Clinical index | Alterations with COVID-19 severity | Ref. |
| Neutrophil-to-lymphocyte ratio | Increased | [84,122-124,131,134-136] |
| CRP | Increased | [122,124,125,128,129,131,134-136,137-144] |
| Platelets | Decreased | [78,83,122,126,129,131-133,136,145,146] |
| Lymphocytes | Decreased | [78,128,129,131,134-136,147,148] |
| D-dimer | Increased | [55,83,84,127,128,131,137,144-146,149-152] |
| Ferritin | Increased | [91,94,128,129,131,134,135,137-139,144,153-155] |
| Procalcitonin | Increased | [83,84,128,144,156-158] |
| Lactate dehydrogenase | Increased | [106,129-131,152,159-173] |
| Albumin | Decreased | [111,116,128,129,136,148,174-186] |
The cumulative incidence of COVID-19-associated VTE risk has raised concerns. Table 3 shows the frequency of venous thromboembolic complications in COVID-19 patients in different studies[64,107,108].
| Ref. | Proportion | Cumulative incidence | Median follow-up | Patients |
| Cui et al[59] | 20/81 (25%) | NR | NR | ICU patients |
| Klok et al[60] | 68/184 (37%) | 57% or 49% adjusted for competing risk of death | 14 d | ICU patients only.19 PE were limited to subsegmental arteries.65/68 venous events were PE (95.6%) |
| Poissy et al[61] | VTE 22.2% of 54 ICU admitted | |||
| Helms et al[44] | 27/150 (18%) | NR | NR | ICU patients with ARDS 25/27 events were PE (92.5%) |
| Poissy et al[61] | PE only 22/107 (20.6%) | 20.4% calculated at ICU day 15 | 6 d | ICU only |
| Middeldorp et al[63] | Venous thromboembolism 39% of COVID-19 ICU cases 74 patients | |||
| Llitjos et al[64] | DVT: 18/26 (69%); PE: 6/26 (23%) | NR | NR | ICU patients. Systematic ultrasound screening |
| Léonard-Lorant et al[183] | PE only 32/106 (30%) | NR | NR | 24/32 (75%) PE-positive patients were in the ICU |
| Grillet et al[184] | PE only 23/100 (23%) | NR | NR | Ward: 6/61 (9.8%); ICU: 17/39 (43.6%) |
| Middeldorp et al[63] | 33/198 (17%) | 15% at 7 d; 34% at 14 d | 5 d | Ward: 4/123 (3.3%); ICU: 35/75 (47%); 11 (5.4%) clots detected on screening 11/33 events were PE (33%) |
| Lodigiani et al[185] | 16/362 (4.4%) | 21% (time not reported) | 10 d | ICU 4/48(8.3%); Ward 12/314 (3.8%) |
| Thomas et al[186] | 6/63 (9%) | 27% | 8 d | ICU patients |
| Cattaneo et al[108] | DVT only 0/388 (0%) | NR | NR | Non-ICU Ward 64 patients had screening ultrasound. All negative |
Many international societies and ministries of health have to publish their interim guidance to overcome this challenging situation[49,65,109-111]
Although the general adoption of many societies[112] of the interim guidance of the ISTH[110], some institutions may vary in their management strategy of thromboembolic complications and would encourage enrollment in clinical trials to determine the best approach[113,114]. The ISTH recommends that all inpatients (ICU, medical non-ICU, and perioperative surgical and obstetric patients with COVID-19) receive prophylactic anticoagulation unless contraindicated after careful stratification with a DIC score. The low prophylactic dose molecular weight (LMW) heparin is preferred [e.g., enoxaparin in a dose of 40 mg to 60 mg once daily for patients with creatinine clearance (CrCl) > 30 mL/min, and 30 mg once daily for patients with CrCl 15 to 30 mL/min]. Dalteparin, nadroparin, and tinzaparin are also recommended. In a retrospective study of 449 patients with severe COVID-19, 99 patients who received enoxaparin in prophylactic doses showed a better prognosis concerning mortality, especially those with high SIC score and markedly elevated D-dimer[115]. Moreover, LMWH could have anti-inflammatory properties that would help in COVID-19 patients where proinflammatory cytokines are markedly elevated[116]. The high incidence (43%) of VTE reported in a multicenter prospective study of ICU patients, mainly PE, despite being on a regular prophylactic dose of LMWH[43], prompted many experts to suggest higher doses and call for more aggressive anticoagulation with intermediate-dose or even therapeutic dose anticoagulation for thromboprophylaxis. For patients with CrCl < 15 mL/min or renal replacement therapy, unfractionated heparin can be used. Doses should be modified according to weight and pregnancy conditions. Full-dose anticoagulation is indicated in those with documented VTE like DVT or PE in the same way as those without COVID-19 infection.
Not all patients have access to confirmatory tests for VTE in real life. The empirical initiation on full-dose anticoagulation can be justified by the local consultation of expertise in hemostasis and thrombosis and clinical evaluation of individual patients. Sudden respiratory status deterioration in a previously stable intubated patient not explained by a cardiac cause indicates a high suspicion of PE. Moreover, those with highly elevated fibrinogen and/or D-dimer and otherwise unexplained respiratory failure, superficial thrombophlebitis, retiform purpura, recurrent clotting of arterial lines, or central venous catheters despite prophylactic anticoagulation are highly indicated for full-dose anticoagulation. The dose dilemma for critically ill ICU COVID-19 patients is still not resolved. Whether the regular prophylactic, intermediate, or therapeutic dose would better treat disease morbidity and mortality needs future clinical trials to improve our practice. This strategy is supported by the American Society of Hema
In contrast, the patients with no evidence of VTE or other indication for therapeutic anticoagulation who require high-flow oxygen, CPAP, NIV for severe ventilatory failure, or invasive ventilation should receive less than therapeutic dosing[118]. Meanwhile, The Italian Society of Thrombosis and Hemostasis strongly recommends prophylactic anticoagulation with LMWH, UFH, or fondaparinux for the entire hospital stay for 7–14 d more after hospital discharge[119]. Furthermore, the American College of Chest Physicians and Global COVID-19 Thrombosis Collaborative Group recommends standard dose anticoagulation for inpatients with COVID-19 disease and ICU/Critical Care patients; meanwhile, SIGN and NICE NG-191 exerts intermediate-dose/ standard dose anticoagulation for those patients[120-122].
Much International and National guidance regarding VTE thromboprophylaxis has been published; however, more extensive studies are required to investigate the potential therapeutic approach. Most of the international guidelines and recommendations (ISTH-IG, ACF, CDC, and ASH) adopt stopping anticoagulation in patients who developed bleeding or severely thrombocytopenic; furthermore, they also do not recommend a particular platelet count threshold[123]. Furthermore, the expert panel reports by CHEST/AIPPD/AABIP stated that empiric use of therapeutic anticoagulation regimens in ICU patients with COVID-19 is not beneficial and may be harmful, while its use in hospitalized, noncritically ill patients with COVID-19 remains uncertain[123].
The catastrophic event of unopposed coagulopathy and DIC is a strong predictor of mortality in patients with COVID-19. On a laboratory basis, a significant elevation in D-dimer and INR with a decrease in fibrinogen level was also observed in non-survivors at days 10-14, and this was considered a poor prognostic sign[55]. For this reason, continuous and close monitoring of their levels is essential to determine prognosis and outcome, D-dimer level above 1 μg/mL was a strong and independent risk factor for death in this population[124]. In an observational study, a mean D-dimer level of 2.12 mg/L was observed in patients who did not survive compared to a concentration of 0.61 mg/L in survivors[55]. Another study revealed that patients admitted to ICU had significantly higher median D-dimer concentrations than patients who did not receive ICU care[84]. A third study reported that D-dimer on admission greater than 1 mg/L resulted in an 18-times increased risk of death[125]. These data provided strong evidence that D-dimer could be used as an excellent prognostic sign[125]. A retrospective study that included 449 patients admitted to the hospital with severe COVID-19 infection showed that the use of prophylactic heparin was associated with a lower mortality rate than in patients who did not receive prophylactic heparin[115]. The available data about coagulopathy in COVID-19 patients suggest that regular monitoring of PT, platelet count, and D-dimer concentrations could predict prognosis and expected complications. Accordingly, there is justifying evidence supporting using a prophylactic dose of LMWH to prevent VTE in critically ill COVID-19 patients[126].
Indeed SARS-CoV-2 is not as pathogenic as other RNA viruses (Ebola and hemorrhagic fever viruses) in causing severe hemorrhagic manifestations[127]. Owing to the abnormal coagulation cascade and subsequent high risk of thrombosis necessitating pharmacologic VTE prophylaxis, especially in severe COVID-19, the risk of bleeding with COVID-19 due to over anticoagulation, SIC, or DIC is inevitable. Although there are few reported data about clinically-overt bleeding in the setting of COVID-19, close observation for the occurrence of bleeding or thrombosis is mandatory for all COVID-19 patients who develop SIC or DIC[128]. In the absence of overt bleeding, the correction of coagulopathy is not mandatory in most COVID-19 patients. It is recommended to monitor full blood count, coagulation profile, and/or TEG and Rotational Thromboelastometry are all needed in cases of minor bleeding. However, in cases of significant bleeding as observed with a decrease in systolic blood pressure to less than 90 mmHg and/or increase of heart rate more than 110 beats per minute, management should be started immediately with FFP (15-25 mg/kg if PT/INR or aPTT ratios are greater than 1.5), platelet transfusion (for platelet count < 50 × 109/L), fibrinogen replacement (when fibrinogen level is < 1.5 g/L).
Additionally, prothrombin complex concentrate will be given if FFP transfusion is not feasible and/or tranexamic acid (in a dose of 1 g over 10 mi) followed by a further dose (of 1 gm) if bleeding persists or restarts in the following 24 h provided that the patient does not have any evidence of DIC and followed by repeated monitoring with coagulation screens[129]. In a unique observation from Thailand on 41 COVID-19 infected patients initially presented with bleeding and petechiae, no specific additional treatment for this hemorrhagic problem was needed, and fortunately, no deaths occurred. This study and other studies may be of great value to raise awareness about the hemorrhagic presentation associated with COVID-19. Therefore, investigation and follow-up for possible hemorrhagic problems induced by COVID-19 are highly recommended[130]. A retrospective study comparing the risk of thrombosis vs the risk of bleeding in COVID-19 patients showed that critically ill patients had an increased incidence of bleeding (26.7%). This was a complicated situation in the setting of VTE prophylaxis and could be explained by dysregulated hemostasis in severe COVID-19. However, in noncritically ill COVID-19 patients, the prediction risk of VTE and major bleeding was minor. Based on that, critically ill COVID-19 patients are predisposed to both high risk of thrombosis and bleeding, so prevention strategies should be individualized according to the assessment of thrombosis vs bleeding risk[131]. Another study reported two cases of a significant hemorrhagic complication in severe COVID-19 patients presented by spontaneous abdominal, internal bleeding. Patients had bilateral interstitial pneumonia, and there were no other apparent predisposing factors for bleeding. Patients were managed with interventional radiology, with no mortalities recorded. These imbalances (or disruption) in platelet production and disorders of the coagulation system induced by SARS-CoV-2 need to be further clarified in extensive prospective studies[132]. Only a few published data about COVID-19 infection with known bleeding disorder patients are available. A case report of mild COVID-19 in a known hemophilia-A patient reported no evidence of bleeding linked to COVID-19 infection, and the patient recovered completely with only home isolation, antiviral agents, empirical antibiotics, and supportive therapies. Indeed, mild COVID-19 is not known to increase the risk of bleeding, even in patients with known bleeding disorders[133]. Transfusion therapy should be restricted for those with active bleeding, requiring an invasive procedure, or at otherwise high risk for bleeding complications and accordingly to be managed similar to those in ISTH guidelines for DIC[134].
In conclusion, and based on all the previously discussed data, we should highlight the importance of using empirical therapeutic anticoagulation for COVID-19 patients to guard against possible thromboembolic events with close observation for the occurrence of bleeding.
Authors acknowledge the great effort and sacrifice of the medical staff and nurses working in COVID-19 quarantine hospitals all over the world.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medical laboratory technology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Krishnan A, United States; Mukhopadhyay A, India S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Bauer K, Weitz J. Laboratory markers of coagulation and fibrinolysis. Hemostasis and Thrombosis: Basic Principles and Clinical Practice, 1994: 1197-1210. |
| 2. | Opal SM. Interactions between coagulation and inflammation. Scand J Infect Dis. 2003;35:545-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Key NS, Vercellotti GM, Winkelmann JC, Moldow CF, Goodman JL, Esmon NL, Esmon CT, Jacob HS. Infection of vascular endothelial cells with herpes simplex virus enhances tissue factor activity and reduces thrombomodulin expression. Proc Natl Acad Sci U S A. 1990;87:7095-7099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, Hensley LE. Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J Infect Dis. 2003;188:1618-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 266] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 5. | Antoniak S, Owens AP 3rd, Baunacke M, Williams JC, Lee RD, Weithäuser A, Sheridan PA, Malz R, Luyendyk JP, Esserman DA, Trejo J, Kirchhofer D, Blaxall BC, Pawlinski R, Beck MA, Rauch U, Mackman N. PAR-1 contributes to the innate immune response during viral infection. J Clin Invest. 2013;123:1310-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | van der Poll T, de Boer JD, Levi M. The effect of inflammation on coagulation and vice versa. Curr Opin Infect Dis. 2011;24:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | van Gorp EC, Suharti C, ten Cate H, Dolmans WM, van der Meer JW, ten Cate JW, Brandjes DP. Review: infectious diseases and coagulation disorders. J Infect Dis. 1999;180:176-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Levi M. Disseminated intravascular coagulation. Crit Care Med. 2007;35:2191-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Huerta-Zepeda A, Cabello-Gutiérrez C, Cime-Castillo J, Monroy-Martínez V, Manjarrez-Zavala ME, Gutiérrez-Rodríguez M, Izaguirre R, Ruiz-Ordaz BH. Crosstalk between coagulation and inflammation during Dengue virus infection. Thromb Haemost. 2008;99:936-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Miller HC, Stephan M. Hemorrhagic varicella: a case report and review of the complications of varicella in children. Am J Emerg Med. 1993;11:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Squizzato A, Gerdes VE, Büller HR. Effects of human cytomegalovirus infection on the coagulation system. Thromb Haemost. 2005;93:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367:1075-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 323] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 13. | Harms PW, Schmidt LA, Smith LB, Newton DW, Pletneva MA, Walters LL, Tomlins SA, Fisher-Hubbard A, Napolitano LM, Park PK, Blaivas M, Fantone J, Myers JL, Jentzen JM. Autopsy findings in eight patients with fatal H1N1 influenza. Am J Clin Pathol. 2010;134:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Armstrong KL, Fraser DK, Faoagali JL. Gastrointestinal bleeding with influenza virus. Med J Aust. 1991;154:180-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Calore EE, Uip DE, Perez NM. Pathology of the swine-origin influenza A (H1N1) flu. Pathol Res Pract. 2011;207:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Wiwanitkit V. Hemostatic disorders in bird flu infection. Blood Coagul Fibrinolysis. 2008;19:5-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Hwang DM, Chamberlain DW, Poutanen SM, Low DE, Asa SL, Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol. 2005;18:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 246] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Hung LS. The SARS epidemic in Hong Kong: what lessons have we learned? J R Soc Med. 2003;96:374-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Ng WF, Wong SF, Lam A, Mak YF, Yao H, Lee KC, Chow KM, Yu WC, Ho LC. The placentas of patients with severe acute respiratory syndrome: a pathophysiological evaluation. Pathology. 2006;38:210-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Yang M, Ng MH, Li CK, Chan PK, Liu C, Ye JY, Chong BH. Thrombopoietin levels increased in patients with severe acute respiratory syndrome. Thromb Res. 2008;122:473-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Chow EY, Chiu WK. Severe acute respiratory syndrome and lupus anticoagulants in children. Br J Haematol. 2003;123:367-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Ng LF, Hibberd ML, Ooi EE, Tang KF, Neo SY, Tan J, Murthy KR, Vega VB, Chia JM, Liu ET, Ren EC. A human in vitro model system for investigating genome-wide host responses to SARS coronavirus infection. BMC Infect Dis. 2004;4:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Ashton AW, Ware JA. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circ Res. 2004;95:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Panigrahi S, Ma Y, Hong L, Gao D, West XZ, Salomon RG, Byzova TV, Podrez EA. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circ Res. 2013;112:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Tang BS, Chan KH, Cheng VC, Woo PC, Lau SK, Lam CC, Chan TL, Wu AK, Hung IF, Leung SY, Yuen KY. Comparative host gene transcription by microarray analysis early after infection of the Huh7 cell line by severe acute respiratory syndrome coronavirus and human coronavirus 229E. J Virol. 2005;79:6180-6193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Crawley JT, Goulding DA, Ferreira V, Severs NJ, Lupu F. Expression and localization of tissue factor pathway inhibitor-2 in normal and atherosclerotic human vessels. Arterioscler Thromb Vasc Biol. 2002;22:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Katayama S, Koyama K, Shima J, Tonai K, Goto Y, Koinuma T, Nunomiya S. Thrombomodulin, Plasminogen Activator Inhibitor-1 and Protein C Levels, and Organ Dysfunction in Sepsis. Crit Care Explor. 2019;1:e0013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Han M, Yan W, Huang Y, Yao H, Wang Z, Xi D, Li W, Zhou Y, Hou J, Luo X, Ning Q. The nucleocapsid protein of SARS-CoV induces transcription of hfgl2 prothrombinase gene dependent on C/EBP alpha. J Biochem. 2008;144:51-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Gralinski LE, Baric RS. Molecular pathology of emerging coronavirus infections. J Pathol. 2015;235:185-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 240] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 30. | Gralinski LE, Bankhead A 3rd, Jeng S, Menachery VD, Proll S, Belisle SE, Matzke M, Webb-Robertson BJ, Luna ML, Shukla AK, Ferris MT, Bolles M, Chang J, Aicher L, Waters KM, Smith RD, Metz TO, Law GL, Katze MG, McWeeney S, Baric RS. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. mBio. 2013;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 31. | Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PRM, Weiss DJ. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. 2020;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 32. | Singh SK. Middle East Respiratory Syndrome Virus Pathogenesis. Semin Respir Crit Care Med. 2016;37:572-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Al-Abdallat MM, Payne DC, Alqasrawi S, Rha B, Tohme RA, Abedi GR, Al Nsour M, Iblan I, Jarour N, Farag NH, Haddadin A, Al-Sanouri T, Tamin A, Harcourt JL, Kuhar DT, Swerdlow DL, Erdman DD, Pallansch MA, Haynes LM, Gerber SI; Jordan MERS-CoV Investigation Team. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 34. | Algahtani H, Subahi A, Shirah B. Neurological Complications of Middle East Respiratory Syndrome Coronavirus: A Report of Two Cases and Review of the Literature. Case Rep Neurol Med. 2016;2016:3502683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 35. | Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1635] [Cited by in RCA: 1597] [Article Influence: 319.4] [Reference Citation Analysis (1)] |
| 36. | Chaturvedi S, Braunstein EM, Yuan X, Yu J, Alexander A, Chen H, Gavriilaki E, Alluri R, Streiff MB, Petri M, Crowther MA, McCrae KR, Brodsky RA. Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS. Blood. 2020;135:239-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 37. | Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133:906-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 463] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 38. | Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 3418] [Article Influence: 683.6] [Reference Citation Analysis (0)] |
| 39. | Iba T, Levy JH, Wada H, Thachil J, Warkentin TE, Levi M; Subcommittee on Disseminated Intravascular Coagulation. Differential diagnoses for sepsis-induced disseminated intravascular coagulation: communication from the SSC of the ISTH. J Thromb Haemost. 2019;17:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Lee A, Connors J, Kreuziger L, Murphy M, Gernsheimer T, Lin Y. COVID-19 and coagulopathy: frequently asked questions. American society of hematology/Covid-19 resources, 2020. |
| 41. | Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18:1559-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 470] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 42. | Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382:e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1532] [Cited by in RCA: 1606] [Article Influence: 321.2] [Reference Citation Analysis (0)] |
| 43. | Bowles L, Platton S, Yartey N, Dave M, Lee K, Hart DP, MacDonald V, Green L, Sivapalaratnam S, Pasi KJ, MacCallum P. Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with Covid-19. N Engl J Med. 2020;383:288-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 358] [Cited by in RCA: 378] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 44. | Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes PM, Meziani F; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1669] [Cited by in RCA: 2051] [Article Influence: 410.2] [Reference Citation Analysis (0)] |
| 45. | Belen-Apak FB, Sarıalioğlu F. Pulmonary intravascular coagulation in COVID-19: possible pathogenesis and recommendations on anticoagulant/thrombolytic therapy. J Thromb Thrombolysis. 2020;50:278-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 46. | O’donnell J, Sharif K, Emery P, Bridgewood CMD, McGonagle D. Why the immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia are distinct from macrophage activation syndrome with disseminated Intravascular coagulation. Autoimmun Rev. 2020;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 599] [Cited by in RCA: 552] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 47. | Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, Dentali F, Montecucco F, Massberg S, Levi M, Abbate A. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 726] [Cited by in RCA: 636] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 48. | McFadyen JD, Stevens H, Peter K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ Res. 2020;127:571-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 425] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 49. | Goeijenbier M, van Wissen M, van de Weg C, Jong E, Gerdes VE, Meijers JC, Brandjes DP, van Gorp EC. Review: Viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84:1680-1696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 50. | Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 800] [Article Influence: 160.0] [Reference Citation Analysis (0)] |
| 51. | Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173:268-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1577] [Cited by in RCA: 1749] [Article Influence: 349.8] [Reference Citation Analysis (0)] |
| 52. | Alon R, Sportiello M, Kozlovski S, Kumar A, Reilly EC, Zarbock A, Garbi N, Topham DJ. Leukocyte trafficking to the lungs and beyond: lessons from influenza for COVID-19. Nat Rev Immunol. 2021;21:49-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 53. | Minet C, Potton L, Bonadona A, Hamidfar-Roy R, Somohano CA, Lugosi M, Cartier JC, Ferretti G, Schwebel C, Timsit JF. Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Crit Care. 2015;19:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 54. | Malato A, Dentali F, Siragusa S, Fabbiano F, Kagoma Y, Boddi M, Gensini GF, Peris A, Crowther M, Napolitano M. The impact of deep vein thrombosis in critically ill patients: a meta-analysis of major clinical outcomes. Blood Transfus. 2015;13:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 55. | Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3992] [Cited by in RCA: 4040] [Article Influence: 808.0] [Reference Citation Analysis (0)] |
| 56. | Chung MK, Zidar DA, Bristow MR, Cameron SJ, Chan T, Harding CV 3rd, Kwon DH, Singh T, Tilton JC, Tsai EJ, Tucker NR, Barnard J, Loscalzo J. COVID-19 and Cardiovascular Disease: From Bench to Bedside. Circ Res. 2021;128:1214-1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 245] [Article Influence: 61.3] [Reference Citation Analysis (2)] |
| 57. | Al-Ani F, Chehade S, Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. 2020;192:152-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 273] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 58. | Zhang X, Yang X, Jiao H, Liu X. Coagulopathy in patients with COVID-19: a systematic review and meta-analysis. Aging (Albany NY). 2020;12:24535-24551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421-1424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1330] [Article Influence: 266.0] [Reference Citation Analysis (0)] |
| 60. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3488] [Cited by in RCA: 3408] [Article Influence: 681.6] [Reference Citation Analysis (0)] |
| 61. | Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S; Lille ICU Haemostasis COVID-19 Group. Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation. 2020;142:184-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 872] [Article Influence: 174.4] [Reference Citation Analysis (0)] |
| 62. | Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, Du B, Aboodi M, Wunsch H, Cecconi M, Koh Y, Chertow DS, Maitland K, Alshamsi F, Belley-Cote E, Greco M, Laundy M, Morgan JS, Kesecioglu J, McGeer A, Mermel L, Mammen MJ, Alexander PE, Arrington A, Centofanti JE, Citerio G, Baw B, Memish ZA, Hammond N, Hayden FG, Evans L, Rhodes A. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020;46:854-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1239] [Cited by in RCA: 1360] [Article Influence: 272.0] [Reference Citation Analysis (0)] |
| 63. | Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995-2002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1017] [Cited by in RCA: 1093] [Article Influence: 218.6] [Reference Citation Analysis (0)] |
| 64. | Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743-1746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 850] [Cited by in RCA: 921] [Article Influence: 184.2] [Reference Citation Analysis (0)] |
| 65. | Han H, Yang L, Liu R, Liu F, Wu KL, Li J, Liu XH, Zhu CL. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 788] [Article Influence: 157.6] [Reference Citation Analysis (0)] |
| 66. | Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, Dangayach NS, Bederson JB, Tuhrim S, Fifi JT. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382:e60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1553] [Cited by in RCA: 1576] [Article Influence: 315.2] [Reference Citation Analysis (0)] |
| 67. | Bellosta R, Luzzani L, Natalini G, Pegorer MA, Attisani L, Cossu LG, Ferrandina C, Fossati A, Conti E, Bush RL, Piffaretti G. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 297] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 68. | Perini P, Nabulsi B, Massoni CB, Azzarone M, Freyrie A. Acute limb ischaemia in two young, non-atherosclerotic patients with COVID-19. Lancet. 2020;395:1546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 69. | Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2428] [Cited by in RCA: 3009] [Article Influence: 601.8] [Reference Citation Analysis (1)] |
| 70. | Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2516] [Cited by in RCA: 2842] [Article Influence: 568.4] [Reference Citation Analysis (0)] |
| 71. | Foy BH, Carlson JCT, Reinertsen E, Padros I Valls R, Pallares Lopez R, Palanques-Tost E, Mow C, Westover MB, Aguirre AD, Higgins JM. Association of Red Blood Cell Distribution Width With Mortality Risk in Hospitalized Adults With SARS-CoV-2 Infection. JAMA Netw Open. 2020;3:e2022058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 72. | Sahu KK, Siddiqui AD. From Hematologist's desk: The effect of COVID-19 on the blood system. Am J Hematol. 2020;95:E213-E215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 73. | Pavord S, Thachil J, Hunt BJ, Murphy M, Lowe G, Laffan M, Makris M, Newland AC, Provan D, Grainger JD, Hill QA. Practical guidance for the management of adults with immune thrombocytopenia during the COVID-19 pandemic. Br J Haematol. 2020;189:1038-1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 74. | Kasinathan G, Sathar J. Haematological manifestations, mechanisms of thrombosis and anti-coagulation in COVID-19 disease: A review. Ann Med Surg (Lond). 2020;56:173-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 75. | Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738-1742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 926] [Cited by in RCA: 952] [Article Influence: 190.4] [Reference Citation Analysis (0)] |
| 76. | Zhan H, Chen H, Liu C, Cheng L, Yan S, Li H, Li Y. Diagnostic Value of D-Dimer in COVID-19: A Meta-Analysis and Meta-Regression. Clin Appl Thromb Hemost. 2021;27:10760296211010976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 77. | Yu B, Li X, Chen J, Ouyang M, Zhang H, Zhao X, Tang L, Luo Q, Xu M, Yang L, Huang G, Liu X, Tang J. Evaluation of variation in D-dimer levels among COVID-19 and bacterial pneumonia: a retrospective analysis. J Thromb Thrombolysis. 2020;50:548-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 78. | Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta. 2020;506:145-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 946] [Cited by in RCA: 1133] [Article Influence: 226.6] [Reference Citation Analysis (0)] |
| 79. | Zhang Y, Zeng X, Jiao Y, Li Z, Liu Q, Ye J, Yang M. Mechanisms involved in the development of thrombocytopenia in patients with COVID-19. Thromb Res. 2020;193:110-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 80. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18877] [Article Influence: 3775.4] [Reference Citation Analysis (7)] |
| 81. | Amgalan A, Othman M. Exploring possible mechanisms for COVID-19 induced thrombocytopenia: Unanswered questions. J Thromb Haemost. 2020;18:1514-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 82. | Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2021;51:1107-1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 290] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 83. | Lippi G, Favaloro EJ. D-dimer is Associated with Severity of Coronavirus Disease 2019: A Pooled Analysis. Thromb Haemost. 2020;120:876-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 394] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 84. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30121] [Article Influence: 6024.2] [Reference Citation Analysis (3)] |
| 85. | Gao Y, Li T, Han M, Li X, Wu D, Xu Y, Zhu Y, Liu Y, Wang X, Wang L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92:791-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 548] [Cited by in RCA: 635] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 86. | Lippi G, Salvagno GL, Ippolito L, Franchini M, Favaloro EJ. Shortened activated partial thromboplastin time: causes and management. Blood Coagul Fibrinolysis. 2010;21:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M, Berelowitz M, Dhillon AP, Thomson MA, Harvey P, Valentine A, Davies SE, Walker-Smith JA. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1813] [Cited by in RCA: 1294] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 88. | Li Q, Cao Y, Chen L, Wu D, Yu J, Wang H, He W, Dong F, Chen W, Li L, Ran Q, Liu Q, Ren W, Gao F, Chen Z, Gale RP, Hu Y. Hematological features of persons with COVID-19. Leukemia. 2020;34:2163-2172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 89. | Jiang SQ, Huang QF, Xie WM, Lv C, Quan XQ. The association between severe COVID-19 and low platelet count: evidence from 31 observational studies involving 7613 participants. Br J Haematol. 2020;190:e29-e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 90. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 91. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5517] [Article Influence: 1103.4] [Reference Citation Analysis (1)] |
| 92. | Escher R, Breakey N, Lämmle B. ADAMTS13 activity, von Willebrand factor, factor VIII and D-dimers in COVID-19 inpatients. Thromb Res. 2020;192:174-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 93. | Blasi A, von Meijenfeldt FA, Adelmeijer J, Calvo A, Ibañez C, Perdomo J, Reverter JC, Lisman T. In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation. J Thromb Haemost. 2020;18:2646-2653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 94. | Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1166] [Article Influence: 233.2] [Reference Citation Analysis (0)] |
| 95. | Cardinal RM, D'Amico F, D'Addezio A, Dakers K, Castelli G. Safety and efficacy of direct oral anticoagulants across body mass index groups in patients with venous thromboembolism: a retrospective cohort design. J Thromb Thrombolysis. 2021;52:567-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Takahashi H, Takakuwa E, Yoshino N, Hanano M, Shibata A. Protein C levels in disseminated intravascular coagulation and thrombotic thrombocytopenic purpura: its correlation with other coagulation parameters. Thromb Haemost. 1985;54:445-449. [PubMed] |
| 97. | Ranucci M, Ballotta A, Di Dedda U, Baryshnikova E, Dei Poli M, Resta M, Falco M, Albano G, Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747-1751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 679] [Cited by in RCA: 680] [Article Influence: 136.0] [Reference Citation Analysis (0)] |
| 98. | Hunt BJ. Bleeding and coagulopathies in critical care. N Engl J Med. 2014;370:2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 690] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 100. | Akima S, McLintock C, Hunt BJ. RE: ISTH interim guidance to recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:2057-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 101. | Kichloo A, Dettloff K, Aljadah M, Albosta M, Jamal S, Singh J, Wani F, Kumar A, Vallabhaneni S, Khan MZ. COVID-19 and Hypercoagulability: A Review. Clin Appl Thromb Hemost. 2020;26:1076029620962853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 102. | Borges AH, O'Connor JL, Phillips AN, Baker JV, Vjecha MJ, Losso MH, Klinker H, Lopardo G, Williams I, Lundgren JD; INSIGHT SMART Study Group; ESPRIT Study Group; SILCAAT Scientific Committee. Factors associated with D-dimer levels in HIV-infected individuals. PLoS One. 2014;9:e90978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 103. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6751] [Article Influence: 1350.2] [Reference Citation Analysis (0)] |
| 104. | Iba T, Levy JH, Yamakawa K, Thachil J, Warkentin TE, Levi M; Scientific and Standardization Committee on DIC of the International Society on Thrombosis and Haemostasis. Proposal of a two-step process for the diagnosis of sepsis-induced disseminated intravascular coagulation. J Thromb Haemost. 2019;17:1265-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 105. | Shang W, Dong J, Ren Y, Tian M, Li W, Hu J, Li Y. The value of clinical parameters in predicting the severity of COVID-19. J Med Virol. 2020;92:2188-2192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 106. | Ramos-Rincon JM, Buonaiuto V, Ricci M, Martín-Carmona J, Paredes-Ruíz D, Calderón-Moreno M, Rubio-Rivas M, Beato-Pérez JL, Arnalich-Fernández F, Monge-Monge D, Vargas-Núñez JA, Acebes-Repiso G, Mendez-Bailon M, Perales-Fraile I, García-García GM, Guisado-Vasco P, Abdelhady-Kishta A, Pascual-Pérez MD, Rodríguez-Fernández-Viagas C, Montaño-Martínez A, López-Ruiz A, Gonzalez-Juarez MJ, Pérez-García C, Casas-Rojo JM, Gómez-Huelgas R; SEMI-COVID-19 Network. Clinical Characteristics and Risk Factors for Mortality in Very Old Patients Hospitalized With COVID-19 in Spain. J Gerontol A Biol Sci Med Sci. 2021;76:e28-e37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 107. | Marchandot B, Trimaille A, Curtiaud A, Carmona A, Matsushita K, Sato C, Leonard-Lorant I, Sattler L, Grunebaum L, Ohana M, Ohlmann P, Jesel L, Morel O. Staging Severity of COVID-19 according to Hemostatic Abnormalities (CAHA Score). Thromb Haemost. 2020;120:1716-1719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 108. | Cattaneo M, Bertinato EM, Birocchi S, Brizio C, Malavolta D, Manzoni M, Muscarella G, Orlandi M. Pulmonary Embolism or Pulmonary Thrombosis in COVID-19? Thromb Haemost. 2020;120:1230-1232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 109. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11507] [Article Influence: 2301.4] [Reference Citation Analysis (0)] |
| 110. | Del Nonno F, Colombo D, Nardacci R, Falasca L. Fatal pulmonary arterial thrombosis in a COVID-19 patient, with asymptomatic history, occurred after swab negativization. Thromb J. 2021;19:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 111. | Barnes G, Cuker A, Gluckman T. Thrombosis and COVID-19: FAQs for current practice. 2020. [cited 15 May 2020]. Available from: https://www.acc.org/Latest-in-cardiology/articles/2020/04/17/14/42/thrombosis-and-coronavirus-disease-2019-covid-19-faqs-for-current-practice. |
| 112. | England N, Improvement N. Virtual Solutions for Managing Cancer Care In a Pandemic Era. Lessons from COVID-19 A Rapid Evidence Review, 2020. |
| 113. | Ciavarella A, Peyvandi F, Martinelli I. Where do we stand with antithrombotic prophylaxis in patients with COVID-19? Thromb Res. 2020;191:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 114. | Barrett CD, Moore HB, Yaffe MB, Moore EE. ISTH interim guidance on recognition and management of coagulopathy in COVID-19: A comment. J Thromb Haemost. 2020;18:2060-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 115. | Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2447] [Cited by in RCA: 2509] [Article Influence: 501.8] [Reference Citation Analysis (1)] |
| 116. | Guan WJ, Zhong NS. Clinical Characteristics of Covid-19 in China. Reply. N Engl J Med. 2020;382:1861-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 259] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 117. | Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, Yaffe MB, Moore HB, Barrett CD. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. J Thromb Haemost. 2020;18:1752-1755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 414] [Cited by in RCA: 395] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 118. | Society BT. BTS guidance on venous thromboembolic disease in patients with COVID-19 (updated 4th May 2020). BTS: 2020. |
| 119. | Marietta M, Ageno W, Artoni A, De Candia E, Gresele P, Marchetti M, Marcucci R, Tripodi A. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus. 2020;18:167-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 163] [Reference Citation Analysis (0)] |
| 120. | Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, Holley AB, Jimenez D, Le Gal G, Rali P, Wells P. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. Chest. 2020;158:1143-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 469] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 121. | Surkhali B, Garbuja CK. Virtual learning during COVID-19 pandemic: pros and cons. J Lumbini Med Coll. 2020;8:154-155. |
| 122. | Gill D, Baker EH, Hitchings AW. We need clinical guidelines fit for a pandemic. BMJ. 2021;373:n1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Mousa N, Abdel-Razik A, Mousa E, Elbadrawy T, Hosni K, Mousa A, Taha A, Elmetwalli A. Coagulopathy in COVID-19 from Pathogenesis until Treatment: A systemic Review. Med J Viral Hep. 2021;5:3-8. |
| 124. | Levi M, Scully M. How I treat disseminated intravascular coagulation. Blood. 2018;131:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 125. | de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 756] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 126. | Nardacci R, Colavita F, Castilletti C, Lapa D, Matusali G, Meschi S, Del Nonno F, Colombo D, Capobianchi MR, Zumla A, Ippolito G, Piacentini M, Falasca L. Evidences for lipid involvement in SARS-CoV-2 cytopathogenesis. Cell Death Dis. 2021;12:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 127. | Schnittler HJ, Feldmann H. Viral hemorrhagic fever--a vascular disease? Thromb Haemost. 2003;89:967-972. [PubMed] |
| 128. | Cannegieter SC, Klok FA. COVID-19 associated coagulopathy and thromboembolic disease: Commentary on an interim expert guidance. Res Pract Thromb Haemost. 2020;4:439-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 129. | Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950-2973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2178] [Cited by in RCA: 2191] [Article Influence: 438.2] [Reference Citation Analysis (0)] |
| 130. | Joob B, Wiwanitkit V. Hemorrhagic Problem Among the Patients With COVID-19: Clinical Summary of 41 Thai Infected Patients. Clin Appl Thromb Hemost. 2020;26:1076029620918308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 131. | Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C, Zhang N, Zhong N, Li S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362-e363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 273] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 132. | Conti CB, Henchi S, Coppeta GP, Testa S, Grassia R. Bleeding in COVID-19 severe pneumonia: The other side of abnormal coagulation pattern? Eur J Intern Med. 2020;77:147-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 133. | Cui D, Zhang A, Liu A, Hu Q. Clinical findings in a patient with haemophilia A affected by COVID-19. Haemophilia. 2020;26:e214-e216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 134. | Wada H, Thachil J, Di Nisio M, Mathew P, Kurosawa S, Gando S, Kim HK, Nielsen JD, Dempfle CE, Levi M, Toh CH; The Scientific Standardization Committee on DIC of the International Society on Thrombosis Haemostasis. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 135. | Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, Psaltopoulou T, Gerotziafas G, Dimopoulos MA. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95:834-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 936] [Cited by in RCA: 1211] [Article Influence: 242.2] [Reference Citation Analysis (0)] |
| 136. | Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 3340] [Article Influence: 668.0] [Reference Citation Analysis (0)] |
| 137. | Deng Y, Liu W, Liu K, Fang YY, Shang J, Zhou L, Wang K, Leng F, Wei S, Chen L, Liu HG. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133:1261-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 445] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 138. | Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2604] [Cited by in RCA: 3194] [Article Influence: 638.8] [Reference Citation Analysis (0)] |
| 139. | Lippi G, Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020;42:116-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 140. | Hahn WH, Song JH, Kim H, Park S. Is procalcitonin to C-reactive protein ratio useful for the detection of late onset neonatal sepsis? J Matern Fetal Neonatal Med. 2018;31:822-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 141. | Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 1221] [Article Influence: 55.5] [Reference Citation Analysis (2)] |
| 142. | Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect. 2020;50:332-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 441] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 143. | Xiong Y, Sun D, Liu Y, Fan Y, Zhao L, Li X, Zhu W. Clinical and High-Resolution CT Features of the COVID-19 Infection: Comparison of the Initial and Follow-up Changes. Invest Radiol. 2020;55:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 289] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 144. | Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 330] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 145. | Huang J, Cheng A, Kumar R, Fang Y, Chen G, Zhu Y, Lin S. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020;92:2152-2158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 146. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18200] [Article Influence: 3640.0] [Reference Citation Analysis (0)] |
| 147. | Gaffney PJ. Breakdown products of fibrin and fibrinogen: molecular mechanisms and clinical implications. J Clin Pathol Suppl (R Coll Pathol). 1980;14:10-17. [PubMed] |
| 148. | Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID-19 - A systematic review. Life Sci. 2020;254:117788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 420] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 149. | Huang Y, Lyu X, Li D, Wang L, Wang Y, Zou W, Wei Y, Wu X. A cohort study of 676 patients indicates D-dimer is a critical risk factor for the mortality of COVID-19. PLoS One. 2020;15:e0242045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 150. | Vargas-Vargas M, Cortés-Rojo C. Ferritin levels and COVID-19. Rev Panam Salud Publica. 2020;44:e72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 151. | Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, Ntaganou M, Kyriakopoulou M, Dimopoulos G, Koutsodimitropoulos I, Velissaris D, Koufargyris P, Karageorgos A, Katrini K, Lekakis V, Lupse M, Kotsaki A, Renieris G, Theodoulou D, Panou V, Koukaki E, Koulouris N, Gogos C, Koutsoukou A. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27:992-1000.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1556] [Article Influence: 311.2] [Reference Citation Analysis (0)] |
| 152. | Velavan TP, Meyer CG. Mild versus severe COVID-19: Laboratory markers. Int J Infect Dis. 2020;95:304-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 337] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 153. | Hu R, Han C, Pei S, Yin M, Chen X. Procalcitonin levels in COVID-19 patients. Int J Antimicrob Agents. 2020;56:106051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 177] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 154. | Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 2342] [Article Influence: 468.4] [Reference Citation Analysis (0)] |
| 155. | Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8473] [Cited by in RCA: 7602] [Article Influence: 1520.4] [Reference Citation Analysis (0)] |
| 156. | Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chim Acta. 2020;505:190-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 375] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 157. | Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 in- fection. Clin Chem Lab Med. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 158. | Ticinesi A, Nouvenne A, Prati B, Guida L, Parise A, Cerundolo N, Bonaguri C, Aloe R, Guerra A, Meschi T. The Clinical Significance of Procalcitonin Elevation in Patients over 75 Years Old Admitted for COVID-19 Pneumonia. Mediators Inflamm. 2021;2021:5593806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 159. | Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383:2255-2273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1324] [Cited by in RCA: 2103] [Article Influence: 420.6] [Reference Citation Analysis (0)] |
| 160. | Chen LD, Zhang ZY, Wei XJ, Cai YQ, Yao WZ, Wang MH, Huang QF, Zhang XB. Association between cytokine profiles and lung injury in COVID-19 pneumonia. Respir Res. 2020;21:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 161. | Elshazli RM, Toraih EA, Elgaml A, El-Mowafy M, El-Mesery M, Amin MN, Hussein MH, Killackey MT, Fawzy MS, Kandil E. Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: A meta-analysis of 6320 patients. PLoS One. 2020;15:e0238160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 162. | Feng X, Li S, Sun Q, Zhu J, Chen B, Xiong M, Cao G. Immune-Inflammatory Parameters in COVID-19 Cases: A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2020;7:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 163. | Akbari H, Tabrizi R, Lankarani KB, Aria H, Vakili S, Asadian F, Noroozi S, Keshavarz P, Faramarz S. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Life Sci. 2020;258:118167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 164. | Malik P, Patel U, Mehta D, Patel N, Kelkar R, Akrmah M, Gabrilove JL, Sacks H. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2021;26:107-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 165. | Shen Y, Cheng C, Zheng X, Jin Y, Duan G, Chen M, Chen S. Elevated Procalcitonin Is Positively Associated with the Severity of COVID-19: A Meta-Analysis Based on 10 Cohort Studies. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 166. | Henry BM, Aggarwal G, Wong J, Benoit S, Vikse J, Plebani M, Lippi G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am J Emerg Med. 2020;38:1722-1726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 355] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 167. | Szarpak L, Ruetzler K, Safiejko K, Hampel M, Pruc M, Kanczuga-Koda L, Filipiak KJ, Jaguszewski MJ. Lactate dehydrogenase level as a COVID-19 severity marker. Am J Emerg Med. 2021;45:638-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 168. | Martha JW, Wibowo A, Pranata R. Prognostic value of elevated lactate dehydrogenase in patients with COVID-19: a systematic review and meta-analysis. Postgrad Med J. 2022;98:422-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 169. | Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1450] [Cited by in RCA: 1438] [Article Influence: 287.6] [Reference Citation Analysis (0)] |
| 170. | Aziz M, Fatima R, Lee-Smith W, Assaly R. The association of low serum albumin level with severe COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 171. | Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J Med Virol. 2020;92:2283-2285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 371] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 172. | Akirov A, Masri-Iraqi H, Atamna A, Shimon I. Low Albumin Levels Are Associated with Mortality Risk in Hospitalized Patients. Am J Med. 2017;130:1465.e11-1465.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 173. | Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J Parenter Enteral Nutr. 2019;43:181-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 709] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 174. | Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX; China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1711] [Cited by in RCA: 2189] [Article Influence: 437.8] [Reference Citation Analysis (0)] |
| 175. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 176. | Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 177. | Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 178. | Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2289] [Cited by in RCA: 2550] [Article Influence: 510.0] [Reference Citation Analysis (2)] |
| 179. | Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92:568-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 972] [Cited by in RCA: 851] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 180. | Xu Y, Yang H, Wang J, Li X, Xue C, Niu C, Liao P. Serum Albumin Levels are a Predictor of COVID-19 Patient Prognosis: Evidence from a Single Cohort in Chongqing, China. Int J Gen Med. 2021;14:2785-2797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 181. | Violi F, Ceccarelli G, Cangemi R, Alessandri F, D'Ettorre G, Oliva A, Pastori D, Loffredo L, Pignatelli P, Ruberto F, Venditti M, Pugliese F, Mastroianni CM. Hypoalbuminemia, Coagulopathy, and Vascular Disease in COVID-19. Circ Res. 2020;127:400-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 182. | Violi F, Cangemi R, Romiti GF, Ceccarelli G, Oliva A, Alessandri F, Pirro M, Pignatelli P, Lichtner M, Carraro A, Cipollone F, D'ardes D, Pugliese F, Mastroianni CM. Is Albumin Predictor of Mortality in COVID-19? Antioxid Redox Signal. 2021;35:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 183. | Léonard-Lorant I, Delabranche X, Séverac F, Helms J, Pauzet C, Collange O, Schneider F, Labani A, Bilbault P, Molière S, Leyendecker P, Roy C, Ohana M. Acute Pulmonary Embolism in Patients with COVID-19 at CT Angiography and Relationship to d-Dimer Levels. Radiology. 2020;296:E189-E191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 385] [Cited by in RCA: 443] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 184. | Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected with Pulmonary CT Angiography. Radiology. 2020;296:E186-E188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 185. | Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, Sandri MT, Barco S; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1535] [Article Influence: 307.0] [Reference Citation Analysis (0)] |
| 186. | Thomas W, Varley J, Johnston A, Symington E, Robinson M, Sheares K, Lavinio A, Besser M. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |