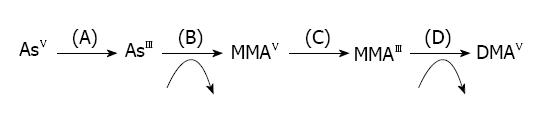Published online Aug 12, 2014. doi: 10.5528/wjtm.v3.i2.96
Revised: June 21, 2014
Accepted: July 17, 2014
Published online: August 12, 2014
Processing time: 113 Days and 20.6 Hours
Health hazards due to the consumption of heavy metals such as arsenic have become a worldwide problem. Metabolism of arsenic produces various intermediates which are more toxic and cause toxicity. Arsenic exposure results in impairment of glucose metabolism, insulin secretion in pancreatic β-cells, altered gene expressions and signal transduction, and affects insulin-stimulated glucose uptake in adipocytes or skeletal muscle cells. Arsenic toxicity causes abnormalities in glucose metabolism through an increase in oxidative stress. Arsenic interferes with the sulfhydryl groups and phosphate groups present in various enzymes involved in glucose metabolism including pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, and contributes to their impairment. Arsenic inhibits glucose transporters present in the cell membrane, alters expression of genes involved in glucose metabolism, transcription factors and inflammatory cytokines which stimulate oxidative stress. Some theories suggest that arsenic exposure under diabetic conditions inhibits hyperglycemia. However, the exact mechanism behind the behavior of arsenic as an antagonist or synergist on glucose homeostasis and insulin secretion is not yet fully understood. The present review delineates the relationship between arsenic and the biochemical basis of its relationship to glucose metabolism. This review also addresses potential therapeutic and nutritional interventions for attenuating arsenic toxicity. Several other potential nutritional supplements are highlighted in the review that could be used to combat arsenic toxicity.
Core tip: This review illustrated the interference caused by arsenic in enzymes, genes and transcription factors involved in glucose metabolism and possible nutritional aspects for attenuating arsenic toxicity.
- Citation: Kulshrestha A, Jarouliya U, Prasad G, Flora S, Bisen PS. Arsenic-induced abnormalities in glucose metabolism: Biochemical basis and potential therapeutic and nutritional interventions. World J Transl Med 2014; 3(2): 96-111
- URL: https://www.wjgnet.com/2220-6132/full/v3/i2/96.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v3.i2.96
Arsenic is a toxic heavy metal and belongs to the 5th group in the periodic table. It is present in both inorganic and organic forms in different surroundings and its level is increased by anthropogenic contamination[1]. It is a ubiquitous element and is found in four oxidation states -3, 0, +3, and +5. It is an environmental contaminant of worldwide concern due to its high toxicity and presence in groundwater aquifers. Arsenic contamination in water has been found in countries such as Canada, India, Bangladesh, United States, China, Taiwan, Mexico, Poland, Japan, Nepal[2] and Iran[3]. Inorganic arsenic is believed to be the major form of arsenic in water, soil and various foods[4] and is said to be a group I carcinogen based on clinical studies[5].
Flora reported that the major exposure route of inorganic arsenic (iAs) is by contaminated drinking water in India, Bangladesh, China and American countries. Argentina (200 ppb), Mexico (400 ppb), Taiwan (50-1980 ppb), and the Indo-Bangladesh region (800 ppb) are countries where arsenic concentration in drinking water is reported to be beyond WHO guidelines maximum permissible value (10 ppb)[6].
Epidemiological studies in various regions of the world with high levels of arsenic in groundwater have associated arsenic exposure with increased risks of different types of cancer (skin, liver, kidney and lung), arteriosclerosis and cardiovascular diseases, diabetes, hypertension and neurological diseases (Alzheimer and Parkinson)[7-13]. Arsenic stimulates alterations in oxidative stress, cell calcium signaling, impairment of cell mitochondrial function and affects cell cycle progression[14-17]. Some of these toxic effects at cellular and molecular levels ultimately lead to cancer[18]. Although arsenic induces adverse health effects, all exposed humans do not develop arsenic symptoms related to exposure, suggesting that genetic susceptibility is also an important aspect involved in the human response to arsenic exposure.
Metabolism of arsenic takes place in the liver where the first step is methylation. The presence of monomethylarsenic acid (MMAV) and dimethylarsenic acid (DMAV) indicates the methylation of arsenic in bile and urine. Monomethylarsenic acid is comparatively more toxic than dimethylarsenic acid[19]. It was previously suggested that arsenic metabolism was a detoxification procedure, but now it is reported that intermediates of arsenic metabolism generate more toxicity. Absorbed arsenic undergoes biomethylation to form MMAV and DMAV (urinary excretion products) and are more toxic than iAs[20]. Pentavalent arsenic (iAsV) is quickly reduced to trivalent arsenic (iAsIII) and is then enzymatically methylated in humans and animals, which is then excreted via urine in the form of the dimethylated metabolite DMAV[21-24]. Methylation of arsenic requires S-adenosylmethionine as the methyl donor and glutathione sulfhydryl as a vital co-factor[25] (Figure 1).
Along with the major metabolite, DMAv, dimethylmonothioarsenic acid (DMMTAv), a thiolated metabolite, is also found in urine as a minor metabolite[26-29]. In addition, DMMTAv and dimethyldithioarsenic acid (DMDTAv) are found in organs in vivo and in vitro[30-32]. Moreover, iAs consumed by marine organisms is converted into arsenosugars and arsenobetaines and their thiolated metabolites are recognized as minor marine arsenic metabolites[33-36]. Arsenic is ingested as arsenate or arsenite, is altered into the dimethylated form for excretion, and inorganic arsenicals and their metabolite viz., DMA. Among these arsenic metabolites, DMDTAV and DMMTAV are the current arsenic metabolites observed in urine and organs in man and animals[26-29,31,32]. It has been suggested that DMMTAV is simply absorbed by organs/tissues and is more toxic in nature[37]. DMMTAV is absorbed efficiently by organs in a different way to that of DMDTAV, although DMMTAV and DMDTAV are both thioarsenicals. In addition, the distribution and metabolism of DMMTAV are similar to DMAIII in hamsters, while the distribution and metabolism of DMDTAV are similar to those of DMAV[38].
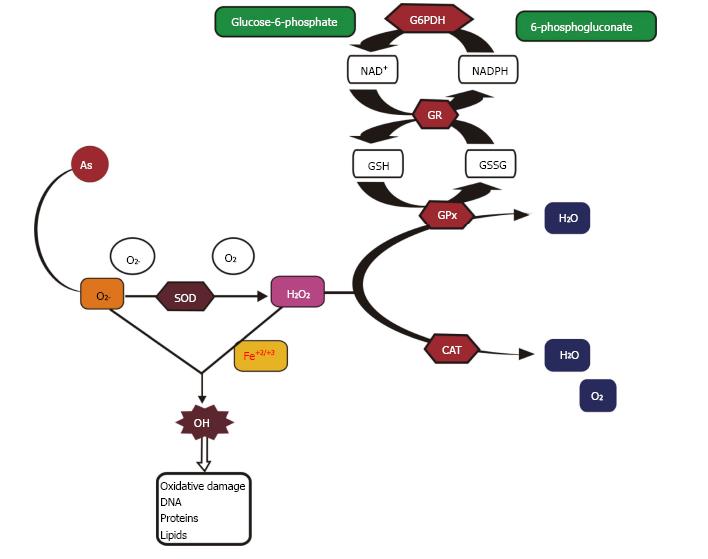
Arsenic causes toxicity via oxidative stress by affecting the antioxidant enzymes[6,39]. It stimulates the production of reactive oxygen species (ROS) which results in the induction of adverse health effects[20,40]. The mitochondrion is the chief site of ROS generation in cells and enhanced ROS formation is due to the abnormal function of electron transfer through the respiratory chain in mitochondria which in turn results in the production of hydrogen peroxide (H2O2), superoxide anion (O2.-) and hydroxyl radicals (OH.)[41]. Furthermore, in the electron transport chain, complexes I and III are the major leak sites for ROS formation, as some of the electrons passing through the mitochondrial respiratory chain leak out to molecular oxygen (O2) to form superoxide radicals and then dismutate to H2O2. Increased ROS causes cellular and metabolic impairment through oxidative damage, which results in physiological abnormalities and deleterious chronic disorders. H2O2 is produced during the oxidation of As(III) to As(V) when intermediary arsine species are formed such as dimethylarsinic radicals [(CH3)2As•] and dimethylarsinic peroxyl [(CH3)2AsOO•] involving O2•−[42]. Arsenic leads to an increase in consumption of oxygen by cells, which results in ROS production and hence an increase in oxidative stress[43]. Hepatic and renal heme oxygenase isoform-1 (HO-1) are also involved in the production of ROS by iAs which in turn results in extra free iron and biliverdin formation[44]. This free iron participates in the Fenton reaction resulting in the formation of hydroxyl free radical (•OH) which attacks DNA[45].
ROS produced intracellularly at the time of physiological processes, regulate cell functions, for instance endocytic pathways, autophagy, gene expression, intracellular Ca2+, glucose homeostasis, hypoxic and inflammatory responses[46-49]. ROS function as second messengers due to stimulation/suppression of numerous signaling features by the oxidation of sulfhydryl groups and by changing the intracellular redox status, therefore inducing cell signaling pathways, downstream gene expression and cell reproduction or death[13,20]. The signaling molecules affected include protein tyrosine kinases and phosphatases, protein serine/threonine kinases and phosphatases, small G proteins, lipid signaling, Ca2+ signaling and transcription factors[50]. Biochemical reactions such as glycation results in the formation of advanced glycation end-products (AGEs) and protein oxidation causes alterations in cells which in turn results in the formation of disulfides between cysteine and methionine residues, cyclization of polyunsaturated fatty acid residues of phospholipids forming malondialdehyde (MDA), lipid peroxidation, 4-hydroxy-2-nonenal (HNE) and nucleic acid oxidation[7,8,51,52]. Free radicals produced during iAs metabolism are the source of oxidative stress[45]. Low concentrations of MMAIII and DMAIII are cytotoxic in human and rat skin, bladder, lung cells and human hepatocytes[53-56]. Cellular offense in response to methylated metabolites is involved in genotoxicity with strong proof of oxidative stress as a causal factor. Genotoxicity of MMAIII and DMAIII can be reversed by ROS inhibitors[57]. Moreover, methylated metabolites mainly DMAIII and trimethylarsenic oxide (TMAO), also play a role in arsenic-induced genotoxicity[58]. Cells having low methylation capabilities are more prone to cyotoxicity by arsenic specifying that other mechanisms are also employed in cytotoxicity induced by arsenic. An in vitro study on mammalian cell lines showed that there was no clear link between arsenic methylation capability by cells and resulting cytotoxicity induced by sodium arsenite[59]. The possible mechanism of arsenic toxicity is depicted in Figure 2.
Diabetes mellitus is one of the world’s oldest known diseases. Type 2 diabetes mellitus (T2DM) is a widespread global metabolic disorder, distinguished by the unusual metabolism of carbohydrates and lipids, mainly resulting either from a fault in insulin secretion and/or insulin action, or adipocyte functioning[60]. In T2DM, the entire body glucose homeostasis is disrupted due to insulin resistance and impaired glucose uptake by peripheral tissues, consisting of skeletal muscle and adipose tissue. In these tissues, glucose homeostasis is regulated by a mechanism involving insulin-dependent stimulation of glucose uptake.
The worldwide incidence of diabetes among people aged 20-79 years was approximately 6.4% in 2010. This rate is supposed to rise to 70% in developing countries and 20% in developed countries from 2010 to 2030[61]. Globally, more than 0.39 million people die every year from diabetes which is due to increase in the next decade[62,63]. T2DM is more prevalent than type 1 diabetes mellitus. In India, the World Health Organization (WHO) reported that about 32 million people suffered from diabetes in 2000. According to the International Diabetes Federation (IDF), the total number of diabetic patients is nearly 40.9 million which is supposed to increase to 69.9 million in 2025[64]. Environmental and lifestyle factors are the main causes of this remarkable increase in T2DM prevalence[65,66].
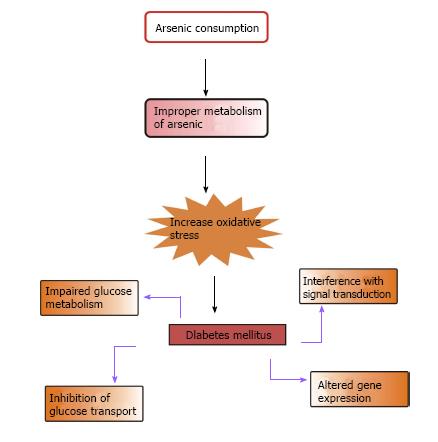
Epidemiological studies suggest that T2DM is one of the most familiar non-cancerous metabolic disorders correlated with chronic exposure to iAs. Lai et al[67] in 1994 first established the link between diabetes and iAs. The correlation between arsenic toxicity and diabetes mellitus is a burning issue. Increased prevalence of T2DM is associated with the use of drinking water containing high levels of iAs and chronic occupational exposure to iAs[68-75]. This is more prevalent in people consuming contaminated water in Bangladesh and Taiwan and in those working in copper smelters and the art glass industry in Sweden[67,70-72,74-76]. According to the American Diabetes Association, diabetes due to arsenic toxicity or arsenic-induced diabetes may be classified under “the other specific types”[77]. In epidemiologic studies, arsenic exposed subjects showed symptoms of diabetes mellitus similar to T2DM[70]. As the symptoms were almost identical to those of T2DM, it is considered that the pathophysiology of diabetes mellitus induced by arsenic is more likely to be similar to that of T2DM[6]. According to Wang et al[78], there is a relationship between increased risk of metabolic syndrome, one of the most important cardiovascular disease risk factors and exposure to iAs in the general population[78]. Figure 3 shows the possible way by which arsenic causes diabetes mellitus.
Arsenate (AsV) replaces the phosphate group in various biochemical reactions owing to their similar structure and properties[79]. Arsenate reacts in vitro with glucose and gluconate[80,81] to form glucose-6-arsenate and 6-arsenogluconate, respectively, which are corresponding similar to glucose-6-phosphate and 6-phosphogluconate. Arsenate also replaces the phosphate group in the sodium pump and anion exchange transport system of human erythrocytes[82]. Arsenate inhibits ATP formation during glycolysis by substituting arsenate for the phosphate anion in a process known as arsenolysis. In one of the steps of glycolysis, the phosphate group is enzymatically linked to D-gylceraldehyde-3-phosphate to form 1,3-diphospho-D-glycerate. In this reaction, phosphate is replaced by arsenate to form an unstable anhydride, 1-arsenato-3-phospho-D-glycerate, and hydrolyzes into arsenate and 3-phosphoglycerate. The instability of arsenic anhydride is due to the longer As-O bond length compared with the P-O bond length[79]. ATP is not generated during glycolysis in the presence of arsenate[83,84]. At the mitochondrial level, arsenolysis may occur during oxidative phosphorylation in the presence of succinate to form adenosine-5’-diphosphate (ADP) arsenate[81]. ADP-phosphate formed during oxidative phosphorylation is difficult to hydrolyze in comparison to ADP-arsenate. During the process of cellular respiration, arsenolysis diminished ATP production by substituting phosphate with arsenate in respiratory pathways. An in vitro study suggested that arsenate exposure caused a reduction in ATP in rabbit and human erythrocytes[85,86]. The activity of hexokinase is inhibited at higher concentrations of arsenate[87]. In contrast, the two pentavalent forms of methylated metabolites, monomethylarsonate and dimethylarsonate do not disturb the metabolism of phosphate or bind to sulfhydryl groups[88].
Arsenic affinity for thiols, especially the vicinal thiols of enzymes, is an accepted mechanism for arsenic toxicity, thereby inhibits catalytic activity of an enzyme by binding to a thiol-containing active site[84]. Trivalent arsenicals easily react in vitro with molecules having a sulfhydryl group, for instance cysteine and reduced glutathione (GSH)[85]. The complex linking vicinal thiols and arsenic is generally strong. Three main pathways by which arsenic decreases cellular GSH level have been suggested: (1) In the reduction of arsenates to arsenites, GSH functions as an electron donor; (2) Arsenite has a strong affinity for GSH; and (3) Arsenic-induced free radicals oxidize GSH.
As a consequence of obstruction of the Kreb’s cycle and disruption of oxidative phosphorylation by arsenic, a reduction in cellular ATP followed by cell death occur. Due to the interaction of arsenic with thiol groups, methylated trivalent arsenicals such as MMAIII inhibits GSH reductase and thioredoxin reductase[89,90]. Cellular redox conditions are modified by the activities of methylated arsenicals, which in turn results in cytotoxicity. GSH protects cells from cytotoxins and is also involved in the metabolism of arsenic, through the formation of GSH conjugates. Numerous proteins with regulatory functions such as nuclear factor kappa B (NFκB) and adiponectin (AP)-1 are susceptible to cellular redox conditions. These proteins are regulated by GSH by altering the redox state of particular sulfhydryl groups of target proteins including stress kinases, transcription factors and caspases[91].
Insulin-independent diabetes is the common form of diabetes mellitus among people chronically exposed to iAs[71]. It has been suggested that at the cellular level, iAs or its metabolites disturb glucose metabolism and insulin signaling. Trivalent arsenicals are moderately effective inhibitors of numerous enzymes involved in glucose metabolism such as succinyl Co-A synthase, α-ketoglutarate dehydrogenase and pyruvate dehydrogenase (PDH)[92,93]. The PDH complex is the most studied enzyme and is considered most sensitive to inhibition by arsenite. During cell respiration, pyruvate is converted into acetyl-CoA in the presence of the pyruvate dehydrogenase enzyme complex (dihydrolipoyl transacetylase, dihydrolipoyl dehydrogenase, pyruvate decarboxylase, thiamine pyrophosphate, lipoic acid, CoASH, FAD, NAD+). Arsenite inhibits PDH by binding to the lipoic acid moiety[94]. It has been reported that MMAIII is a stronger inhibitor of PDH than arsenite[93]. The Kreb’s cycle provides reducing power in the electron transport chain for ATP generation. Inhibition of PDH leads to decreased generation of ATP and energy resulting in cell damage and cell death.
In addition, an organic derivative of arsenite, phenylarsine oxide (PAO) inhibits basal or insulin stimulated glucose uptake by canine kidney cells, adipocytes and intact skeletal muscle[95-100]. Arsenic interferes with sulfhydryl-containing enzymes such as pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, competes with the phosphate binding sites on glycolytic enzymes, uncouples oxidative phosphorylation and impairs glucose metabolism[101,102]. Arsenic interferes with phosphate binding sites in ATP resulting in the formation of ADP-arsenate which inhibits metabolic pathways which require ATP. Glucose-6-phosphate is an essential mediator for glycolysis, glycogenesis, gluconeogenesis and glycogenolysis, and the pentose phosphate pathway (PPP). The PPP generates nicotinamide adenine dinucleotide phosphate (NADPH), an essential cofactor for glutathione reduction. Insufficient production of NADPH from the PPP further interrupts the cell’s ability to deal with oxidative stress[103]. Glucose-6-phosphate dehydrogenase (G6PDH) activity in mice exposed to arsenic, was significantly reduced in a time-related manner[104]. G6PDH is an enzyme of the PPP, an alternate metabolic pathway for glucose. Reduced blood activity of G6PDH can lead to oxidative stress-induced diabetes and diminished nitric oxide generation[105,106]. Exposure to arsenic also results in an increase in glycosylated hemoglobin level which indicates high blood glucose level and was reported in Danish people working in the wood industry[73]. Thus, individuals exposed to iAs both from the environment and occupationally exposed are prone to diabetes mellitus.
Signal transduction pathways activated by insulin which result in glucose uptake have been widely studied. This consists of binding of the insulin molecule to the α-subunit of the insulin receptor followed by activation of the tyrosine kinase moiety leading to autophosphorylation of the β-subunit of the insulin receptor, and consequent phosphorylation of insulin receptor substrate 1 or 2, phosphorylation and activation of phosphatidylinositol 3-kinase, and phosphorylation of phosphatidylinositol-4,5-biphosphate at the cell membrane to phosphatidylinositol-3,4,5-triphosphate (PIP3)[107-109]. PIP3 promotes phosphorylation of protein kinase B (PKB/AKt) and protein kinase C (PKC) enzymes that is, PKC λ and PKC ζ[110,111]. The phosphorylation of PKB/AKT results in the transport of GLUT4 from the perinuclear space to the plasmalemma and the activation of glucose uptake[112,113].
iAsIII and methylated arsenicals interfere with the main signal transduction pathways in human cells[114]. Arsenic exposed cells show inhibition of the expression or activation of PKB/AKT, which is an essential component of the insulin stimulated signal transduction pathway. Thus, insulin-dependent signal transduction at the PKB/AKT level is inhibited, which is responsible for hyperglycemia in humans exposed to iAs. An in vitro study showed that iAsIII interrupts the expression and phosphorylation of PKB/AKT and inhibits insulin-stimulated glucose uptake and mobilization of GLUT4[115]. Arsenic is also involved in the modulation of the mitogen-activated protein kinases (MAPK) pathway and related growth factors[114,116]. The MAPK signaling pathway regulates stepwise phosphorylation of protein kinases and terminates the activation of transcription factors needed for cellular proliferation, differentiation or apoptosis.
Sulfhydryl groups play an essential role in insulin-dependent and insulin-independent mediated glucose transport (GLUT). The thiol component forms a structural bond linking the A and B polypeptide chains of insulin, the α and β subunits of the insulin receptor and the exofacial sulfhydryl moiety present on glucose transporters at the plasma membrane[6]. PAO forms stable cyclic thio-arsenite complexes with vicinal or paired sulfhydryl groups of cellular proteins and inhibits glucose transport in adipocytes[95,117]. Moreover, PAO prevents insulin-stimulated glucose transport without affecting insulin binding to its receptor[117]. PAO only affects insulin-dependent GLUT4 present in adipocytes and myocytes[118].
However, the effect of arsenite on glucose transport is dose-dependent. Studies have shown that arsenite stimulates glucose uptake at higher concentrations, while at low level, glucose uptake decreases[119]. Arsenite does not disturb regulation of GLUT4 gene expression, thus overall GLUT4 quantity does not alter. Walton et al[115] examined the dose-dependent decrease in insulin-stimulated glucose uptake in 3T3-L1 adipocytes treated with iAs and its metabolites.
There are many studies which support that diabetes is induced by arsenic via alteration in gene expression. When isolated rat pancreatic β-cells were exposed to 5 μmol/L arsenite for 72 h, mRNA expression and insulin secretion decreased[9]. An in vitro study showed that exposure to arsenite decreased the gene expression and activity of catalase, whereas the production of ROS increased[120]. Peroxisome proliferative-activated receptor-γ (PPAR-γ) (a transcription factor) controls the main gene expression for insulin sensitivity. When mouse adipocytes from the C3H 10T1/2 cell line were exposed to 6 μmol/L arsenite, alteration in the expression of PPAR-γ and AP-2 genes occurred which resulted in the inhibition of mRNA and reversal of adipocyte differentiation[121]. The transcription of cytokines, namely tumor necrosis factor-α (TNF-α) and interleukins (IL), required in insulin resistance, are regulated by NF-κB[122]. When human bronchial epithelial cell lines were exposed to 18 μmol/L arsenite for 12 h, NF-κB dependent genes were activated. In contrast, exposure to 12.5 μmol/L arsenic in TNF-α stimulated HeLa cells for 2 h resulted in inhibition of NF-κB activation and IκB degradation[123,124]. When human GM847 fibroblast cells were exposed to 0.1 and 5 μmol/L arsenite for 24 h, upregulation and expression of c-fos and c-jun genes and DNA binding activity of AP-1 takes place[125].
Arsenic upregulates inflammatory cytokines from mononuclear cells. When human peripheral mononuclear cells were exposed to very low arsenite levels, TNF-α production increased 2-fold[126]. Studies have shown that when blood arsenic level ranged from 0.128 to 0.62 μmol/L, the expression of IL-6 increased 3-fold[127]. Expression and phosphorylation of AKT were suppressed when 3T3-L1 adipocytes were exposed to trivalent arsenicals[115]. Activation of AKT by PDK-1 phosphorylation, is also inhibited by arsenite[128]. In adipocyte cells, exposure to arsenite at high levels reduced the expression and phosphorylation of AKT genes, while at low levels expression was stimulated[115,129]. In addition, the expression of phosphoenol pyruvate carboxykinase (PEPCK) increased in chick embryos after exposure to high dose arsenite, which may be due to the interaction of arsenic with glucocorticoid receptor complexes[130,131].
T2DM is hypothetically related to variations in blood arsenic concentration and there is evidence to suggest that arsenic metabolism is modified in people with T2DM and these factors have precise roles in the pathogenesis and progression of this disorder[132]. Many in vivo and in vitro studies have shown that iAs induces diabetes, but some experiments contradict these reports. There are convincing reports that diabetes alters the pharmacodynamics and pharmacokinetics of drugs/xenobiotics in humans and animals[133,134]. Our previous studies showed that arsenic exposure causes the inhibition of hyperglycemia in diabetic rats and mice (Kulshrestha et al, Unpublished observation). Arsenic exposure in diabetic rat results in the promotion of insulin secretion and decrement of arsenic concentrations[135,136].
The causal correlation between arsenic exposure and diabetes mellitus is still debated. Various epidemiological studies performed in arsenic-contaminated regions proved the relationship between chronic arsenic exposure and diabetes mellitus, however, the exact mechanism is not known[137]. Various animal studies on the effects of exposure to arsenic on glucose metabolism and insulin secretion show inconsistent results due to variations in animal species, dose and time of exposure[128,137]. The studies carried out by Wang et al[138] in both humans and rats suggested that glucose metabolism is altered by arsenic.
Hsueh et al[139,140] suggested that arsenic toxicity is associated with nutritional status in residents living in arsenic-contaminated areas such as Taiwan. As arsenic causes toxicity via oxidative stress, thereby decreasing antioxidant enzyme activity, it is possible that there is a link between arsenic-induced diabetes mellitus and antioxidant deficiency, and that the individual consuming less antioxidants has an increased risk of diabetes mellitus and cardiovascular disease[141]. Thus, good nutritional status with sufficient antioxidant intake reduces the chance of arsenic-induced diseases. As arsenic interferes with GSH, people with diabetes have a lower level of GSH[142]. It was found that selenium, which is required for GSH biosynthesis, is significantly lower in arsenic exposed subjects than in normal controls[143,144]. GSH is required for the correct action of insulin and increased uptake of glucose, and is obligatory for the excretion of arsenic[145]. Animal studies have shown that nutritional status modifies arsenic toxicity. A choline or methionine deficient diet (source of methyl donor group) results in a reduction in arsenic methylation, which leads to high retention of arsenic in the body and an increase in toxicity[146-148]. Therefore, by consuming methionine rich diets, arsenic toxicity can be alleviated.
Preventive and therapeutic measures are available against arsenic toxicity. The roles of chelating agents, antioxidants, natural/herbal remedies as protective/therapeutic agents against arsenic toxicity are discussed below.
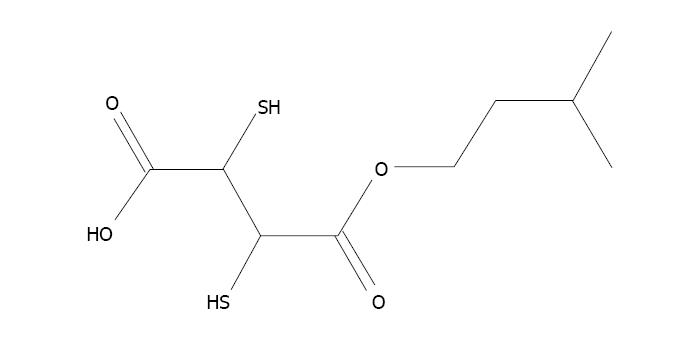
The formation of a metal ion complex is known as chelation in which two or more separate coordinate bonds are formed between monodentate or polydentate ligands and metal ions. These ligands are referred to as chelators or chelating agents and are organic compounds able to link together metal ions to form a complex structure called chelates. In chelation therapy, chelating agents are used to detoxify toxic heavy metals such as arsenic, and convert them to a chemically inert form with greater water solubility, which increases their excretion by the kidney without further interaction within the body. Various chelating agents are used to treat arsenic toxicity[149]. The first chelating agent used was British Anti Lewisite (BAL) which was used during World War II. This is a dithiol compound used as a therapeutic agent against heavy metal toxicity. Despite its capacity to treat metal toxicity, its use is limited due to a low therapeutic index[150]. Other metal chelators such as meso-2,3-dimercaptosuccinic acid (DMSA) and 2,3-dimercapto-1-propanesulphonic acid (DMPS) can be administered for a much longer time due to their very low toxicity[151]. DMSA decreases the arsenic burden in cells by inhibiting the constant formation of ROS[152]. Subsequently, numerous esters of DMSA have been produced to achieve more advantageous chelation. Flora et al[153] found that administration of dimethyl DMSA (DMDMSA), diethyl DMSA (DEDMSA), diisoamyl DMSA and diisopropyl DMSA (DiPDMSA) led to a decrease in arsenic content in blood and soft tissues, but was less effective in recovering biochemical alterations following sub-chronic arsenic exposure in rats[153]. Kreppel et al[154] observed that administration of the monoesters, mono isoamyl DMSA (MiADMSA), mono n-amyl DMSA (MnDMSA), mono n-butyl DMSA (MnBDMSA) and mono i-butyl DMSA (MiBDMSA) were able to reduce the arsenic concentration in tissues, of which MiADMSA and MnADMSA were found to be most effective in mice[154]. Administration of MiADMSA (Figure 4) and mono methyl DMSA (MmDMSA) (Figure 5) resulted in a reduction in arsenic concentration in blood and soft tissues in experimental animals[155,156]. Despite the beneficial effects of chelating agents against arsenic toxicity, they have some drawbacks, such as non-specificity, low therapeutic index, failure to permeate the plasma membrane and metal redeployment, and induce side effects including headache, nausea, and vomiting, thus their use has been limited[157]. Although these chelating agents enhance arsenic excretion, these agents have numerous drawbacks. Therefore, the identification of novel therapies without side-effects and complete medical recovery in terms of altered biochemical variables such as complete removal of metals are necessary.
Arsenic exposure results in the production of ROS, thus cellular antioxidants are reduced. To prevent the increased production of ROS and their deleterious effects, the body’s antioxidant system which consists of superoxide dismutase (SOD), glutathione reductase (GR), catalase, glutathione peroxidase (GPx), and reduced glutathione (GSH) scavenge ROS. In addition to this endogenous system, antioxidant status is improved by the administration of exogenous antioxidants such as vitamin C and E, quercetin, N-acetylcysteine (NAC), and α-lipoic acid.
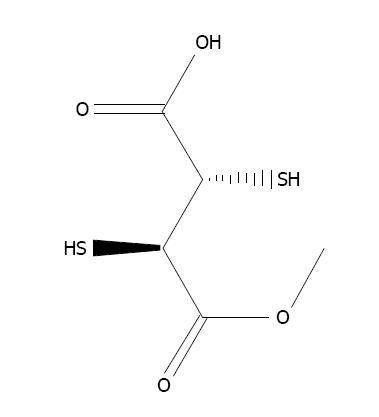
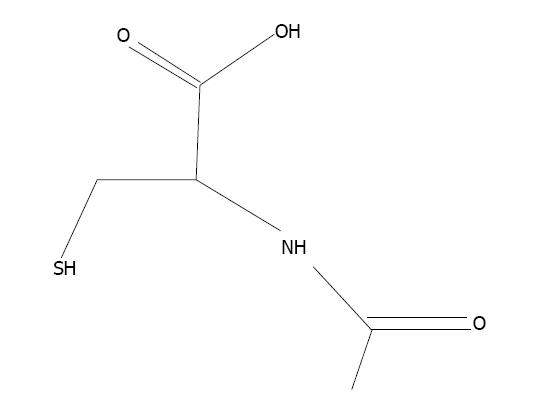
N-acetylcysteine (NAC), the thiol-based antioxidant, is an originator of L-cysteine and GSH and stimulates glutathione synthesis (Figure 6). It protects cellular components against oxidative stress[6,158]. It stimulates the production of GSH, hence retaining intracellular GSH level[159]. NAC plays an essential role in the chelation of toxic metals[160,161]. Co-administration of NAC and zinc alleviates arsenic-induced hepatic and renal toxicity[162]. Flora et al[163] developed a new treatment strategy consisting of combination therapy with DMSA and NAC, to achieve better results against arsenic toxicity in rats. NAC is effective against arsenic toxicity and recovered the level of hepatic malondialdehyde[164]. The protective effect of NAC against arsenic toxicity in animals has been suggested by Hemalatha et al[165] and Reddy et al[158].
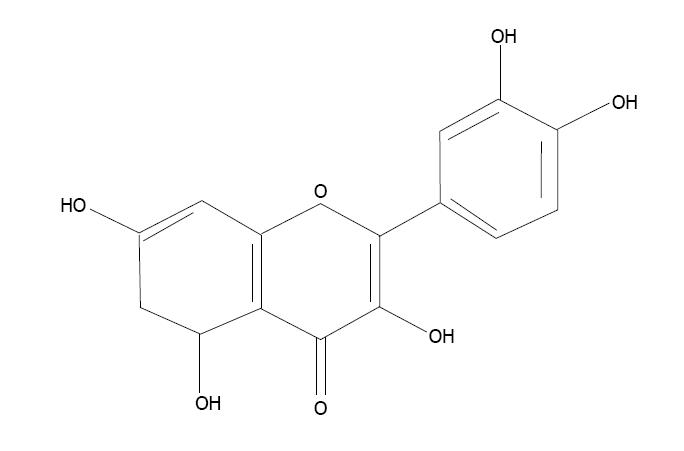
Quercetin (3,3’,4’,5,7-pentahydroxyflavon) is a bioflavonoid found in fruits, vegetables, seeds and flowers (Figure 7). It has very strong antioxidant properties and prevents cell apoptosis caused by oxidative stress[166]. Quercetin scavenges superoxide radicals and protects against lipid peroxidation and chelates metal ions. An in vitro study showed that quercetin prevented cytotoxicity due to low-density lipoproteins[167]. Quercetin co-administration with a thiol chelator was found to be more efficient in reducing body arsenic burden[168].
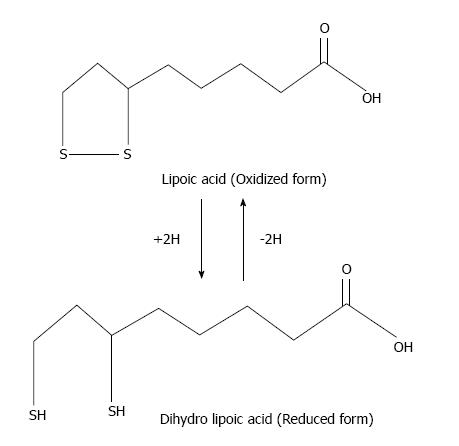
α-lipoic acid (LA, 1,2-dithiolane-3-pentanoic acid) is a dithiol antioxidant produced from octanoic acid in the mitochondria. LA is an essential cofactor for α-ketoacid dehydrogenase in mitochondria. In addition to production, LA is also consumed in the diet from wheat germ, beer, yeast, and red meat[169]. After consumption, it is taken up into the circulatory system and traverses the blood-brain barrier, where it is reduced to dihydrolipoate[170]. LA and dihydrolipoic acid (DHLA) are able to scavenge free radicals and chelate metals (Figure 8). LA treatment reduces arsenic-induced oxidative damage in vivo due to its chelation and free radical scavenging properties[171,172].
A range of vitamins possess antioxidant properties against arsenic poisoning. The consumption of vitamins A, C and E plays a protective role against arsenic toxicity[173]. Vitamin C is hydrophilic and is an intracellular and extracellular antioxidant capable of scavenging ROS in vivo and in vitro by electron transfer to inhibit lipid peroxidation. It traps free radicals and protects biomembranes from oxidative damage. Vitamin C alleviates arsenic-induced oxidative stress in mouse liver[174]. Its advantageous effect is due to its capability to form a complex with arsenic[6].
Vitamin E is a lipid soluble vitamin and its active form is α-tocopherol. It is assembled in lipophilic sites of the cell membrane and protects the membrane against oxidative damage. It donates an electron to the peroxyl radical, which is produced during lipid peroxidation[175]. Vitamin E has the ability to scavenge free radicals, hence protects against arsenic toxicity. An in vivo study showed that vitamin E treatment is effective against hepatotoxicity, nephrotoxicity and regulates altered variables of the heme synthesis pathway[6].
Co-administration of vitamin C and E in combination with a chelator was found to be more effective than chelator alone in sub-chronically arsenic-exposed rats[39]. Administration of vitamin C and E reduced the rate of DNA fragmentation in arsenic exposed rats[176]. Some other vitamins such as A and B have also been reported to be effective against arsenic poisoning. Therapy with folic acid and vitamin B12 alleviated oxidative damage induced by arsenic in cardiac tissue[177]. A cross-sectional study performed in Bangladesh, reported that the intake of B-vitamins and antioxidants may reduce the risk of arsenic-related skin lesions[178]. Other antioxidants such as taurine can be very useful in reducing oxidative stress induced by arsenic[179].
For several years, herbal/natural remedies have been used all over the world as therapeutic and prophylactic agents. The synergistic action of a broad range of antioxidants from natural sources is better than the activity of a single or synthetic antioxidant[180]. The use of conventional remedies, obtained from plants has been very important in managing arsenic toxicity. Numerous plants/spices or their extracts possess antioxidant effects. The administration of various plant/spice extracts, such as Spirulina, Curcumin, Moringa oleifera, Hippophae rhamnoides, Centella asiatica, Allium sativum, Mentha piperita, and Aloe vera barbadensis, has shown preventive and therapeutic effects against arsenic exposure in animals[179,181].
With regard to natural and bio-available sources of antioxidants, we explored the beneficial effects of Spirulina in arsenic exposed diabetic rats. Spirulina administration was found to be associated with the alleviation of various metabolic disorders such as diabetes mellitus and drug-metal-induced toxicities[181-183]. Our studies on arsenic toxicity showed that the administration of Spirulina suspension for one week resulted in a reduction in arsenic burden, restoration of blood glucose and insulin level in rats (Kulshrestha et al; Unpublished observations). Spirulina has significant antioxidant activity due to the presence of an enormous amount of phycobiliproteins, phycocyanin and allophycocyanin, phenolic compounds, γ-linoleic acid, minerals, tocopherols, β-carotenes, vitamin E & C and selenium[184,185]. Spirulina possesses free radical scavenging properties in addition to its biosorption effect against heavy metal toxicity[186-189]. Rahman et al[190] and Karkos et al[191] studied the efficacy of Spirulina in patients with chronic arsenicosis and found that Spirulina reversed the changes caused by arsenic[190,191].
Centella or Indian Pennywort, Centella asiatica (L.) Urban Syn. and Hydrocotyle asiatica L. belong to the family Apiaceae. C. asiatica is useful for restoring biochemical alterations in arsenic-induced toxicity. It depletes tissue arsenic concentrations, to some extent, in rats[192]. Sea buckthorn (Hippophae rhamnoides L.) Elaeagnaceae, is a nitrogen fixing shrub found in Europe and Asia. It is now cultivated in various parts of the world for nutritional and remedial purposes. The whole plant is an excellent source of various bioactive compounds such as carotenoids (α, β, δ-carotene, lycopene), vitamins (A, C, E, K, riboflavin, folic acid), organic acids (malic acid, oxalic acid), phytosterols (ergosterol, stigmasterol, lanosterol, amyrins), and a few vital amino acids[193-195]. In vitro and in vivo studies have shown the antioxidant and immunomodulatory properties of Sea buckthorn[196]. The antioxidant activities of H. rhamnoides extract is due to the presence of flavanoids and phenolic compounds which exhibit free radical scavenging properties[197]. Gupta and Flora evaluated the protective role of the aqueous extract of H. rhamnoides fruit against arsenic toxicity. However, this extract does not have the ability to chelate arsenic, and it is recommended that it should be administered along with an effective chelating agent to achieve the best possible outcome in chelation treatment[198].
Garlic, Allium sativum L. belongs to the family Alliaceae, and contains a high concentration of sulfur compounds. Some biologically active sulfur-containing lipophilic compounds are allicin (diallyl thiosulfinate or diallyl disulfide, DADS), S-allylycysteine (SAC), and diallylsulfide (DAS) and hydrophilic compounds include s-ethyl cysteine (SEC) and N-acetylcysteine (NAC), which are responsible for antioxidant activities due to the stimulation and modification of enzymes such as 3-hydroxy-3 methylglutaryl-CoA reductase, glutathione-s-transferase and catalase[199,200]. In vitro and in vivo studies demonstrated that administration of the aqueous extract of garlic resulted in the reduction of tissue arsenic burden and enhanced urinary arsenic excretion, which was due to the chelating properties of thiosulfur components such as allicin[165,201].
Moringa oleifera is another plant belonging to the Moringaceae family, which exhibits antioxidant and chelating properties. The seed powder of M. oleifera protected animals from arsenic-induced oxidative damage and reduced arsenic concentrations[202]. This protection may be due to the presence of ascorbic acid, and cysteine and methionine rich proteins in the seed powder[203,204]. Curcumin, a polyphenolic compound, is another herbal product which is a major constituent of Curcuma longa (Zingiberacea family). It possesses numerous pharmacological activities including antioxidant and anti-inflammatory. Curcumin protects the hepatic tissues from arsenic-induced imbalance in antioxidants and oxidants. It also reduces hepatic arsenic burden[205]. Curcumin prevents arsenic-induced neurotoxicity and hepatotoxicity during embryonic development[206,207]. Other herbal products including Mentha piperita leaf extract and Aloe vera barbadensis also showed protective effects against arsenic toxicity[208,209].
Arsenic is omnipresent in the environment, however, drinking water, including both groundwater and surface water supplies, is regarded as a major route of human exposure to iAs in arsenic-contaminated regions. Arsenic ingestion through the food chain may affect physiological and biochemical processes in the body. Although human exposure to arsenic is known to induce adverse health effects, the level and time of exposure as well as genetic susceptibility are important factors in outcome. Arsenic exposure plays an etiological role in diabetes development. Low or moderate arsenic exposure plays a positive role, while a high level of arsenic is associated with the risk of developing type-2 diabetes. Studies associated with the biochemical mechanism(s) in relation to arsenic exposure and risk of developing diabetes are still contentious and need to be delineated further. Although various therapeutic and nutritional strategies are available to alleviate arsenic toxicity, more preventive and therapeutic measures against arsenic toxicity are required.
P- Reviewer: Brunetti A, Shimizu Y S- Editor: Ji FF L- Editor: Webster JR E- Editor: Lu YJ
| 1. | Villa-Lojo MC, Alonso-Rodríguez E, López-Mahía P, Muniategui-Lorenzo S, Prada-Rodríguez D. Coupled high performance liquid chromatography-microwave digestion-hydride generation-atomic absorption spectrometry for inorganic and organic arsenic speciation in fish tissue. Talanta. 2002;57:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 2. | Jain CK, Ali I. Arsenic: occurrence, toxicity and speciation Techniques. Water Res. 2000;34:4304-4312. [DOI] [Full Text] |
| 3. | Mosaferi M, Mesdaghinia AR, Yunesian M. Occurrence of arsenic in Kurdistan Province of I.R.Iran. Dhaka: Proceeding of Conference on Fate of Arsenic in the Environment 2003; BUET. |
| 4. | Babel S, Opiso EM. Removal of Cr from synthetic wastewater by sorption into volcanic ash soil. Int J Environ Sci Technol. 2007;4:99-108. [DOI] [Full Text] |
| 5. | Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett. 2002;133:1-16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1110] [Cited by in RCA: 961] [Article Influence: 41.8] [Reference Citation Analysis (1)] |
| 6. | Flora SJS. Arsenic-induced oxidative stress and its reversibility. Free Radical Biol Med. 2011;51:257-281. [RCA] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 581] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 7. | Kligerman AD, Tennant AH. Insights into the carcinogenic mode of action of arsenic. Toxicol Appl Pharmacol. 2007;222:281-288. [RCA] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (2)] |
| 8. | Szymańska-Chabowska A, Antonowicz-Juchniewicz J, Andrzejak R. The concentration of selected cancer markers (TPA, TPS, CYFRA 21-1, CEA) in workers occupationally exposed to arsenic (As) and some heavy metals (Pb, Cd) during a two-year observation study. Int J Occup Med Environ Health. 2007;20:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 9. | Díaz-Villaseñor A, Burns AL, Hiriart M, Cebrián ME, Ostrosky-Wegman P. Arsenic-induced alteration in the expression of genes related to type 2 diabetes mellitus. Toxicol Appl Pharmacol. 2007;225:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Navas-Acien A, Sharrett AR, Silbergeld EK, Schwartz BS, Nachman KE, Burke TA, Guallar E. Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol. 2005;162:1037-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 280] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 11. | Prozialeck WC, Edwards JR, Nebert DW, Woods JM, Barchowsky A, Atchison WD. The vascular system as a target of metal toxicity. Toxicol Sci. 2008;102:207-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 211] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 12. | Schmuck EM, Board PG, Whitbread AK, Tetlow N, Cavanaugh JA, Blackburn AC, Masoumi A. Characterization of the monomethylarsonate reductase and dehydroascorbate reductase activities of Omega class glutathione transferase variants: implications for arsenic metabolism and the age-at-onset of Alzheimer’s and Parkinson’s diseases. Pharmacogenet Genomics. 2005;15:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Vahidnia A, van der Voet GB, de Wolff FA. Arsenic neurotoxicity--a review. Hum Exp Toxicol. 2007;26:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Florea AM, Büsselberg D. Arsenic trioxide in environmentally and clinically relevant concentrations interacts with calcium homeostasis and induces cell type specific cell death in tumor and non-tumor cells. Toxicol Lett. 2008;179:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Baysan A, Yel L, Gollapudi S, Su H, Gupta S. Arsenic trioxide induces apoptosis via the mitochondrial pathway by upregulating the expression of Bax and Bim in human B cells. Int J Oncol. 2007;30:313-318. [PubMed] |
| 16. | Paul MK, Kumar R, Mukhopadhyay AK. Dithiothreitol abrogates the effect of arsenic trioxide on normal rat liver mitochondria and human hepatocellular carcinoma cells. Toxicol Appl Pharmacol. 2008;226:140-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Laparra JM, Vélez D, Barberá R, Farré R, Montoro R. As2O3-induced oxidative stress and cycle progression in a human intestinal epithelial cell line (Caco-2). Toxicol In Vitro. 2008;22:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Vahter M. Health effects of early life exposure to arsenic. Basic Clin Pharmacol Toxicol. 2008;102:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Islam K, Haque A, Karim R, Fajol A, Hossain E, Salam KA, Ali N, Saud ZA, Rahman M, Rahman M. Dose-response relationship between arsenic exposure and the serum enzymes for liver function tests in the individuals exposed to arsenic: a cross sectional study in Bangladesh. Environ Health. 2011;10:64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Kumagai Y, Sumi D. Arsenic: signal transduction, transcription factor, and biotransformation involved in cellular response and toxicity. Annu Rev Pharmacol Toxicol. 2007;47:243-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Mandal BK, Suzuki KT. Arsenic round the world: a review. Talanta. 2002;58:201-235. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 1709] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 22. | Vahter M, Marafante E. Intracellular interaction and metabolic fate of arsenite and arsenate in mice and rabbits. Chem Biol Interact. 1983;47:29-44. [RCA] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 119] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Yamauchi H, Yamamura Y. Dynamic change of inorganic arsenic and methylarsinic compounds in human urine after oral intake as arsenic trioxide. Ind Health. 1979;17:79-83. [DOI] [Full Text] |
| 24. | Yoshida K, Inoue Y, Kuroda K, Chen H, Wanibuchi H, Fukushima S, Endo G. Urinary excretion of arsenic metabolites after long term oral administration of various arsenic compounds to rats. J Tocxicol Environ Health Part A. 1998;54:179-192. [RCA] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Hsueh YM, Chung CJ, Shiue HS, Chen JB, Chiang SS, Yang MH, Tai CW, Su CT. Urinary arsenic species and CKD in a Taiwanese population: a case-control study. Am J Kidney Dis. 2009;54:859-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 26. | Hansen HR, Raab A, Jaspars M, Milne BF, Feldmann J. Sulfur-containing arsenical mistaken for dimethylarsinous acid [DMA(III)] and identified as a natural metabolite in urine: major implications for studies on arsenic metabolism and toxicity. Chem Res Toxicol. 2004;17:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Raml R, Goessler W, Traar P, Ochi T, Francesconi KA. Novel thioarsenic metabolites in human urine after ingestion of an arsenosugar, 2’,3’-dihydroxypropyl 5-deoxy-5-dimethylarsinoyl-beta-D-riboside. Chem Res Toxicol. 2005;18:1444-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Yoshida K, Kuroda K, Inoue Y, Chen H, Date Y, Wanibuchi H, Fukushima S, Endo G. Metabolism of dimethylarsinic acid in rats: production of unidentified metbaloites in vivo. Appl Organomet Chem. 2001;15:539-547. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Yoshida K, Kuroda K, Zhou X, Inoue Y, Date Y, Wanibuchi H, Fukushima S, Endo G. Urinary sulfur-containing metabolite produced by intestinal bacteria following oral administration of dimethylarsinic acid to rats. Chem Res Toxicol. 2003;16:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Fricke MW, Zeller M, Sun H, Lai VW, Cullen WR, Shoemaker JA, Witkowski MR, Creed JT. Chromatographic separation and identification of products from the reaction of dimethylarsinic acid with hydrogen sulfide. Chem Res Toxicol. 2005;18:1821-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Naranmandura H, Suzuki N, Suzuki KT. Trivalent arsenicals are bound to proteins during reductive methylation. Chem Res Toxicol. 2006;19:1010-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Suzuki KT, Mandal BK, Katagiri A, Sakuma Y, Kawakami A, Ogra Y, Yamaguchi K, Sei Y, Yamanaka K, Anzai M. Dimethylthioarsenicals as arsenic metabolites after long-term oral administration of various arsenic compounds to rats. J Toxicol. 2004;17:914-921. |
| 33. | Hansen HR, Jaspars M, Feldmann J. Arsinothioyl-sugars produced by in vitro incubation of seaweed extract with liver cytosol analysed by HPLC coupled simultaneously to ES-MS and ICP-MS. Analyst. 2004;129:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Kahn M, Raml R, Schmeisser E, Vallant B, Francesconi KA, Goessler W. Two movel thio-arsenosugars in scallops identified with HPLC-ICPMS and HPLC-ESMS. Environ Chem. 2005;2:171-176. [DOI] [Full Text] |
| 35. | Meier J, Kienzl N, Goessler W, Francesconi KA. The occurrence of thio-arsenosugars in some samples of marine algae. Environ Chem. 2005;2:304-307. [DOI] [Full Text] |
| 36. | Schmeisser E, Raml R, Francesconi KA, Kuehnelt D, lindberg AL, Soros C, Goessler W. Thio arsenosugars identified as natural constituents of mussels by liquid chromatography mass spectrometry. Chem Commun. 2003;10:1824-1825. |
| 37. | Suzuki KT, Iwata K, Naranmandura H, Suzuki N. Metabolic differences between two dimethylthioarsenicals in rats. Toxicol Appl Pharmacol. 2007;218:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Naranmandura H, Iwata K, Suzuki KT, Ogra Y. Distribution and metabolism of four different dimethylated arsenicals in hamsters. Toxicol Appl Pharmacol. 2010;245:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Kannan GM, Flora SJS. Chronic arsenic poisoning in the rats: treatment with combined administration of succimers and an antioxidants. Ecotoxicol Environ Safety. 2004;58:37-43. [RCA] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (2)] |
| 40. | Shi H, Shi X, Liu KJ. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem. 2004;255:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 455] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 41. | Naranmandura H, Xu S, Sawata T, Hao WH, Liu H, Bu N, Ogra Y, Lou YJ, Suzuki N. Mitochondria are the main target organelle for trivalent monomethylarsonous acid (MMA(III))-induced cytotoxicity. Chem Res Toxicol. 2011;24:1094-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Yamanaka K, Okada S. Induction of lung-specific DNA damage by metabolically methylated arsenics via the production of free radicals. Environ. Health Perspect. 1994;102:37-40. [DOI] [Full Text] |
| 43. | Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radical Biol Med. 1999;27:1405-1412. [RCA] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 189] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Liu SX, Athar M, Lippai I, Waldren C, Hei TK. Induction of oxyradicals by arsenic: implication for mechanism of genotoxicity. Proc Natl Acad Sci. 2001;98:1643-1648. [RCA] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 269] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 45. | Kitchin KT, Ahmad S. Oxidative stress as a possible mode of action for arsenic carcinogenesis. Toxicol Lett. 2003;137:3-13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 189] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 46. | Higaki Y, Mikami T, Fujii N, Hirshman MF, Koyama K, Seino T, Tanaka K, Goodyear LJ. Oxidative stress stimulates skeletal muscle glucose uptake through a phosphatidylinositol 3-kinase-dependent pathway. Am J Physiol Endocrinol Metab. 2008;294:E889-E897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 47. | Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 564] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 48. | Xu S, Touyz RM. Reactive oxygen species and vascular remodelling in hypertension: still alive. Can J Cardiol. 2006;22:947-951. [RCA] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Pouysségur J, Mechta-Grigoriou F. Redox regulation of the hypoxia-inducible factor. Biol Chem. 2006;387:1337-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 50. | Cross JV, Templeton DJ. Regulation of signal transduction through protein cysteine oxidation. Antioxid Redox Signal. 2006;8:1819-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Dalle-Donne I, Milzani A, Gagliano N, Colombo R, Giustarini D, Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid Redox Signal. 2008;10:445-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 52. | Goetz ME, Luch A. Reactive species: a cell damaging rout assisting to chemical carcinogens. Cancer Lett. 2008;266:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 53. | Cohen SM, Arnold LL, Uzvolgyi E, Cano M, St John M, Yamamoto S, Lu X, Le XC. Possible role of dimethylarsinous acid in dimethylarsinic acid-induced urothelial toxicity and regeneration in the rat. Chem Res Toxicol. 2002;15:1150-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 659] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 55. | Vega L, Styblo M, Patterson R, Cullen W, Wang C, Germolec D. Differential effects of trivalent and pentavalent arsenicals on cell proliferation and cytokine secretion in normal human epidermal keratinocytes. Toxicol Appl Pharmacol. 2001;172:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 171] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 56. | Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol. 2000;163:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 443] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 57. | Nesnow S, Roop BC, Lambert G, Kadiiska M, Mason RP, Cullen WR, Mass MJ. DNA damage induced by methylated trivalent arsenicals is mediated by reactive oxygen species. Chem Res Toxicol. 2002;15:1627-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 58. | Andrewes P, Kitchin KT, Wallace K. Dimethylarsine and trimethylarsine are potent genotoxins in vitro. Chem Res Toxicol. 2003;16:994-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Stýblo M, Drobná Z, Jaspers I, Lin S, Thomas DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic: a research update. Environ Health Perspect. 2002;110 Suppl 5:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Mahajan S, Singh N, Subramanian SK, Chauhan P, Saxena S, Goswamy HM, Prasad GBKS, Bisen PS. “Diabegon”, a safe and effective polyherbal therapy for type 2 diabetes mellitus. World J Transl Med. 2013;2:75-82. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4438] [Cited by in RCA: 4379] [Article Influence: 291.9] [Reference Citation Analysis (4)] |
| 62. | IDF-International Diabetes Federation. Diabetes Atlas. 5th ed. Brussels: International Diabetes Federation 2009; . |
| 63. | WHO. World Health Organization. Fact Sheet No. 312: What is Diabetes 2009; . |
| 64. | Mohan V, Deepa M, Farooq S, Narayan KM, Datta M, Deepa R. Anthropometric cut points for identification of cardiometabolic risk factors in an urban Asian Indian population. Metabolism. 2007;56:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1801] [Cited by in RCA: 1745] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 66. | Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3804] [Cited by in RCA: 3689] [Article Influence: 153.7] [Reference Citation Analysis (0)] |
| 67. | Lai MS, Hsueh YM, Chen CJ, Shyu MP, Chen SY, Kuo TL, Wu MM, Tai TY. Ingested inorganic arsenic and prevalence of diabetes mellitus. Am J Epidemiol. 1994;139:484-492. [PubMed] |
| 68. | Nizam S, Kato M, Yatsuya H, Khalequzzaman M, Ohnuma S, Naito H, Nakajima T. Differences in urinary arsenic metabolites between diabetic and non-diabetic subjects in Bangladesh. Int J Environ Res Public Health. 2013;10:1006-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Wang SL, Chiou JM, Chen CJ, Tseng CH, Chou WL, Wang CC, Wu TN, Chang LW. Prevalence of non-insulin-dependent diabetes mellitus and related vascular diseases in southwestern arseniasis-endemic and nonendemic areas in Taiwan. Environ Health Perspect. 2003;111:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 70. | Tseng CH, Tai TY, Chong CK, Tseng CP, Lai MS, Lin BJ, Chiou HY, Hsueh YM, Hsu KH, Chen CJ. Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: a cohort study in arseniasis-hyperendemic villages in Taiwan. Environ Health Perspect. 2000;108:847-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 238] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 71. | Tseng CH, Tseng CP, Chiou HY, Hsueh YM, Chong CK, Chen CJ. Epidemiologic evidence of diabetogenic effect of arsenic. Toxicol Lett. 2002;133:69-76. [RCA] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 72. | Rahman M, Tondel M, Ahmad SA, Axelson O. Diabetes mellitus associated with arsenic exposure in Bangladesh. Am J Epidemiol. 1998;148:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 190] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Jensen EG, Hansen ML. Occupational arsenic exposure and glycosylated haemoglobin. Analyst. 1998;123:77-80. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 74. | Rahman M, Axelson O. Diabetes mellitus and arsenic exposure: a second look at case-control data from a Swedish copper smelter. Occup Environ Med. 1995;52:773-774. [RCA] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Rahman M, Wingren G, Axelson O. Diabetes mellitus among Swedish art glass workers--an effect of arsenic exposure? Scand J Work Environ Health. 1996;22:146-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Rahman M, Tondel M, Chowdhury IA, Axelson O. Relations between exposure to arsenic, skin lesions, and glucosuria. Occup Environ Med. 1999;56:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | American Diabetes Association ; Clinical practice recommendations, Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27 Suppl 1:S5-S10. [PubMed] |
| 78. | Wang SL, Chang FH, Liou SH, Wang HJ, Li Dennis W, Hsieh PH. An inorganic arsenic exposure and its relation to metabolic syndrome in an industrial area of Taiwan. Environ Int. 2007;33:805-811. |
| 79. | Dixon HBF. The biochemical action of arsenic acids especially as phosphate analogues. Adv Inorg Chem. 1997;44:191-227. [RCA] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 91] [Article Influence: 3.1] [Reference Citation Analysis (3)] |
| 80. | Lagunas R. Sugar-arsenate esters: thermodyanimcs and biochemical behavior. Arch Biochem Biophys. 1980;205:67-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 81. | Gresser MJ. ADP-arsenate. Formation by submitochondrial particles under phosphorylating conditions. J Biol Chem. 1981;256:5981-5983. [PubMed] |
| 82. | Kenny LJ, Kaplan JH. Arsenate substitutes for phosphate in the human red cell sodium pump and anion exchanger. J Biol Chem. 1988;263:7954-7960. |
| 83. | Crane RK, Lipmann F. The effect of arsenate on aerobic phosphorylation. J Biol Chem. 1953;201:235-243. [PubMed] |
| 84. | Aposhian HV. Biochemical toxicology of arsenic. Biochemical Toxicology. New York: Elsevier Science 1989; 265-299. |
| 85. | Delnomdedieu M, Basti MM, Otvos JD, Thomas DJ. Reduction and binding of arsenate and dimethylarsinate by glutathione: a magnetic resonance study. Chem Biol Interact. 1994;90:139-155. [RCA] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 190] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 86. | Winski SL, Carter DE. Arsenate toxicity in human erythrocytes: characterization of morphologic changes and determination of the mechanism of damage. J Toxicol Environ Health. 1998;53:345-355. [RCA] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 87. | Moore SA, Moennich DM, Gresser MJ. Synthesis and hydrolysis of ADP-arsenate by beef heart submitochondrial particles. J Biol Chem. 1983;258:6266-6271. [PubMed] |
| 88. | Delnomdedieu M, Styblo M, Thomas DJ. Time dependence of accumulation and binding of inorganic and organic arsenic species in rabbit erythrocytes. Chem Biol Interact. 1995;98:69-83. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Styblo M, Serves SV, Cullen WR, Thomas DJ. Comparative inhibition of yeast glutathione reductase by arsenicals and arsenothiols. Chem Res Toxicol. 1997;10:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 189] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 90. | Lin S, Cullen WR, Thomas DJ. Methylarsenicals and arsinothiols are potent inhibitors of mouse liver thioredoxin reductase. Chem Res Toxicol. 1999;12:924-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 137] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 91. | Habib GM, Shi ZZ, Lieberman MW. Glutathione protects cells against arsenite-induced toxicity. Free Radic Biol Med. 2007;42:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 92. | Boquist L, Boquist S, Ericsson I. Structural beta-cell changes and transient hyperglycemia in mice treated with compounds inducing inhibited citric acid cycle enzyme activity. Diabetes. 1988;37:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 93. | Petrick JS, Jagadish B, Mash EA, Aposhian HV. Monomethylarsonous acid (MMA(III)) and arsenite: LD(50) in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem Res Toxicol. 2001;14:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 222] [Article Influence: 9.3] [Reference Citation Analysis (2)] |
| 94. | Chouhan S, Flora SJ. Arsenic and fluoride: two major ground water pollutants. Indian J Exp Biol. 2010;48:666-678. [PubMed] |
| 95. | Douen AG, Kacem R, Jones MN. Direct interactions of phenylarsine oxide with hexose transporters in isolated rat adipocytes. Biochimica et Biophysica Acta. 1988;944:444-450. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Frost SC, Kohanski RA, Lane MD. Effect of phenylarsine oxide on insulin-dependent protein phosphorylation and glucose transport in 3T3-L1 adipocytes. J Biol Chem. 1987;262:9872-9876. [PubMed] |
| 97. | Liebl B, Muckter H, Doklea E, Fichtl B, Forth W. Influence of organic and inorganic arsenicals on glucose uptake in Madin-Darby canine kidney (MDCK) cells. Analyst. 1992;117:681-684. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 98. | Liebl B, Mückter H, Doklea E, Reichl FX, Fichtl B, Forth W. Influence of glucose on the toxicity of oxophenylarsine in MDCK cells. Arch Toxicol. 1995;69:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 99. | Henriksen EJ, Hollotszy JO. Effects of phenylarsine oxide on stimulation of glucose transport in rat skeletal muscle. Am J Physiol. 1990;258:648-653. |
| 100. | Sowell MO, Robinson KA, Buse MG. Phenylarsine oxide and denervation effects on hormone-stimulated glucose transport. Am J Physiol. 1988;255:159-165. |
| 101. | Brazy PC, Balaban RS, Gullans SR, Mandel LJ, Dennis VW. Inhibition of Renal Metabolism. Relative effects of arsenate on sodium, phosphate, and glucose transport by the rabbit proximal tubule. J Clin Invest. 1980;66:1211-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 102. | Liebl B, Muckter H, Nguyen PT, Doklea E, Islambouli S, Fichtl B, Forth W. Differential effects of various trivalent and pentavalent organic and inorganic arsenic species on glucose metabolism in isolated kidney cells. Appl Organomet Chem. 1995;9:531-540. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 103. | Tseng CH. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol Appl Pharmacol. 2004;197:67-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 104. | Santra A, Maiti A, Chowdhury A, Mazumder DN. Oxidative stress in liver of mice exposed to arsenic-contaminated water. Indian J Gastroenterol. 2000;19:112-115. [PubMed] |
| 105. | Wan GH, Tsai SC, Chiu DT. Decreased blood activity of glucose-6-phosphate dehydrogenase associates with increased risk for diabetes mellitus. Endocrine. 2002;19:191-195. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Gaskin RS, Estwick D, Peddi R. G6PD deficiency: its role in the high prevalence of hypertension and diabetes mellitus. Ethn Dis. 2001;11:749-754. [PubMed] |
| 107. | Farese RV. Insulin-sensitive phospholipid signaling systems and glusoce transport. Update II. Exp Biol Med. 2001;226:283-295. |
| 108. | Ruderman NB, Kapeller R, White MF, Cantley LC. Activation of phosphatidylinositol 3-kinase by insulin. Proc Natl Acad Sci USA. 1990;87:1411-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 421] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 109. | White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994;269:1-4. [PubMed] |
| 110. | Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 863] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 111. | Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075-30082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 344] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 112. | Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372-31378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 975] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 113. | Tanti JF, Grillo S, Grémeaux T, Coffer PJ, Van Obberghen E, Le Marchand-Brustel Y. Potential role of protein kinase B in glucose transporter 4 translocation in adipocytes. Endocrinology. 1997;138:2005-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 114. | Simeonova PP, Luster MI. Arsenic carcinogenicity: relevance of c-Src activation. Mol Cell Biochem. 2002;234/235:277-282. [DOI] [Full Text] |
| 115. | Walton FS, Harmon AW, Paul DS, Drobná Z, Patel YM, Styblo M. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: possible mechanism of arsenic-induced diabetes. Toxicol Appl Pharmacol. 2004;198:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 131] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 116. | Luster MI. Proposed mechanisms for arsenic carcinogenicity: implications for the shape of the dose-response curve. Abstract presented at 2003 Society of Toxicology Conference. Toxicol Sci. 2003;72:Abstract 546. |
| 117. | Frost SC, Lane MD. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J Biol Chem. 1985;260:2646-2652. [PubMed] |
| 118. | Jhun BH, Hah JS, Jung CY. Phenylarsine oxide causes an insulin-dependent, GLUT4-specific degradation in rat adipocytes. J Biol Chem. 1991;266:22260-22265. [PubMed] |
| 119. | Bazuine M, Ouwens DM, Gomes de Mesquita DS, Maassen JA. Arsenite stimulated glucose transport in 3T3-L1 adipocytes involves both Glut4 translocation and p38 MAPK activity. Eur J Biochem. 2003;270:3891-3903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 120. | Sun X, Li B, Li X, Wang Y, Xu Y, Jin Y, Piao F, Sun G. Effects of sodium arsenite on catalase activity, gene and protein expression in HaCaT cells. Toxicol In Vitro. 2006;20:1139-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 121. | Wauson EM, Langan AS, Vorce RL. Sodium arsenite inhibits and reverses expression of adipogenic and fat cell-specific genes during in vitro adipogenesis. Toxicol Sci. 2002;65:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 122. | Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med (Berl). 2004;82:434-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 715] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 123. | Kapahi P, Takahashi T, Natoli G, Adams SR, Chen Y, Tsien RY, Karin M. Inhibition of NF-kappa B activation by arsenite through reaction with a critical cysteine in the activation loop of Ikappa B kinase. J Biol Chem. 2000;275:36062-36066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 286] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 124. | Chen F, Lu Y, Zhang Z, Vallyathan V, Ding M, Castranova V, Shi X. Opposite effect of NF-kappa B and c-Jun N-terminal kinase on p53-independent GADD45 induction by arsenite. J Biol Chem. 2001;276:11414-11419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 125. | Hu Y, Jin X, Snow ET. Effect of arsenic on transcription factor AP-1 and NF-kappaB DNA binding activity and related gene expression. Toxicol Lett. 2002;133:33-45. [RCA] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 126. | Yu HS, Liao WT, Chang KL, Yu CL, Chen GS. Arsenic induces tumor necrosis factor alpha release and tumor necrosis factor receptor 1 signaling in T helper cell apoptosis. J Invest Dermatol. 2002;119:812-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 127. | Wu MM, Chiou HY, Ho IC, Chen CJ, Lee TC. Gene expression of inflammatory molecules in circulating lymphocytes from arsenic-exposed human subjects. Environ Health Perspect. 2003;111:1429-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 128. | Paul DS, Hernández-Zavala A, Walton FS, Adair BM, Dedina J, Matousek T, Stýblo M. Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: development of a mouse model for arsenic-induced diabetes. Toxicol Appl Pharmacol. 2007;222:305-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 129. | Wang ZX, Jiang CS, Liu L, Wang XH, Jin HJ, Wu Q, Chen Q. The role of Akt on arsenic trioxide suppression of 3T3-L1 preadipocyte differentiation. Cell Res. 2005;15:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 130. | Hamilton JW, Kaltreider RC, Bajenova OV, Ihnat MA, McCaffrey J, Turpie BW, Rowell EE, Oh J, Nemeth MJ, Pesce CA. Molecular basis for effects of carcinogenic heavy metals on inducible gene expression. Environ Health Perspect. 1998;106 Suppl 4:1005-1015. [PubMed] [DOI] [Full Text] |
| 131. | Kaltreider RC, Davis AM, Lariviere JP, Hamilton JW. Arsenic alters the function of the glucocorticoid receptor as a transcription factor. Environ Health Perspect. 2001;109:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 132. | Serdar MA, Bakir F, Haşimi A, Celik T, Akin O, Kenar L, Aykut O, Yildirimkaya M. Trace and toxic element patterns in nonsmoker patients with noninsulin-dependent diabetes mellitus, impaired glucose tolerance, and fasting glucose. Int J Diabetes Dev Ctries. 2009;29:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 133. | Chawalit K, Sretarugsa P, Thithapandha A. Comparative effects of diabetogenic agents on hepatic drug metabolism. Drug Metab Dispos. 1982;10:81-86. [PubMed] |
| 134. | Longhurst PA, Lacagnin LB, Staats DE, Colby HD. Changes in hepatic drug metabolism in alloxan diabetic male rabbits. Biochem Pharmacol. 1986;35:1768-1771. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 135. | Singh N, Rana SV. Effect of insulin on arsenic toxicity in diabetic rats-liver function studies. Biol Trace Elem Res. 2009;132:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 136. | Singh S, Gadhok G, Rana SVS. Effect of alloxan induced diabetes on liver and kidney function in arsenic treated rats. Toxicol Environ Chem. 2006;88:673-682. [DOI] [Full Text] |
| 137. | Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E. Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ Health Perspect. 2006;114:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 138. | Wang JP, Wang SL, Lin Q, Zhang L, Huang D, Ng JC. Association of arsenic and kidney dysfunction in people with diabetes and validation of its effects in rats. Environ Int. 2009;35:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 139. | Hsueh YM, Cheng GS, Wu MM, Yu HS, Kuo TL, Chen CJ. Multiple risk factors associated with arsenic-induced skin cancer: effects of chronic liver disease and malnutritional status. Br J Cancer. 1995;71:109-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 119] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 140. | Hsueh YM, Wu WL, Huang YL, Chiou HY, Tseng CH, Chen CJ. Low serum carotene level and increased risk of ischemic heart disease related to long-term arsenic exposure. Atherosclerosis. 1998;141:249-257. [RCA] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 92] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 141. | Ford ES, Mokdad AH, Giles WH, Brown DW. The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes. 2003;52:2346-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 320] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 142. | Dincer Y, Akcay T, Alademir Z, Ilkova H. Assessment of DNA base oxidation and glutathione level in patients with type 2 diabetes. Mutat Res. 2002;505:75-81. [RCA] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 143. | Horng CJ, Lin SR. Determination of urinary trace elements (As, Hg, Zn, Pb, Se) in patients with blackfoot disease. Talanta. 1997;45:75-83. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 144. | Lin SM, Yang MH. Arsenic, selenium, and zinc in patients with Blackfoot disease. Biol Trace Elem Res. 1988;15:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 145. | Kala SV, Neely MW, Kala G, Prater CI, Atwood DW, Rice JS, Lieberman MW. The MRP2/cMOAT transporter and arsenic-glutathione complex formation are required for biliary excretion of arsenic. J Biol Chem. 2000;275:33404-33408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 222] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 146. | Gebel TW, Leister , M , Schumann W, Hirsch-Ernst K. Low-level self-tolerance to arsenite in human HepG2 cells is associated with a depressed induction of micronuclei. Mutat Res. 2002;514:245-255. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 147. | Tice RR, Yager JW, Andrews P, Crecelius E. Effect of hepatic methyl donor status on urinary excretion and DNA damage in B6C3F1 mice treated with sodium arsenite. Mutat Res. 1997;386:315-334. [RCA] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 148. | Vahter M, Marafante E. Effects of low dietary intake of methionine, choline or proteins on the biotransformation of arsenite in the rabbit. Toxicol Lett. 1987;37:41-46. [RCA] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 118] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 149. | Guha Mazumder DN. Chronic arsenic toxicity & amp; human health. Indian J Med Res. 2008;128:436-447. [PubMed] |
| 150. | Flora SJS, Flora G, Saxena G, Mishra M. Arsenic and lead induced free radical generation and their reversibility following chelation. Cell Mol Biol. 2007;53:26-47. [DOI] [Full Text] |
| 151. | Andersen O. Principles and recent developments in chelation treatment of metal intoxication. Chem Rev. 1999;99:2683-2710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 236] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 152. | Kokilavani V, Devi MA, Sivarajan K, Panneerselvam C. Combined efficacies of dl-α-lipoic acid and meso 2,3 dimercaptosuccinic acid against arsenic induced toxicity in antioxidant systems of rats. Toxicol Lett. 2005;160:1-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 153. | Flora SJS, Pant BP, Tripathi N, Kannan GM, Jaiswal DK. Therapeutic efficacy of a few diesters of meso 2,3- dimercaptosuccinic acid during sub-chronic arsenic intoxication in rats. J Occup Hlth. 1997;39:119-123. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 154. | Kreppel H, Reichl FX, Kleine A, Szinicz L, Singh PK, Jones MM. Antidotal efficacy of newly synthesized dimercaptosuccinic acid (DMSA) monoesters in experimental arsenic poisoning in mice. Toxicol Appl Pharmacol. 1995;26:239-245. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 155. | Modi M, Pathak U, Kalia K, Flora SJ. Arsenic antagonism studies with monoisoamyl DMSA and zinc in male mice. Environ Toxicol Pharmacol. 2005;19:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 156. | Flora SJ, Mehta A, Rao PV, Kannan GM, Bhaskar AS, Dube SN, Pant BP. Therapeutic potential of monoisoamyl and monomethyl esters of meso 2,3-dimercaptosuccinic acid in gallium arsenide intoxicated rats. Toxicology. 2004;195:127-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 157. | Flora SJ, Mehta A. Haematological, hepatic and renal alterations after repeated oral and intraperitoneal administration of monoisoamyl DMSA. II. Changes in female rats. J Appl Toxicol. 2003;23:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 158. | Reddy PS, Rani GP, Sainath SB, Meena R, Supriya Ch. Protective effects of N-acetylcysteine against arsenic-induced oxidative stress and reprotoxicity in male mice. J Trace Elem Med Biol. 2011;25:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 159. | Flora SJ, Bhadauria S, Kannan GM, Singh N. Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: a review. J Environ Biol. 2007;28:333-347. [PubMed] |
| 160. | Banner W, Koch M, Capin DM, Hopf SB, Chang S, Tong TG. Experimental chelation therapy in chromium, lead, and boron intoxication with N-acetylcysteine and other compounds. Toxicol Appl Pharmacol. 1986;83:142-147. [RCA] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 161. | Franklin WA, Veve R, Hirsch FR, Helfrich BA, Bunn PA. Epidermal growth factor receptor family in lung cancer and premalignancy. Semin Oncol. 2002;29:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 193] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 162. | Modi M, Kaul RK, Kannan GM, Flora SJ. Co-administration of zinc and n-acetylcysteine prevents arsenic-induced tissue oxidative stress in male rats. J Trace Elem Med Biol. 2006;20:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 163. | Flora SJ. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid Med Cell Longev. 2009;2:191-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 432] [Cited by in RCA: 325] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 164. | Patrick L. Toxic metals and antioxidants: Part II. The role of antioxidants in arsenic and cadmium toxicity. Altern Med Rev. 2003;8:106-128. [PubMed] |
| 165. | Hemalatha P, Reddy AG, Reddy YR, Shivakumar P. Evaluation of protective effect of N-acetylcysteine on arsenic induced hepatotoxicity. J Nat Sci Biol Med. 2013;4:393-395. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 166. | Choi YJ, Kang JS, Park JH, Lee YJ, Choi JS, Kang YH. Polyphenolic flavonoids differ in their antiapoptotic efficacy in hydrogen peroxide-treated human vascular endothelial cells. J Nutr. 2003;133:985-991. [PubMed] |
| 167. | Nègre-Salvayre A, Salvayre R. Quercetin prevents the cytotoxicity of oxidized LDL on lymphoid cell lines. Free Radic Biol Med. 1992;12:101-106. [RCA] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 168. | Mishra D, Flora SJS. Quercetin administration during chelation therapy protects arsenic-induced oxidative stress in mice. Biol Trace Elem Res. 2008;122:137-147. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 169. | Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. α-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790:1149-1160. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 653] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 170. | Gonzalez-Pereza O, Gonzaleza-Castaneda RE. Therapeutic perspectives on the combination of alpha-lipoic acid and vitamin e. Nutr Res. 2006;26:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 171. | Bhatt K, Flora SJ. Oral co-administration of α-lipoic acid, quercetin and captopril prevents gallium arsenide toxicity in rats. Environ Toxicol Pharmacol. 2009;28:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 172. | Samuel S, Kathirvel R, Jayavelu T, Chinnakkannu P. Protein oxidative damage in arsenic induced rat brain: influence of DL-alpha-lipoic acid. Toxicol Lett. 2005;155:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 173. | Khandker S, Dey RK, Maidul Islam AZM, Ahmad SA, Ifthaker-Al-Mahmud . Arsenic-safe drinking water and antioxidants for the management of arsenicosis patients. Bangladesh J Pharmacol. 2006;1:42-50. |
| 174. | Banerjee P, Bhattacharyya SS, Bhattacharjee N. Ascorbic Acid Combats Arsenic-Induced Oxidative Stress in Mice Liver. Ecotoxicol Environ Safety. 2009;72:639-649. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 175. | Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9-19. |
| 176. | Ramanathan K, Anusuyadevi M, Shila S, Panneerselvam C. Ascorbic acid and alpha-tocopherol as potent modulators of apoptosis on arsenic induced toxicity in rats. Toxicol Lett. 2005;156:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 177. | Bhattacharjee S, Sarkar C, Pal S. Additive beneficial effect of folic acid vitamin B12 coadministration on arsenic induced oxidative damage in cardiac tissue in vivo. Asian J Pharm Clin Res. 2013;6:64-69. |
| 178. | Zablotska LB, Chen Y, Graziano JH, Parvez F, van Geen A, Howe GR, Ahsan H. Protective effects of B vitamins and antioxidants on the risk of arsenic-related skin lesions in Bangladesh. Environ Health Perspect. 2008;116:1056-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 179. | Kalia K, Flora SJS. Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. J Occup Health. 2005;47:1-21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 180. | Benedetti S, Benvenuti F, Pagliarani S, Francogli S, Scoglio S, Canestrari F. Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci. 2004;75:2353-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 181. | Kulshreshtha A, Zacharia AJ, Jarouliya U, Bhadauriya P, Prasad GB, Bisen PS. Spirulina in health care management. Curr Pharm Biotechnol. 2008;9:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 182. | Jatav SK, Kulshrestha A, Zacharia A, Singh N, Tejovathi G, Bisen PS, Prasad GBKS. Spirulina maxima protects liver from Isoniazid and Rifampicin drug toxicity. Evidence based complementary and alternative medicine. New York: Elsevier Science 2014; . [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 183. | Jarouliya U, Zacharia JA, Kumar P, Bisen PS, Prasad GB. Alleviation of metabolic abnormalities induced by excessive fructose administration in Wistar rats by Spirulina maxima. Indian J Med Res. 2012;135:422-428. [PubMed] |
| 184. | Dartsch PC. Antioxidant potential of selected Spirulina platensis preparations. Phytother Res. 2008;22:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 185. | Khan Z, Bhadouria P, Bisen PS. Nutritional and therapeutic potential of Spirulina. Curr Pharm Biotechnol. 2005;6:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 186. | Estrada JEP, Bescos PB, Villar Del Fresno AM. Antioxidant activity of different fractions of Spirulina platensis protean extract. II Farmaco. 2001;56:497-500. [RCA] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 187. | Wu LC, Ho JA, Shieh MC, Lu IW. Antioxidant and antiproliferative activities of Spirulina and Chlorella water extracts. J Agric Food Chem. 2005;53:4207-4212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 188. | Chen H, Pan SS. Bioremediation potential of spirulina: toxicity and biosorption studies of lead. J Zhejiang Univ Sci B. 2005;6:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 189. | Rangasaytor N, Upatham ES, Krutrachure M, Pokethitiyook P, Lanza G. Phytoremediation potential of Spirulina (Arthrospira) platensis: biosorption and toxicity studies of cadmium. Environ Poll. 2002;119:45-53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 190. | Rahman MH, Sikder MS, Islam AZM, Wahab MA. Spirulina as food supplement is effective in arsenicosis. J Pakistan Assoc Dermatol. 2006;16:86-92. |
| 191. | Karkos PD, Leong SC, Karkos CD, Sivaji N, Assimakopoulos DA. Spirulina in clinical practice: evidence-based human applications. Evid Based Complement Alternat Med. 2011;2011:531053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 192. | Gupta R, Flora SJ. Effect of Centella asiatica on arsenic induced oxidative stress and metal distribution in rats. J Appl Toxicol. 2006;26:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 193. | Beveridge T, Li TS, Oomah BD, Smith A. Sea buckthorn products: manufacture and composition. J Agric Food Chem. 1999;47:3480-3488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 159] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 194. | Yang B, Kallio HP. Fatty acid composition of lipids in Sea buckthorn (Hippophae rhamnoides L.) berries of different origins. J Agric Food Chem. 2001;49:1939-1947. [RCA] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 195. | Pintea A, Varga A, Stepnowski P, Socaciu C, Culea M, Diehl HA. Chromatographic analysis of carotenol fatty acid esters in Physalis alkekengi and Hippophae rhamnoides. Phytochem Anal. 2005;16:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 196. | Suryakumar G, Gupta A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J Ethnopharmacol. 2011;138:268-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 197. | Kim JS, Kwon YS, Sa YJ, Kim MJ. Isolation and identification of sea buckthorn (Hippophae rhamnoides) phenolics with antioxidant activity and α-glucosidase inhibitory effect. J Agric Food Chem. 2011;59:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 198. | Gupta R, Flora SJ. Protective effects of fruit extracts of Hippophae rhamnoides L. against arsenic toxicity in Swiss albino mice. Hum Exp Toxicol. 2006;25:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 199. | Tsai TH, Tsai PJ, Ho SC. Antioxidant and anti-inflammatory activities of several commonly used spices. J Food Sci. 2005;70:93-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 200. | Yin MC, Cheng WS. Antioxidant and antimicrobial effects of four garlic-derived organosulfur compounds in ground beef. Meat Sci. 2003;63:23-28. [RCA] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 201. | Chowdhury R, Dutta A, Chaudhuri SR, Sharma N, Giri AK, Chaudhuri K. In vitro and in vivo reduction of sodium arsenite induced toxicity by aqueous garlic extract. Food Chem Toxicol. 2008;46:740-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 202. | Gupta R, Kannan GM, Sharma M, S Flora SJ. Therapeutic effects of Moringa oleifera on arsenic-induced toxicity in rats. Environ Toxicol Pharmacol. 2005;20:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 203. | Mishra D, Gupta R, Pant SC, Kushwah P, Satish HT, Flora SJ. Co-administration of monoisoamyl dimercaptosuccinic acid and Moringa oleifera seed powder protects arsenic-induced oxidative stress and metal distribution in mice. Toxicol Mech Methods. 2009;19:169-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 204. | Gupta R, Dubey DK, Kannan GM, Flora SJ. Concomitant administration of Moringa oleifera seed powder in the remediation of arsenic-induced oxidative stress in mouse. Cell Biol Int. 2007;31:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 205. | El-Demerdash FM, Yousef MI, Radwan FM. Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem Toxicol. 2009;47:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 206. | Reddy MVB, Sasikala P, Karthik A, Sudheer SD, Murthy LN. Protective role of curcumin against arsenic trioxide toxicity during gestation and lactational periods. Global Veterinaria. 2012;9:270-276. [DOI] [Full Text] |
| 207. | Yadav RS, Sankhwar ML, Shukla RK, Chandra R, Pant AB, Islam F, Khanna VK. Attenuation of arsenic neurotoxicity by curcumin in rats. Toxicol Appl Pharmacol. 2009;240: 367-376. [RCA] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 208. | Sharma A, Sharma MK, Kumar M. Protective effect of Mentha piperita against arsenic-induced toxicity in liver of awiss albino mice. Basic Clin Pharmacol Toxicol. 2006;100:249-257. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 209. | Gupta R, Flora SJ. Protective value of Aloe vera against some toxic effects of arsenic in rats. Phytother Res. 2005;19:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |