Published online Dec 12, 2015. doi: 10.5528/wjtm.v4.i3.69
Peer-review started: January 28, 2015
First decision: April 10, 2015
Revised: April 27, 2015
Accepted: August 30, 2015
Article in press: August 31, 2015
Published online: December 12, 2015
Processing time: 321 Days and 19.4 Hours
Targeting apoptosis is one of the major strategies for cancer therapy. Essentially, most of the conventional cancer therapeutic drugs that are in the clinical use induce apoptosis and in part necrosis of malignant cells and therefore prevent cancer progression and metastasis. Although these cytotoxic anticancer drugs are important weapons for killing cancers, their toxic side effects limited their application. The molecularly targeted therapeutics that are based on the deeper understanding of the defects in the apoptotic signaling in cancers are emerging and have shown promising anticancer activity in selectively killing cancers but not normal cells. The examples of molecular targets that are under exploration for cancer therapy include the cell surface receptors such as TNFR family death receptors, the intrinsic Bcl-2 family members and some other intracellular molecules like p53, MDM2, IAP, and Smac. The advance in the high-throughput bio-technologies has greatly accelerated the progress of cancer drug discovery.
Core tip: Chemotherapy and radiotherapy are important approaches for cancer therapy and have prolonged the lifespan and reduced the mortality of cancer patients. But chemotherapy and radiotherapy induce apoptosis in both cancer and normal cells, therefore possessing severe toxic side effects. It appears quite important to develop the biological mechanism-based drugs that that can selectively kill tumor cells but not normal cells. Molecular targets in the apoptotic signaling pathways such as p53, TRAIL, and Bcl-2 have been identified, and molecularly targeting drugs for a variety of tumors based on these pathways are currently under development. Dissecting the genetic alterations in a particular tumor type and designing the rational drug combinations targeting different pathways can help achieve synergy in eradicating cancer cells and reversing drug resistance, and this holds great promise for the personalized treatment of cancer patients.
- Citation: Liu XC, Gao JM, Liu S, Liu L, Wang JR, Qu XJ, Cai B, Wang SL. Targeting apoptosis is the major battle field for killing cancers. World J Transl Med 2015; 4(3): 69-77
- URL: https://www.wjgnet.com/2220-6132/full/v4/i3/69.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v4.i3.69
The most important concern facing current cancer therapy is the lack of tumor selective agents that kill cancer cells effectively but do not harm normal cells. In the past decade, many of the efforts have been contributed to dissecting the mechanisms underlying the pathogenesis of cancer, particularly the altered signal transduction pathways in different cancers[1-5]. Recent advances in our understanding of how a normal cell is mutated to become cancerous and the accumulation of mutations in cancerous cells leads to malignancy, progression and metastasis have greatly promoted the identification of new molecular targets for developing tumor selective therapeutics[6-10].
Apoptosis is a programmed physiological process to eradicate unwanted cells for maintaining homeostasis[11-14]. The signal transduction pathways mediating apoptosis are frequently deregulated in cancers, enabling cancer cells to evade from apoptosis and become hyper-proliferated. Most of the genetically altered signaling components in the intrinsic and extrinsic apoptotic pathways in cancers are usually the central regulators of apoptosis, which play a key role in arbitrating the fate of a cell[15,16]. The key components in the apoptotic machinery are the Bcl-2 family members including Bcl-2, Bax and Bcl-xl in the mitochondrial pathway, the extrinsic cell surface receptors such as death receptor KILLER/DR5, and some other intracellular molecules such as p53[17-25]. These signaling molecules are frequently mutated or deleted in cancers and therefore are the ideal targets for developing novel cancer targeting drugs[24-28]. Targeting of Bcl-2 with RNAi and BH3 mimetics are currently underway in the clinical trial studies and have shown significant clinical activity in selectively killing cancer cells[29-32]. High-throughput screening of chemical libraries have identified some small molecule compounds that can target p53 to activate or restore p53 functions, which already showed success in killing some of the cancer cells[33,34]. Loss of p53 in cancers confers resistance to some chemotherapeutics and tumors bearing p53 mutations are less sensitive to radiotherapy[35-37]. Optimal combination of agents targeting different targets in the signal transduction pathways in cancers leads to a synergistic effect on killing tumors and overcoming resistance to single agent treatment[25,38].
In this review, we summarize the basic understanding of the apoptotic signal transduction cascades in cancers and the crosstalk between different pathways in which the signaling components are mutated in cancers. We also introduce some of the knowledge about the mechanisms of tumor targeted drugs and chemotherapeutic agents in killing cancers. Moreover, we highlight recent advances in the technologies that are applied for identification of cancer drug targets and the strategies for cancer drug screening. The translational designs of drug combination for achieving synergy in eradicating cancer cells and reversing drug resistance will also be discussed.
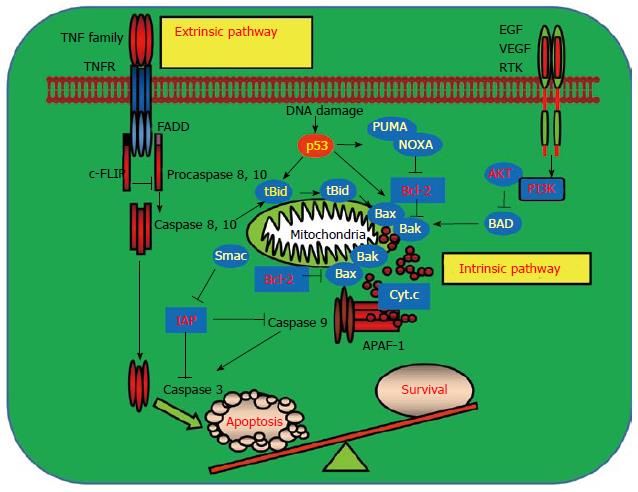
The complex apoptotic signaling networks play a key regulatory role in maintaining homeostatic cellular progresses in living organisms[39]. There are two major signaling pathways that mediate apoptosis in mammalian cells: The extrinsic apoptotic signaling pathway and the intrinsic apoptotic signaling pathway (Figure 1). Some intracellular stimuli, such as DNA damaging agents, irradiation or oncogene activation, trigger apoptosis primarily through the intrinsic pathway which requires the activation of apoptotic regulators in the mitochondrion. Extracellular apoptotic pathway is initiated through apoptotic signaling cascades mediated by members of the tumor necrosis factor (TNF) superfamily, usually generated by the cytotoxic cells of the immune system, for example TNF, TRAIL, and FasL. Both pathways require the activation of proapoptotic cysteine proteases, caspases, to execute the cell death process[40-43]. Most of the genetically mutated intracellular signaling molecules in the intrinsic and extrinsic apoptotic pathways in cancers are the central regulators of apoptosis which arbitrate life-or-death decision of a cell. Mutations in these signaling components allow cancer cells to escape from eradicating by the host immune system and accelerates the proliferation of transformed tumor cells[44,45].
Some of the mutated molecules in the apoptotic machinery have been identified as important markers for the distinction between normal and cancer cells, and therefore are the targets for developing tumor therapeutic drugs. The alterations in the apoptotic pathways in cancers include the Bcl-2 superfamily members in the mitochondrion. All the Bcl-2 pro-survival family members like Bcl-2 and Bcl-XL are likely to be oncogenic [15,17,18]. Bcl-2 was found to be overexpressed and implicated in the pathogenesis of myeloid and T-cell leukemia[21]. Conversely, members of the pro-apoptotic subfamily such as Bax and Bak are probably tumor suppressors. Bax or Bak is frequently mutated or deleted in some gastric and colorectal cancers. Cell surface death receptor KILLER/DR5 is mutated in some of the head and neck cancers and lung cancers[46,47]. P53 is mutated or deleted in more than 50% of the human cancers. P53 induces apoptosis through regulating its downstream targets including the proapoptotic Bax, KILLER/DR5 and p53 upregulated modulator of apoptosis (PUMA). Loss of p53 impairs the apoptotic signaling pathways by aberrant control of its downstream apoptotic target genes and confers resistance to chemotherapy or radiotherapy-induced apoptosis[35-37,48-50]. Oncogenic mutations impair apoptosis indirectly by prompting or repressing the expression of the signaling molecules in cell death pathways and promote tumor progression and metastasis.
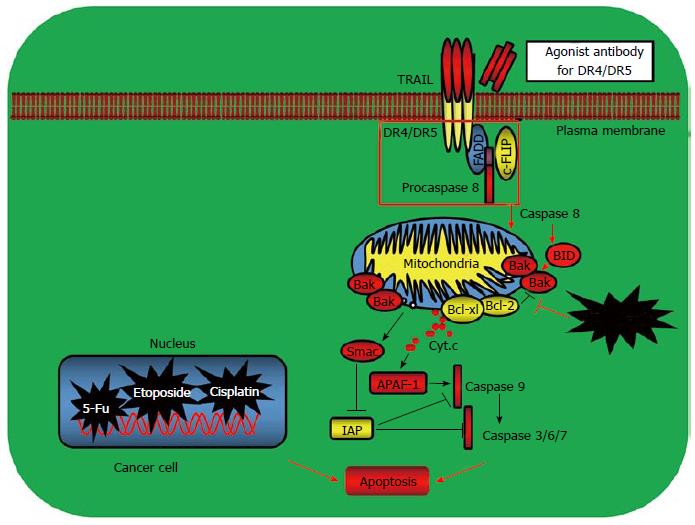
Recent advances in our understanding of how apoptosis is activated and the mechanisms how cancers evade apoptosis have paved new revenues for developing the molecularly targeted cancer drugs[10,19,26-28]. The first link between apoptosis and cancer emerged when Bcl-2, the gene that is linked to an immunoglobulin locus by chromosome translocation in follicular lymphoma, was found to inhibit cell death[51]. Later on, many other Bcl-2 family proteins including the proapoptotic Bax, Bak and Bok, and anti-apoptotic Bcl-2, Bcl-XL and Mcl-1 were discovered. The BH3-only proteins such as the proapoptotic Bid, Bad, Bim, PUMA and phorbol-12- myristate-13-acetate induced protein 1 (NOXA) were identified as the other key regulators which control the mitochondria-mediated intrinsic apoptotic pathway[52]. These Bcl-2 family proteins are important targets for cancer therapy (Figure 2). Several strategies have been developed by targeting the Bcl-2 family members. One approach for modulating Bcl-2 function is to target Bcl-2 with antisense Bcl-2 deoxyoligonucleotides. The other approach is to mimic the binding of a BH3 peptide to its surface groove. For example, ABT-737 and ABT-263 are BH3-mimetics that inhibit Bcl-2, Bcl-XL and Bcl-w to liberate Bax and Bak to induce apoptosis (Table 1). Preclinical studies have shown that ABT-737 was effective as a single agent in killing small cell lung cancer (SCLC) and causing SCLC tumor regression[29-31,51-55]. ABT-737 has also been shown to synergize with other conventional chemotherapy, radiotherapy or tyrosine kinase inhibitors imatinib and gefitinib to reverse drug resistance and enhance cancer cell apoptosis[56,57]. One advantage of the BH-3-memitics as cancer therapeutics is to target specific Bcl-2 family members such as Bcl-2 or Bcl-XL which is overexpressed in certain cancer types, therefore increasing the tumor selectivity and reducing the toxicity of the anticancer drugs (Figure 2).
| Drug name | Mechanism of action | Drug targets |
| CP-31398 | Restoration of wild type p53 function | Mutant p53 |
| PRIMA-1 | Restoration of wild type p53 function | Mutant p53 |
| Nutlins | Prevent p53 degradation | p53/MDM2 |
| RITA | Prevent p53 degradation | p53/MDM2 |
| Oblimersen sodium | Antisense inhibitors | Bcl-2 |
| ABT-737 | BH3 mimetics | Bcl-2, Bcl-XL,Bcl-w |
p53 is the most commonly mutated gene in human cancers and more than 50% of cancers carry mutations or deletions in p53, making p53 an important target for cancer therapy[58-60]. Growing evidence has shown that reactivation or restoration of p53 function in cancers will have significant therapeutic benefit. Several strategies are being explored to target tumors expressing mutant p53. Small molecules that bind to a site in mutant p53 (Y220C) increase the level of p53 with wild type conformation and activity. Other compounds (e.g., PRIMA-1, CP-31398) bind to multiple mutant p53 proteins and interact with DNA binding domain, thereby promoting the proper folding of the mutant protein and restoration of p53 function[61,62]. Of all the compounds that restore wild type activity, the most progress has been made with PRIMA analogs, with the demonstration of safety in phase I clinical trial study[63] (Table 1). An alternative approach to target mutant p53 is to remove the protein by enhancing its degradation. HDAC inhibitors such as SAHA show promise in destabilizing mutant p53 by preventing HDAC6 from interacting with Hsp90[64]. Small molecule activators of SIRT1 have also been shown to lead to the deacetylation of p53 and reduction of overall mutant p53 levels[65].
Oncogenic mutations of proteins in the intrinsic or extrinsic apoptotic pathways often lead to resistance to anticancer agents, among these molecules are p53, the Bcl-2 family members, death receptors, inhibitor of apoptosis proteins (IAPs), and the prosurvival factors such as AKT, PI3K and c-FLIP[66-69]. Mutant p53-carrying tumors showed increased resistance to commonly used chemotherapeutic agents. Overexpression of Bcl-2 confers the resistance of leukemic cells to cytotoxic chemotherapeutic agents. Similarly, other Bcl-2 family members including Bcl-XL, Bax, and BH3-only proteins such as P53 Upregulated Modulator of Apoptosis and NOXA also play regulatory roles in determining the sensitivity of cancer cells to therapeutic agents[48,49,53]. In addition to the defects in the cell apoptotic and survival machineries, there are still some other mechanisms that contribute to cancer drug resistance including the rates of drug efflux, alterations in drug metabolism and drug targets, DNA damage repair capacity, and the changes of the local tumor microenvironment. Drug resistance can also be acquired during treatment of tumors that were initially sensitive as well as the adaptive responses such as activation of other compensatory pathways. Further investigation of the molecular basis of drug resistance will help to overcome the hurdle that limits the clinical usage of cancer chemotherapeutic drugs and design rational drug combinations to restore or enhance the sensitivity of cancer drugs and improve the tumor selectivity and efficacy of cancer therapeutics[70,71].
The initial identification of a chemical compound or small molecule inhibitors to be translated into medicine generally occurred by screening molecules in the animal models and cellular assay systems with functional biomarkers or molecular targets which are specifically mutated in cancers. Therefore, the level of mechanistic understanding of the alterations in cancers and the suitable targets used determine the success of a design for cancer drug discovery. The emergence of the high-throughput and high-content technologies has driven modern cancer therapy to the molecular era that new generations of molecularly targeted cancer drugs were continuously discovered. In the past decades, new technologies have been developed for dissecting the mechanisms of cancer development and metastasis and the molecular targets have been identified for designing new cancer selective therapeutic drugs. Large scale profiling approaches such as transcriptomics and proteomics and the high-throughput sequencing techniques which comparatively quantify the expression levels of transcripts and proteins have uncovered numerous genes, proteins and biomarkers that correlate with tumorigenesis. Some of these genes and proteins have been used as good targets for developing cancer therapeutics. The most prominent examples are EGFR, BCR-ABL, VEGF, BRAF, PDGFR, p53, BCL2, MDM2, and ERBB2[24,58-65,72-78].
Target-based screening strategy focused on the rational molecular targets that are hypothesized to have a role in cancers and represents the predominant approach for cancer drug discovery. Alternatively, there is another cancer drug discovery strategy called phenotypic screening approach which is referred to as phenotypic measurement of responses upon drug treatments in the animals or cellular models and is based on the cellular phenotype or functional endpoints rather than target potency alone. Much of the early pharmaceutical and drug discovery is based on the phenotypic screening. A recent report showed that phenotypic screening achieved more success in first-in-class medicine than target-based screening. One of the successful examples of cell based phenotypic drug screening was the discovery of vorinostat, an HDAC inhibitor, for inducing differentiation of cancer cells[79].
The integration of advanced high-content imaging system into the drug screening field has aided the rapid development of cancer drug discovery[80-82]. Light microscope imaging methods evolving in the mid-90s have provided multiple cellular measurements from living cells but require continuous user intervention. The development of fluorescent proteins permitted the tracking of proteins in living cells and the use of green fluorescent protein to tag functional proteins allowed many different fluorescent analogs to be created quickly and be used as markers to study the functions, subcellular localization, trafficking and activities of particular proteins. The automation of light microscope imaging, particularly the laser-directed multicolor fluorescence of arrays of cells, formed the high content screening system. Image-based screening techniques provide invaluable readouts, for example, the changes in the morphology, proliferation, cell cycle progression, cell death, differentiation, cell migration and invasion in the cellular models before and after drug treatment. Multiplexed high content screening assays integrated measurements of multiple cellular targets in a single assay, can be applied in numerous cell types which were subjected to libraries of chemical compounds or other experimental treatments such as RNAi, and emerged as an important cancer target and drug discovery platform[83,84]. In addition, intravital imaging has gained the cellular details by tagging single cells, tissues and subcellular compartments with fluorescent proteins, through direct labelling of cells before exogenous inoculations in vivo or by using the specific promoters to drive the expression of genes of interest. These methods have been applied to monitor cancer drug response in vivo and determine the effect of cancer drugs on particular targets in animal models. Whole-body imaging techniques such as in vivo bioluminescence system have been used to monitor tumor progression and regression rates from multiple tissue sites during drug treatment[82,85].
Other fluorescence-based assays are also the important platforms for cancer drug screening. Examples of these dynamic technologies include the fluorescence resonance energy transfer which can be used to measure protein-protein interactions and is suitable for lead compound identification. This approach can provide a precise measurement of drug activity and insights of drug mechanism by determining the biomolecular interactions or activations[86,87]. Surface plasmon resonance imaging (SPRi) is an optical technology which allows the label-free and real-time detection of biomolecule interaction. It offers the possibility of monitoring hundreds of biological interactions simultaneously and from the binding profiles, allows the estimation of the kinetic parameters of the interactions between immobilized probes and the ligands in solution. SPRi has been applied in a variety of affinity systems, including protein/protein, protein/DNA, antibody/antigen, ligand/receptor, DNA/DNA, carbohydrate/protein, and cell/cell interactions. SPRi imaging as an affinity-based biosensor technology has been adopted for cancer drug screening, food and environment evaluation, and clinical diagnostics[88-91]. Enzymatic activation of caspases, which are a class of cysteine protease, was determined by using the peptide arrays based on the SPR imaging. This strategy used streptavidin to amplify the SPR signals of the surface-immobilized substrate peptides labeled with biotin at the C-terminus and the cleavage of the substrate peptides by caspases was detected by the increased SPRi signal. This method allowed the examination of the activities of purified caspases and caspase in cell lysate and therefore can be applicable to cell-based drug screening[92].
Enhancing or restoring apoptotic pathways in cancers are important strategies for developing cancer drugs. Some of the aberrant components in the apoptotic pathways in cancers have been identified as suitable targets for cancer therapy. These targets can be the cell surface death receptors, Bcl-2 family members, and some other intracellular molecules like p53, MDM2, IAP, and Smac. Although some of the chemical compounds or small molecule inhibitors have been discovered to modulate the activity of these targets and achieve varying degrees of success in killing cancers, drug resistance, limited patient responses and the toxic side effects still remained problematic and limited their clinic application. Further investigation of the mechanisms of apoptosis evasion in cancers and designing the rational drug combinations are still the challenges for cancer researchers in the world.
A number of high-throughput approaches including the genomic, proteomic and multiplexed imaging technologies have been applied to identify new targets in the signaling networks that are involved in cancer pathogenesis and led to discovery of new generations of target-directed chemical compounds and small molecule drugs. The natural products from plants, Chinese herbal medicine and marine bioproducts are invaluable sources for cancer drug development and many studies need to be done to discover more druggable natural products, study the mechanism of action and cure cancer patients more efficiently and less costly.
P- Reviewer: Baldi E, Chello M, Weber GF S- Editor: Tian YL L- Editor: Wang TQ E- Editor: Wu HL
| 1. | Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009;9:167-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 398] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 2. | Letai AG. Diagnosing and exploiting cancer’s addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 440] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 3. | Long JS, Ryan KM. New frontiers in promoting tumour cell death: targeting apoptosis, necroptosis and autophagy. Oncogene. 2012;31:5045-5060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 792] [Cited by in RCA: 778] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 5. | Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 964] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 6. | Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3451] [Cited by in RCA: 3575] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 7. | Gerl R, Vaux DL. Apoptosis in the development and treatment of cancer. Carcinogenesis. 2005;26:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 280] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 960] [Cited by in RCA: 937] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 9. | Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1680] [Cited by in RCA: 1698] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 10. | Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9960] [Cited by in RCA: 10008] [Article Influence: 188.8] [Reference Citation Analysis (0)] |
| 12. | Horvitz HR. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 1999;59:1701s-1706s. [PubMed] |
| 13. | Meier P, Finch A, Evan G. Apoptosis in development. Nature. 2000;407:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 698] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 14. | Nagata S. Apoptosis by death factor. Cell. 1997;88:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3739] [Cited by in RCA: 3644] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 15. | Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3914] [Cited by in RCA: 3938] [Article Influence: 145.9] [Reference Citation Analysis (0)] |
| 16. | Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6842] [Cited by in RCA: 6815] [Article Influence: 252.4] [Reference Citation Analysis (0)] |
| 17. | Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2769] [Cited by in RCA: 2760] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 18. | Vander Heiden MG, Thompson CB. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol. 1999;1:E209-E216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 504] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 19. | Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7:1001-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 328] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 20. | Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 669] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 21. | Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2187] [Cited by in RCA: 2230] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 22. | Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4399] [Cited by in RCA: 4503] [Article Influence: 140.7] [Reference Citation Analysis (0)] |
| 23. | Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1083] [Cited by in RCA: 1120] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 24. | Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10:760-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 872] [Cited by in RCA: 858] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 25. | Wang S, El-Deiry WS. Requirement of p53 targets in chemosensitization of colonic carcinoma to death ligand therapy. Proc Natl Acad Sci USA. 2003;100:15095-15100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002;1:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 456] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 27. | Kaufmann SH, Vaux DL. Alterations in the apoptotic machinery and their potential role in anticancer drug resistance. Oncogene. 2003;22:7414-7430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 489] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 29. | Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA. 2000;97:7124-7129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 908] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 30. | Degterev A, Lugovskoy A, Cardone M, Mulley B, Wagner G, Mitchison T, Yuan J. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat Cell Biol. 2001;3:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 31. | Tzung SP, Kim KM, Basañez G, Giedt CD, Simon J, Zimmerberg J, Zhang KY, Hockenbery DM. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nat Cell Biol. 2001;3:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 334] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 32. | Delbridge AR, Strasser A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015;22:1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 394] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 33. | Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3508] [Cited by in RCA: 3664] [Article Influence: 174.5] [Reference Citation Analysis (0)] |
| 34. | Yang Y, Ludwig RL, Jensen JP, Pierre SA, Medaglia MV, Davydov IV, Safiran YJ, Oberoi P, Kenten JH, Phillips AC. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell. 2005;7:547-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 248] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 35. | Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2022] [Cited by in RCA: 2067] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 36. | Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1995] [Cited by in RCA: 2021] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 37. | Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE, Housman DE, Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994;266:807-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1034] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 38. | Chin L, Andersen JN, Futreal PA. Cancer genomics: from discovery science to personalized medicine. Nat Med. 2011;17:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 398] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 39. | Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1184] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 40. | Coultas L, Strasser A. The role of the Bcl-2 protein family in cancer. Semin Cancer Biol. 2003;13:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 160] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4271] [Cited by in RCA: 4194] [Article Influence: 155.3] [Reference Citation Analysis (0)] |
| 42. | Green DR. Apoptotic pathways: ten minutes to dead. Cell. 2005;121:671-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 596] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 44. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19834] [Cited by in RCA: 19515] [Article Influence: 780.6] [Reference Citation Analysis (0)] |
| 45. | McKenzie S, Kyprianou N. Apoptosis evasion: the role of survival pathways in prostate cancer progression and therapeutic resistance. J Cell Biochem. 2006;97:18-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Pai SI, Wu GS, Ozören N, Wu L, Jen J, Sidransky D, El-Deiry WS. Rare loss-of-function mutation of a death receptor gene in head and neck cancer. Cancer Res. 1998;58:3513-3518. [PubMed] |
| 47. | Wang S, El-Deiry WS. Inducible silencing of KILLER/DR5 in vivo promotes bioluminescent colon tumor xenograft growth and confers resistance to chemotherapeutic agent 5-fluorouracil. Cancer Res. 2004;64:6666-6672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 675] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 49. | Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 921] [Cited by in RCA: 959] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 50. | Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1662] [Cited by in RCA: 1760] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 51. | Cory S, Vaux DL, Strasser A, Harris AW, Adams JM. Insights from Bcl-2 and Myc: malignancy involves abrogation of apoptosis as well as sustained proliferation. Cancer Res. 1999;59:1685s-1692s. [PubMed] |
| 52. | Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2873] [Cited by in RCA: 2942] [Article Influence: 127.9] [Reference Citation Analysis (0)] |
| 53. | Huang Z. Bcl-2 family proteins as targets for anticancer drug design. Oncogene. 2000;19:6627-6631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 54. | Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2612] [Cited by in RCA: 2716] [Article Influence: 135.8] [Reference Citation Analysis (0)] |
| 55. | Cragg MS, Harris C, Strasser A, Scott CL. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat Rev Cancer. 2009;9:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 56. | Cragg MS, Jansen ES, Cook M, Harris C, Strasser A, Scott CL. Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J Clin Invest. 2008;118:3651-3659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 57. | Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007;4:1681-1689; discussion 1690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 277] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 58. | Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5100] [Cited by in RCA: 5117] [Article Influence: 204.7] [Reference Citation Analysis (0)] |
| 59. | Finlay CA, Hinds PW, Levine AJ. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989;57:1083-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1290] [Cited by in RCA: 1370] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 60. | Baker SJ, Markowitz S, Fearon ER, Willson JK, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1206] [Cited by in RCA: 1281] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 61. | Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, Bergman J, Wiman KG, Selivanova G. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002;8:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 795] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 62. | Foster BA, Coffey HA, Morin MJ, Rastinejad F. Pharmacological rescue of mutant p53 conformation and function. Science. 1999;286:2507-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 577] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 63. | Lehmann S, Bykov VJ, Ali D, Andrén O, Cherif H, Tidefelt U, Uggla B, Yachnin J, Juliusson G, Moshfegh A. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol. 2012;30:3633-3639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 306] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 64. | Li D, Marchenko ND, Schulz R, Fischer V, Velasco-Hernandez T, Talos F, Moll UM. Functional inactivation of endogenous MDM2 and CHIP by HSP90 causes aberrant stabilization of mutant p53 in human cancer cells. Mol Cancer Res. 2011;9:577-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 238] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 65. | Yi YW, Kang HJ, Kim HJ, Kong Y, Brown ML, Bae I. Targeting mutant p53 by a SIRT1 activator YK-3-237 inhibits the proliferation of triple-negative breast cancer cells. Oncotarget. 2013;4:984-994. [PubMed] |
| 66. | Martelli AM, Tazzari PL, Tabellini G, Bortul R, Billi AM, Manzoli L, Ruggeri A, Conte R, Cocco L. A new selective AKT pharmacological inhibitor reduces resistance to chemotherapeutic drugs, TRAIL, all-trans-retinoic acid, and ionizing radiation of human leukemia cells. Leukemia. 2003;17:1794-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 67. | Thakkar H, Chen X, Tyan F, Gim S, Robinson H, Lee C, Pandey SK, Nwokorie C, Onwudiwe N, Srivastava RK. Pro-survival function of Akt/protein kinase B in prostate cancer cells. Relationship with TRAIL resistance. J Biol Chem. 2001;276:38361-38369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Kim KM, Lee YJ. Amiloride augments TRAIL-induced apoptotic death by inhibiting phosphorylation of kinases and phosphatases associated with the P13K-Akt pathway. Oncogene. 2005;24:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Bortul R, Tazzari PL, Cappellini A, Tabellini G, Billi AM, Bareggi R, Manzoli L, Cocco L, Martelli AM. Constitutively active Akt1 protects HL60 leukemia cells from TRAIL-induced apoptosis through a mechanism involving NF-kappaB activation and cFLIP(L) up-regulation. Leukemia. 2003;17:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 70. | Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3291] [Cited by in RCA: 3384] [Article Influence: 282.0] [Reference Citation Analysis (0)] |
| 71. | Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 700] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 72. | Riemenschneider MJ, Bell DW, Haber DA, Louis DN. Pulmonary adenocarcinomas with mutant epidermal growth factor receptors. N Engl J Med. 2005;352:1724-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 73. | Bell DW, Gore I, Okimoto RA, Godin-Heymann N, Sordella R, Mulloy R, Sharma SV, Brannigan BW, Mohapatra G, Settleman J. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005;37:1315-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 383] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 74. | Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3081] [Cited by in RCA: 3208] [Article Influence: 160.4] [Reference Citation Analysis (0)] |
| 75. | Morris SW, Kirstein MN, Valentine MB, Dittmer K, Shapiro DN, Look AT, Saltman DL. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1995;267:316-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 76. | Burgess DJ. Therapy: Multiple bypass problem for BRAF inhibition. Nat Rev Cancer. 2011;11:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 77. | Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1220] [Cited by in RCA: 1177] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 78. | Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, Gondi V, Hsu KT, Harari PM. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944-3956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 441] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 79. | Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov. 2011;10:507-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1265] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 80. | Conway JR, Carragher NO, Timpson P. Developments in preclinical cancer imaging: innovating the discovery of therapeutics. Nat Rev Cancer. 2014;14:314-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 81. | Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580-589. [PubMed] |
| 82. | Timpson P, McGhee EJ, Anderson KI. Imaging molecular dynamics in vivo--from cell biology to animal models. J Cell Sci. 2011;124:2877-2890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 83. | Cappella P, Gasparri F. Highly multiplexed phenotypic imaging for cell proliferation studies. J Biomol Screen. 2014;19:145-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Young DW, Bender A, Hoyt J, McWhinnie E, Chirn GW, Tao CY, Tallarico JA, Labow M, Jenkins JL, Mitchison TJ. Integrating high-content screening and ligand-target prediction to identify mechanism of action. Nat Chem Biol. 2008;4:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 85. | Beerling E, Ritsma L, Vrisekoop N, Derksen PW, van Rheenen J. Intravital microscopy: new insights into metastasis of tumors. J Cell Sci. 2011;124:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 86. | Fruhwirth GO, Fernandes LP, Weitsman G, Patel G, Kelleher M, Lawler K, Brock A, Poland SP, Matthews DR, Kéri G. How Förster resonance energy transfer imaging improves the understanding of protein interaction networks in cancer biology. Chemphyschem. 2011;12:442-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Nouar R, Devred F, Breuzard G, Peyrot V. FRET and FRAP imaging: approaches to characterise tau and stathmin interactions with microtubules in cells. Biol Cell. 2013;105:149-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Kodoyianni V. Label-free analysis of biomolecular interactions using SPR imaging. Biotechniques. 2011;50:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 89. | Lausted C, Hu Z, Hood L. Quantitative serum proteomics from surface plasmon resonance imaging. Mol Cell Proteomics. 2008;7:2464-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 90. | Lee HJ, Nedelkov D, Corn RM. Surface plasmon resonance imaging measurements of antibody arrays for the multiplexed detection of low molecular weight protein biomarkers. Anal Chem. 2006;78:6504-6510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 91. | Goodrich TT, Wark AW, Corn RM, Lee HJ. Surface plasmon resonance imaging measurements of protein interactions with biopolymer microarrays. Methods Mol Biol. 2006;328:113-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 92. | Inoue Y, Mori T, Yamanouchi G, Han X, Sonoda T, Niidome T, Katayama Y. Surface plasmon resonance imaging measurements of caspase reactions on peptide microarrays. Anal Biochem. 2008;375:147-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |