Published online Dec 12, 2013. doi: 10.5528/wjtm.v2.i3.67
Revised: November 11, 2013
Accepted: November 20, 2013
Published online: December 12, 2013
Processing time: 149 Days and 22.2 Hours
AIM: To generate DNA-aptamers binding to Methicillin-resistant Staphylococcus aureus (MRSA).
METHODS: The Cell-Systematic Evolution of Ligands by Exponential Enrichment (SELEX) technology was used to run the selection against MRSA bacteria and develop target-specific aptamers. MRSA bacteria were targeted while Enterococcus faecalis bacteria were used for counter selection during that process. Binding assays to determine the right aptamer candidates as well as binding assays on clinical samples were performed through flow cytometry and analyzed using the FlowJo software. The characterization of the aptamers was done by determination of their Kd values and determined by analysis of flow data at different aptamer concentration using SigmaPlot. Finally, the recognition of the complex Gold-nanoparticle-aptamer to the bacteria cells was observed using transmission electron microscopy (TEM).
RESULTS: During the cell-SELEX selection process, 17 rounds were necessary to generate enrichment of the pool. While the selection was run using fixed cells, it was shown that the binding of the pools with live cells was giving similar results. After sequencing and analysis of the two last pools, four sequences were identified to be aptamer candidates. The characterization of those aptamers showed that based on their Kd values, DTMRSA4 presented the best binding with a Kd value of 94.61 ± 18.82 nmol/L. A total of ten clinical samples of MRSA, S. aureus and Enterococcus faecalis were obtained to test those aptamers and determine their binding on a panel of samples. DTMRSA1 and DTMRSA3 showed the best results regarding their specificity to MRSA, DTMRSA1 being the most specific of all. Finally, those aptamers were coupled with gold-nanoparticle and their binding to MRSA cells was visualized through TEM showing that adduction of nanoparticles on the aptamers did not change their binding property.
CONCLUSION: A total of four aptamers that bind to MRSA were obtained with Kd values ranking from 94 to 200 nmol/L.
Core tip:Methicillin-resistant Staphylococcus aureus (MRSA) is a nosocomial bacterium that has developed resistance to beta-lactam antibiotics and can now be contracted in community settings. A tool that would enable the recognition of MRSA through its membrane structure could lead to new therapeutic approaches to eradicate the MRSA superbug. This paper presents four MRSA aptamers that can be easily modified as molecular probes for bioanalysis or antibiotics-free therapy. The Cell-SELEX technology was used to develop target-specific aptamers and binding studies of those aptamers were performed by flow cytometry on a panel of clinical strains. A total of four aptamers that bind to MRSA were obtained.
- Citation: Turek D, Simaeys DV, Johnson J, Ocsoy I, Tan W. Molecular recognition of live methicillin-resistant staphylococcus aureus cells using DNA aptamers. World J Transl Med 2013; 2(3): 67-74
- URL: https://www.wjgnet.com/2220-6132/full/v2/i3/67.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v2.i3.67
The resistance of bacteria to antibiotics is a major public health concern. In particular, Staphylococcus aureus (SA) is a bacterium harmlessly carried in the nose or on the skin of about 33% of the population[1]. Nevertheless, this nosocomial-acquired pathogen sometimes causes an infection. This microbe’s primary mode of transmission is by direct contact, usually skin-to-skin, and it can cause skin and wound infections, as well as life-threatening infections, including pneumonia, bacteremia or endocarditis[2]. SA infections are usually treated using β-lactam antibiotics, e.g., methicillin and penicillin, which inhibit the construction of the cell wall of Gram-positive bacteria, such as SA[3]. Unfortunately, SA easily develops antibiotic-resistant strains, and is, therefore, often referred to as a “superbug”. With the discovery of penicillin by Alexander Fleming in 1928, a dramatic reduction in mortality rates for SA infections occurred until resistant strains began to appear not long thereafter[4]. Many more antibiotics have been developed since then, but antibiotic-resistant SA strains continue to emerge. Aside from resistance, the use of antibiotics, in general, raises safety concerns since long-term therapy can result in damage to patients’ commensal flora[5-7]. The impact on human health is becoming very important since Methicillin-resistant Staphylococcus aureus (MRSA), which used to be confined to hospital settings, is now prevalent in community settings[8,9]. MRSA is currently detected using multiplexed PCR primers that detect specific genes for S. aureus (e.g., nuc or fem) and mecA for detection of methicillin resistance[10]. The eradication of multidrug-resistant bacteria is very difficult; thus, it is essential to look for treatment options other than antibiotics. To address this challenge, we used a technology known as Systematic Evolution of Ligands by Exponential Enrichment (SELEX) to generate four MRSA strain-specific aptamers that can easily be modified as an antibiotics-free therapeutic modality. This cell-based selection strategy generates ssDNA aptamers that bind to unknown targets on the surface of the cell membrane.
The library consisted of a 40 bases randomized region flanked by primer regions, each consisting of 18 nucleotides (5’-ATC CAG AGT GAC GCA GCA (N)40 TGG ACA CGG TGG CTT AGT-3’). The forward primers were labeled with 5’-FITC, and the reverse primers were labeled with 5’-biotin. Fluorescein isothiocyanate (FITC) labeling enabled fluorescence monitoring during selection, while the biotin was used for separation of the sense strand from the antisense strand after polymerase chain reaction (PCR) amplification through streptavidin-biotin interaction and subsequent alkaline denaturation. All oligonucleotides were synthesized by standard phosphoramidite chemistry using an ABI 3400 DNA synthesizer (Applied Biosystems) and purified by reverse phase High-performance liquid chromatography (HPLC) (Varian Prostar).
All PCR mixtures contained 50 mmol/L KCl, 10 mmol/L Tris HCl (pH 8.3), 1.5 mmol/L MgCl2, deoxyribonucleoside triphosphates (each at 2.5 mmol/L), 0.5 μmol/L each primer, and Hot start Taq DNA polymerase (5 units/μL) (TaKaRa). Amplification was carried out on a BIO-RAD thermocycler at 95 °C for 30 s, 60.7 °C for 30 s, and 72 °C for 30 s, followed by the final extension for 3 min at 72 °C. Pool enrichment was monitored by flow cytometry analysis using a FACScan cytometer (BD Immunocytometry Systems). Dulbecco’s Phosphate Buffered Saline (PBS) was used to prepare the washing buffer (4.5 g/L glucose and 5 mL MgCl2 at 1 mol/L) and binding buffer (4.5 g/L glucose, 5 mL MgCl2, 1.0 g/L bovine serum albumin and 100 mg/L tRNA).
MRSA standard strain 43300, was purchased from ATCC, and a clinical strain of Enterococcus faecalis was obtained from the Emergency Pathogen Institute at the University of Florida. MRSA was cultured at 37 °C in ATCC medium 18 - Trypticase soy agar with addition of 4 mg/L of sterile methicillin in order to maintain the resistance structural property of the bacteria. Enterococcus faecalis was cultured at 37 °C in ATCC medium 260 - Trypticase soy agar with defibrinated sheep blood and in the corresponding broth with no blood. Stock solutions of each cell line were prepared for the selection study and optimized throughout four solutions. The best results were obtained by the following procedure. Cell lines were incubated overnight at 37 °C on their respective agar plate. Then 3-4 bacterial colonies were transferred from the agar plate to 15 mL Corning centrifuge tubes filled with 6-7 mL of corresponding broth and incubated overnight at 37 °C. From this stock, 3 drops of bacterial solution were transferred to another 15 mL Corning centrifuge tube containing 4 mL of corresponding broth and incubated for 3 h. These two batches of incubated cells were separately washed twice with PBS and fixed in 70:30 methanol: DNase-free water. As a precaution to minimize the clumping, DNase-free water was added first, and cells were resuspended, followed by the corresponding volume of methanol. After 2 h of fixation at 4 °C, cells were stored in 10% PBS: DNase-free water. Finally, OD600 was measured for both batches, and a mixture was made to get the lowest OD600 measured out of the two batches. Four stock solutions of each bacterium were used for the entire selection. For MRSA, stock solution 1: OD600 = 2.08, 15 mL; stock solution 2: OD600 = 1.03, 22 mL; stock solution 3: OD600 = 1.73, 18.7 mL; stock solution 4: OD600 = 1.58, 59.5 mL. For Enterococcus faecalis, stock solution 1: OD600 = 1.18, 12.5 mL; stock solution 2: OD600 = 0.762, 17.5 mL; stock solution 3: OD600 = 2.49, 14 mL; stock solution 4: OD600 = 2.27, 66 mL.
In this selection, Methicillin-resistant MRSA standard strain 43300 was used as the target with Enterococcus faecalis as a negative control. Besides the first round where 107 cells were incubated with 22 nmol of naive ssDNA library dissolved in binding buffer, all other rounds were incubated with 25 pmol of the library obtained from the previous round. Before incubation, the DNA library was denatured at 95 °C for 5 min and quickly cooled on ice for 10 min, allowing each sequence to form the most stable secondary structure. Each round was performed with the counter selection first with an incubation of 30 min in an orbital shaker at 4 °C. The supernatant containing the unbound DNA sequence was then incubated with the positive cell line for 1 h in the same conditions. The pellet obtained was then washed two or three times, depending on the round - the stringency of the washes being increased up to 3 washes with 1 mL of washing buffer for 3 min. After suspension of the last pellet in DNase-free water, the pool was denatured at 95 °C for 15 min and centrifuged at 14000 g for 2 min. The supernatant containing the ssDNA was recovered and amplified by PCR using FITC- and biotin-labeled primers to increase the number of copies of individual sequences. A preparative PCR was performed using the amplified pool as the template. Amplifications were carried out at 95 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s, followed by final extension for 3 min at 72 °C. The selected sense ssDNA strands were separated from the biotinylated antisense ssDNA by streptavidin-coated Sepharose beads (GE Healthcare Bioscience). The ssDNA was eluted from the beads by melting in a 0.2 mol/L NaOH solution. This was desalted using NAP5 columns, dried and resuspended in binding buffer to a concentration of 250 nmol/L. The selection process was repeated until the level of enrichment, as assayed by flow cytometry, reached a plateau. Once the plateau was reached, pools of interest were submitted for sequencing using the Ion Torrent technique. The alignment was processed using MAFFT software.
Binding assays were used for two purposes: to assess the potential aptamer candidates and determine their apparent dissociation constant and to screen the aptamers against different cell lines. Binding assays to assess the potential aptamers and screen them against different cell lines used the same protocol. That protocol is based on the target cells, here MRSA (107 cells), which were incubated with various concentrations of 5’-FITC-labeled aptamers at 4 °C for 20 min in 100 μL of binding buffer. The fluorescence intensity was determined by FACScan cytometry (BD Immunocytometry Systems) by counting 60000 events for binding assays and 30000 for Kd determination. Cells only and random library (Library 0) were used as the background signal. The specific binding was obtained by subtracting the mean fluorescence intensity of the random library from the mean fluorescence intensity of the aptamers. The equilibrium dissociation constant (Kd) was obtained by fitting a plot of the specific binding intensity (Y) vs the aptamer concentration (X) to the equation Y = BmaxX/(Kd + X), using SigmaPlot (Jandel, San Rafael, CA). Cell specificity of the selected aptamers was determined using binding assays by flow cytometry monitoring, using MRSA, SA and Enterococcus faecalis clinical cell lines.
The four 5’ thiol-labeled aptamers were synthesized for these experiments, as explained in the instrumentation section, and fixed MRSA cells were used to run the binding assay. 40 μmol/L initial concentration of the 5’ thiol-modified aptamers were conjugated to 1 mL of gold nanoparticles (AuNPs) by incubation for 12 h in 50 μL of 10 mmol/L HEPES buffer (pH 7.5). Then, 100 μL of MRSA were mixed with 100 μL of aptamer-AuNPs conjugates and incubated for 1 h at room temperature. After removal of the unbound sequences with water by centrifugation at 3000 g, analysis was done by transmission electron microscopy (TEM) using 1% uranyl acetate staining. Assays were run with incubation of cells only, AuNPs only, sgc8-AuNPs only (sgc8 is used here as a random aptamer) and DTMRSA-AuNPs conjugates. Note that the centrifugation speed applied to obtain these results was low (3000 g), compared to the usual speed applied when using Au-NPs (10000 g). This makes it easier to observe the NPs upon target binding since they can be collected with the cell pellet, whereas nonbinding NPs observed in the control tend to remain in the discarded supernatant.
In this study, we selected aptamers binding to MRSA. Using the SELEX technique, a cell-selection was carried out using a random library of ssDNA that was subjected to sequential binding with the object of selecting those aptamers from the pool of DNA sequences having high binding affinity to surface markers on the target SA cell. Counter-selection using the Gram-positive commensal bacterium Enterococcus faecalis was performed on each round, allowing us to eliminate common surface markers, while, at the same time, enriching specific markers on the target bacteria. Both negative and positive cell lines were fixed with methanol before being used in the selection process. Four stock solutions of each fixed bacterium were prepared, as explained in the Experimental Section. The first stock solution was used from round 1 to 11; the second from round 12 to 14; and the third from round 15 to 17. The eluted pool of each round was amplified by PCR and monitored by flow cytometry. Since the pools were enriched throughout different rounds with binding aptamers toward MRSA, an increase in fluorescent signal was observed. The flow cytometry analysis of enrichment of the libraries and the binding assays with individual aptamers were performed on batch four. By the end of the 14th round of selection, a significant increase of specific pool enrichment was observed. After 15 rounds, a plateau was reached, and the selection was run until round 17 to maximize enrichment and homology within binding sequence families.
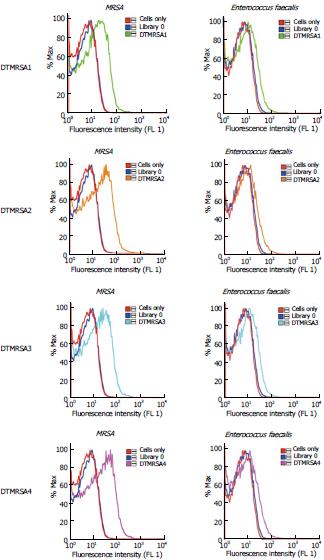
After successful enrichment, selection pools 15 and 17 were chosen and prepared for sequencing. Pool 15 represented the point at which enrichment started a plateau observable by flow cytometry, and pool 17 was the end of the plateau. Sequencing was done with IonTorrent, which identified 600000 sequences. In analyzing the data, the 40 nt random regions were aligned using the MAFTT alignment program. The alignment generated several different homologous families, and representative sequences were identified using the MAFTT and mfold oligo analyzer software programs. Putative DNA aptamers were then synthesized, labeled with FAM (carboxyfluorescein), purified by HPLC and quantified. Binding assays were performed for each sequence on MRSA cells and Enterococcus faecalis cells to determine the relevant aptamer sequences. Initially, we chose all candidates showing observable binding to the target cell MRSA when compared with the control - the random library. Positive sequences were further screened with Enterococcus faecalis to select the aptamers showing specific binding to MRSA. All flow cytometry data were run with unlabeled cells and random library (Library 0) as negative control. As shown in Figure 1 and Table 1, four of those sequences showed specific binding to MRSA.
| Name of aptamers | Percentage of total sequences | Sequence |
| DTMRSA1 | 2.57% | ATCCAGACGTGACGCAGC(N)38TGGACACGGTGGCTTAGTA(N)38 = ATGCGGTTGGTTGCGGTTGGGCATGATGTATTTCTGTG |
| DTMRSA2 | 33.74% | ATCCAGAGTGACGCAGCA(N)36TGGACACGGTGGCTTA(N)36 = CGACACGTTAGGTTGGTTAGGTTGGTTAGTTTCTTG |
| DTMRSA3 | 10.05% | ATCCAGAGTGACGCAGCA(N)40TGGACACGGTGGCTTAGTA(N)40 = GTAGATGGTTTGGTTGGTGTGGTTTCCTACTGATGTTGGG |
| DTMRSA4 | 0.32% | ATCCAGAGTGACGCAGCA(N)39TGTGGACACGGTGGCTTA(N)39 = TTATGGGGTGTGGTGGGGGGTTAATGCGTTGGTTATCCG |
Ten clinical strains from Shands Hospital (Florida, United States) were tested against the four aptamers developed (Table 2). DTMRSA1 and DTMRSA3 showed the best specificity and appeared to be the best candidates for MRSA treatment investigation, DTMRSA1 being the best one of the two, while DTMRSA2 and DTMRSA4 bound to all three types of clinical bacteria strains.
| Clinical strains | DTMRSA1 | DTMRSA2 | DTMRSA3 | DTMRSA4 |
| MRSA 2 | +++ | ++++ | +++ | ++++ |
| MRSA 4 | +++ | +++ | +++ | ++++ |
| MRSA 6 | ++++ | ++++ | +++ | ++++ |
| MRSA 7 | - | ++++ | +++ | ++++ |
| S. aureus 164 | - | ++++ | + | +++ |
| S. aureus 165 | - | ++++ | + | +++ |
| S. aureus 166 | - | ++++ | + | +++ |
| E. faecalis 43 | - | ++++ | + | +++ |
| E. faecalis 44 | - | ++++ | + | ++ |
| E. faecalis 45 | - | ++++ | + | +++ |
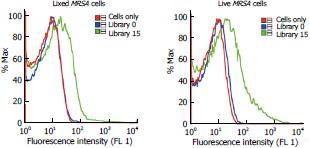
Over the course of the past two decades, mammalian cells have been the focus of most aptamer studies[11-13]. As presented in the result section, we generated four aptamers binding to the MRSA bacterium using the SELEX method. The selection was carried out using fixed cells for safety purposes and further investigation was performed to check whether the enriched pools generated from fixed cells could also bind to live MRSA cells. In order to verify whether methanol had an adverse effect on the binding of the enriched pools, we compared the binding of fixed cells with that of live cells. As can be observed in Figure 2, the binding was maintained when using live cells, demonstrating little permanent adverse effect in the structure of the membrane proteins of the bacteria and the possibility to use fixed cell when running a selection with no counter effect on the binding.
Since the binding was conserved whether live or fixed were used, the pools were sent to sequencing. Through the sequencing analysis, one tends to select for the more abundant sequences. Even though it is the most obvious strategy, the aptamer structure itself is as meaningful for its binding properties as it can be observed with DTMRSA4 that represent only 0.32% of the total sequences but shows a binding as important as DTMRSA2, the most abundant aptamer sequence as shown in Table 1.
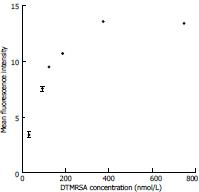
Apparent dissociation constants (Kd) are shown in Table 3 and Figure 3: DTMRSA3 and DTMRSA4 show very good binding with apparent dissociation constant (Kd) of 1.3 ± 0.5 × 102 nmol/L and 9.5 ± 2 × 101 nmol/L, respectively.
| Name of the aptamer | Kd (nmol/L) |
| DTMRSA1 | 1.6 ± 0.5 × 102 |
| DTMRSA2 | 2.0 ± 0.6 × 102 |
| DTMRSA3 | 1.3 ± 0.5 × 102 |
| DTMRSA4 | 9.5 ± 2 × 101 |
Apparent dissociation constants have been studied in the past through cell-SELEX performed on bacteria[14,15], and values typically ranged between 30 and 250 nmol/L, similar to those discovered here. We believe that these affinities are sufficient for MRSA detection assays. The other important and interesting property of these aptamers is their ability to selectively bind around whole cells, which presents a potential clinical use of the selected aptamers as nanocarriers for the identification of antibiotic-resistant cell lines in patients. The successful development of an assay that can differentiate the resistance of SA colonies can facilitate the search for a more effective treatment and management of the disease in individual treatment or in the population.
The latest estimations of the occurrence MRSA are staggering, rising to epidemic proportions in hospitals where, by an estimate provided by the European Antimicrobial Resistance Surveillance System, 40%-60% of all infections were caused by S. aureus in the United States and United Kingdom[16,17]. The ability of the MRSA membrane to structurally change through time in order to survive is a characteristic that increases its lethality and decreases its controllability[18,19]. Consequently, the binding ability of these four aptamers was compared in the context of different clinical MRSA strains, as well as SA and Enterococcus faecalis (Table 2). We are encouraged that the selected aptamers bound to all MRSA clinical strains tested, suggesting that the proteins targeted by those aptmers are common for the different MRSA strains. MRSA, SA and Enterococcus aureus are all gram-positive bacteria. Based on their similarities, some common binding can be expected by the commonality of proteins among the strains, whereas others are more specific to each type of bacterium. DTMRSA1 and DTMRSA3 show the best specificity and therefore appear to be the best candidates for MRSA treatment investigation, DTMRSA1 being the best one of the two, while DTMRSA2 and DTMRSA4 bind to all three types of bacteria.
Therefore, DTMRSA1-4 collectively represent a powerful tool by which to study the membrane structure of MRSA, as well as develop potential treatment modalities to combat this pathogen. As an empirical example of such use, we ran a prelimanary study to visualize the binding sites of MRSA aptamers on the bacteria external membrane. To accomplish this, we conjugated MRSA aptamers to AuNPs, and we were able to observe their target binding via TEM. Flow cytometry is one of the best tools to measure the abundance of a membrane protein on cells[20]. The introduction of a nanoparticle-aptamer conjugate detectable by TEM instead of a dye, as used in flow cytometry, could be a valuable adjunct to complement the information we get from the expression of aptamer targets on the bacteria. This technique allows the visualization of both the binding site of aptamers upon target binding and the structure of the cell wall[21-23]. No binding between MRSA and bare gold or random aptamer conjugated to gold nanoparticles was observed, whereas DTMRSA2-AuNP conjugates were attached to the surface of MRSA, as detected by TEM. With this successful visualization of the aptamers on MRSA cells, we showed that the adduction of nanoparticles on the aptamers did not change their binding property. Since nanoparticles have unique properties of their own[24,25], we believe this approach can be push further and lies on the choice of nanoparticles to either study the morphology of the bacteria, detect it or eradicate it.
In conclusion, once confined to hospital settings, MRSA can now be contracted in community settings as well. Although many new antibiotics against MRSA are in phase II and III clinical trials, a tool that would enable the recognition of MRSA through its membrane structure could lead to new therapeutic approaches to eradicate the MRSA superbug, either without the use of antibiotics or with a strain-specific antibiotic. The possibility of using the SELEX technique on fixed bacteria cells to develop aptamers binding to MRSA that will show the same results on live bacteria cells was shown in this paper. Four aptamers were found to recognize the bacteria membrane, DTMRSA1 and DTMRSA3 being the most specific when a wide range of clinical bacteria strains were tested, and DTMRSA4 presented the strongest binding to MRSA cells based on its Kd value. The binding of those aptamers were confirmed after modification with nanoparticles and visually observed through transmission electron microscopy showing those aptamers could be ealily modified to serve as molecular probes for bioanlysis or antibiotics-free therapy. Further studies would expand the present work to an optimized shorter aptamer length and a better understanding of the aptamer target on the membrane of MRSA bacteria.
We thank Mohammed Rashid for the bacteria training and scientific discussions that contributed to this work. We also thank David Nolan and Dr. Marco Salemi for providing clinical cell lines. Finally, we thank Dr. Kwame Sefah and Dr Kathryn R. Williams for their critical review of the manuscript.
Methicillin-resistant Staphylococcus aureus (MRSA) is any strain of Staphylococcus aureus that has developed resistance to beta-lactam antibiotics, including the penicillins and the cephalosporins. Once confined to hospital settings, MRSA can now be contracted in community settings as well.
Many new antibiotics against MRSA are in phase II and III clinical trials, nevertheless, a tool that would enable the recognition of MRSA through its membrane structure could lead to new therapeutic approaches to eradicate the MRSA superbug, either without the use of antibiotics or with a strain-specific antibiotic.
In the recent years, a cell selection has been done on Staphylococcus aureus and a protein selection has been done on Enterotoxin B, but has not been tested on whole cells. Antibodies are available against MRSA but do not present as much flexibility and advantages as aptamers.
The development of MRSA aptamer would present a great tool to new therapeutic approaches to eradicate the MRSA superbug, either without the use of antibiotics or with a strain-specific antibiotic.
This article is considered to be useful for others research scientists working in the fields of aptamers or MRSA bacteria.
P- Reviewers: Grizzi F, Pranjal C S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Whitt DD, Salyers AA. Bacterial Pathogenesis: A Molecular Approach. USA: ASM Press 2002; 428-434. |
| 2. | Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4167] [Cited by in RCA: 4277] [Article Influence: 158.4] [Reference Citation Analysis (0)] |
| 3. | Millar BC, Loughrey A, Elborn JS, Moore JE. Proposed definitions of community-associated meticillin-resistant Staphylococcus aureus (CA-MRSA). J Hosp Infect. 2007;67:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | LANE WR. Methicillin resistance in staphylococci. Med J Aust. 1962;49:962-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 863] [Cited by in RCA: 763] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 5. | Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334-339. [PubMed] |
| 6. | Levy S. The Antibiotic Paradox: How the Misuse of Antibiotics Destroys Their Curative Powers. Cambridge, MA: Perseus Publishing 2002; . |
| 7. | Marteau P, Seksik P, Jian R. Probiotics and intestinal health effects: a clinical perspective. Br J Nutr. 2002;88 Suppl 1:S51-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10:S122-S129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2467] [Cited by in RCA: 2487] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 9. | O’Brien FG, Lim TT, Chong FN, Coombs GW, Enright MC, Robinson DA, Monk A, Saïd-Salim B, Kreiswirth BN, Grubb WB. Diversity among community isolates of methicillin-resistant Staphylococcus aureus in Australia. J Clin Microbiol. 2004;42:3185-3190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Barski P, Piechowicz L, Galiński J, Kur J. Rapid assay for detection of methicillin-resistant Staphylococcus aureus using multiplex PCR. Mol Cell Probes. 1996;10:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Van Simaeys D, López-Colón D, Sefah K, Sutphen R, Jimenez E, Tan W. Study of the molecular recognition of aptamers selected through ovarian cancer cell-SELEX. PLoS One. 2010;5:e13770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Shangguan D, Li Y, Tang Z, Cao ZC, Chen HW, Mallikaratchy P, Sefah K, Yang CJ, Tan W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc Natl Acad Sci USA. 2006;103:11838-11843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1235] [Cited by in RCA: 1172] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 13. | Sefah K, Tang ZW, Shangguan DH, Chen H, Lopez-Colon D, Li Y, Parekh P, Martin J, Meng L, Phillips JA. Molecular recognition of acute myeloid leukemia using aptamers. Leukemia. 2009;23:235-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Cao X, Li S, Chen L, Ding H, Xu H, Huang Y, Li J, Liu N, Cao W, Zhu Y. Combining use of a panel of ssDNA aptamers in the detection of Staphylococcus aureus. Nucleic Acids Res. 2009;37:4621-4628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 252] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Chen F, Zhang X, Zhou J, Liu S, Liu J. Aptamer inhibits Mycobacterium tuberculosis (H37Rv) invasion of macrophage. Mol Biol Rep. 2012;39:2157-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Zapun A, Contreras-Martel C, Vernet T. Penicillin-binding proteins and beta-lactam resistance. FEMS Microbiol Rev. 2008;32:361-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 455] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 17. | Zapun A; Anonymous. European Antimicrobial Resistance Surveillance System. Bilthoven, The Netherlands: EARSS Annual Report Publishing 2002; . |
| 18. | Dancer SJ. The effect of antibiotics on methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2008;61:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2027] [Cited by in RCA: 1823] [Article Influence: 113.9] [Reference Citation Analysis (0)] |
| 20. | Longobardi Givan A. Flow Cytometry First Principles. 2nd edition. New York: John Wiley & Sons publishing 2001; 180-192. |
| 21. | Hayden SC, Zhao G, Saha K, Phillips RL, Li X, Miranda OR, Rotello VM, El-Sayed MA, Schmidt-Krey I, Bunz UH. Aggregation and interaction of cationic nanoparticles on bacterial surfaces. J Am Chem Soc. 2012;134:6920-6923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 22. | Carrillo-Carrión C, Simonet BM, Valcárcel M. Colistin-functionalised CdSe/ZnS quantum dots as fluorescent probe for the rapid detection of Escherichia coli. Biosens Bioelectron. 2011;26:4368-4374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Joo J, Yim C, Kwon D, Lee J, Shin HH, Cha HJ, Jeon S. A facile and sensitive detection of pathogenic bacteria using magnetic nanoparticles and optical nanocrystal probes. Analyst. 2012;137:3609-3612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alvarez PJ. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012;12:4271-4275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1667] [Cited by in RCA: 1281] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 25. | Lara HH, Ayala-Nunez NV, Ixtepan Turrent L, Rodriguez Padilla C. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J Microbiol Biotechmol. 2010;26:615-621. [RCA] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 404] [Article Influence: 25.3] [Reference Citation Analysis (0)] |