Published online Dec 12, 2013. doi: 10.5528/wjtm.v2.i3.32
Revised: September 4, 2013
Accepted: September 13, 2013
Published online: December 12, 2013
Processing time: 165 Days and 23.2 Hours
Dipeptidyl boronic acids are suitable candidates for the design of “pro-soft” drugs because recent studies have proven that these acids undergo a pH-dependent cyclization equilibrium, generating an inactive cyclic form under physiological conditions. Dipeptidyl boronic acids possess a wide range of potential targets, and the 26S proteasome appears to be one of the main targets. This multicatalytic complex is involved in intracellular protein turnover and is overexpressed in certain pathological conditions, such as malignancies, autoimmune diseases and neurodegenerative diseases. Bortezomib is the first-in-class derivative approved by the Food and Drug Administration for the treatment of hematological malignancies (i.e., relapsed and refractory multiple myeloma and mantle cell lymphoma) but is inactive against solid tumors due to an insufficient tissue distribution. The present study suggests a possible strategy for enhancing the in vivo performance of dipeptidyl boronic acids endowed with promising proteasome-inhibiting properties and their applicability as anticancer agents. In particular, dipeptidyl boronic acids might have a fruitful application as pro-soft drugs when an appropriate recognition motif serves as a substrate for a tumor-specific protease, generating the active form of the drug in situ and preventing systemic side effects after diffusion through cells and tissues.
Core tip: The design of “pro-soft” drugs is a promising strategy for enhancing the tissue specificity of drugs and for avoiding systemic adverse effects. This strategy might be applied to dipeptidyl boronic acids for use as proteasome inhibitors.
- Citation: Micale N. Peptide-based boronates: How to achieve tissue specificity in anticancer therapy. World J Transl Med 2013; 2(3): 32-35
- URL: https://www.wjgnet.com/2220-6132/full/v2/i3/32.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v2.i3.32
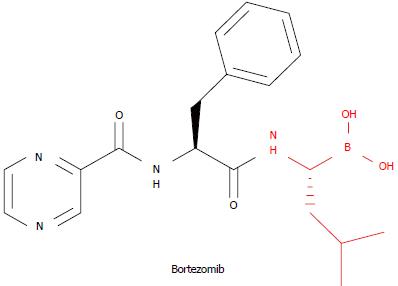
The approval of the 20S proteasome inhibitor bortezomib (Velcade®) by the Food and Drug Administration in 2003 for the treatment of multiple myeloma marked a historical moment in proteasome research[1]. This relatively small drug is a dipeptide boronate protected at its N-terminus by a pyrazinyl group that preferentially inhibits the chymotrypsin-like (β5) enzymatic sites of the proteasome, which are mainly involved in intracellular protein breakdown (Figure 1)[2]. Later, in 2006, the drug was approved for the treatment of another hematological malignancy, mantle cell lymphoma, further underscoring the importance of the 20S proteasome as a drug target in anticancer therapy[3]. Since this time, several research groups have focused their efforts on attempting to obtain similar compounds with a better pharmacological profile compared with this first-in-class derivative[4-11]. In fact, bortezomib presents certain shortcomings as a therapeutic agent, including its route of administration (intravenous bolus); its limited activity against solid tumors; the development of tumor resistance to bortezomib; and the drug’s dose-limiting adverse effect (reversible peripheral neuropathy), caused primarily by “on-target” inhibition of the proteasome in normal cells[12].
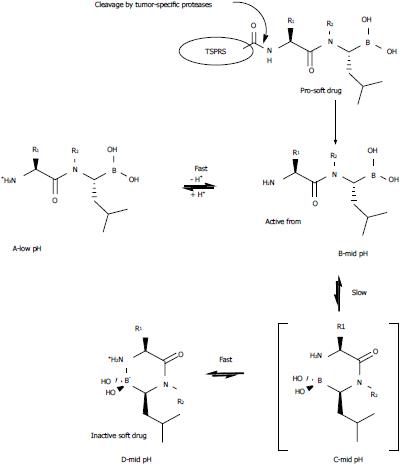
Based on the current literature, a dipeptide sequence represents the smallest chemical frame that affords efficacious proteasome inhibition, and a C-terminal Leu-boronic acid moiety ensures specificity for the β5 catalytic sites of the proteasome and influences the nature of the inhibition (covalent and slowly reversible; Figure 2). Moreover, the poor affinity between boron and sulfur atoms makes peptide boronates targetable by human cysteine proteases and suitable for application in vivo[13].
The inefficacy of bortezomib against solid tumors prompted Milo et al[14] to evaluate a strategy for enhancing the tissue specificity of various dipeptidyl Leu-boronic acids and to verify the applicability of this strategy. The strategy consists of designing a longer peptide-based prodrug in which the active boroLeu dipeptide fragment at the C-terminus can be released by a tumor-specific protease. This tumor specificity is relative because these proteases (generally glycoproteins) are also present in normal cells/tissues at a lower level. Regarding solid tumors, several specific proteases may undertake this activation role (e.g., fibroblast activation protein, prostate-specific antigen, and prostate-specific membrane antigen)[15-17]. However, the main issue with this strategy is represented by the free N-terminal amino group that is generated after the peptidic cleavage. Are the resulting dipeptides of boroLeu sufficiently potent, cell penetrating, and stable against degradation by cellular peptidases? Based on previous studies, the same authors knew that free NH2-terminal dipeptidyl boroPro undergoes a pH-dependent and reversible cyclization reaction that implies the nucleophilic attack of the amino group on the boron atom[18]. Milo et al[14] demonstrated that this pH-dependent equilibrium also exists for the dipeptides of boroLeu, although the cyclization is relatively modest compared with that exhibited by the dipeptides of boroPro. At physiological pH, the inactive cyclic-form D predominates, whereas at low pH, the active open-form A prevails. The loss of pharmacological activity with time is characteristic of compounds termed “soft drugs”, and the above-mentioned pH-dependent equilibrium might be exploited to obtain “pro-soft” drugs. The pro-soft drug will act as a substrate for a tumor-specific protease, which in turn will release the drug in its active open form; cyclization subsequent to release might limit adverse effects as excess inhibitor diffuses from the tumor site. Notably, tumor cellular pH is more acidic than the pH in a normal cell, which might favor the pH-dependent equilibrium of “pro-soft” drugs to selectively gain activity in tumor cells. The strategy of generating the active form of a dipeptidyl boroLeu from a pro-soft drug and its pH-dependent cyclization reaction are depicted in Figure 2.
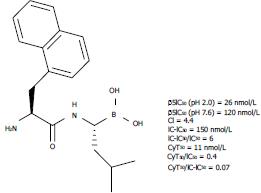
Given this information, Milo et al[14] synthesized and evaluated a wide series of dipeptides of boroLeu in vitro, demonstrating that despite the presence of a free amino group, these small molecules were comparable with bortezomib in terms of potency and cell-penetrating ability. Furthermore, these molecules were sufficiently cytotoxic and stable against degradation by aminopeptidases. The substitution pattern at P2 consisted of both natural and non-natural amino acids[14]. Structural and other significant data for the most druggable candidate (P2 = 1-naphthylalanyl) in the construction of pro-soft drugs are reported in Figure 3.
Pro-soft drug design is a strategy that, in principle, can be applied to wide variety of other inhibitors and targets. However, this strategy deserves special attention in cases, such as the case presented here, in which the tissue specificity of the first target (i.e., the protease that causes the removal of the recognition sequence and the release of the active drug) might play a decisive role in reducing side effects arising from the systemic activity of the drug. The pro-soft strategy is also more reliable when the activating and target enzymes are not the same, allowing enough time for the formation of the soft drug and its correct measurement. It is well known that dipeptidyl boronic acids may act as substrates for a wide range of enzymes[14,18], so the potential for the application of these molecules in drug design is very high. Proteasome inhibition may represent the research area with the best potential, and this strategy, when applied to dipeptidyl boronic acids, may extend the application of the proteasome inhibitors currently under study to solid tumors.
P- Reviewers: Dou QP, Morais C S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Field-Smith A, Morgan GJ, Davies FE. Bortezomib (Velcadetrade mark) in the Treatment of Multiple Myeloma. Ther Clin Risk Manag. 2006;2:271-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Kisselev AF, Callard A, Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem. 2006;281:8582-8590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 337] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 3. | Kane RC, Dagher R, Farrell A, Ko CW, Sridhara R, Justice R, Pazdur R. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13:5291-5294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 287] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 4. | Zhu Y, Zhao X, Zhu X, Wu G, Li Y, Ma Y, Yuan Y, Yang J, Hu Y, Ai L. Design, synthesis, biological evaluation, and structure-activity relationship (SAR) discussion of dipeptidyl boronate proteasome inhibitors, part I: comprehensive understanding of the SAR of alpha-amino acid boronates. J Med Chem. 2009;52:4192-4199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Zhu Y, Zhu X, Wu G, Ma Y, Li Y, Zhao X, Yuan Y, Yang J, Yu S, Shao F. Synthesis, in vitro and in vivo biological evaluation, docking studies, and structure--activity relationship (SAR) discussion of dipeptidyl boronic acid proteasome inhibitors composed of beta-amino acids. J Med Chem. 2010;53:1990-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Zhu Y, Wu G, Zhu X, Ma Y, Zhao X, Li Y, Yuan Y, Yang J, Yu S, Shao F. Synthesis, in vitro and in vivo biological evaluation, and comprehensive understanding of structure-activity relationships of dipeptidyl boronic acid proteasome inhibitors constructed from β-amino acids. J Med Chem. 2010;53:8619-8626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Watanabe T, Momose I, Abe M, Abe H, Sawa R, Umezawa Y, Ikeda D, Takahashi Y, Akamatsu Y. Synthesis of boronic acid derivatives of tyropeptin: proteasome inhibitors. Bioorg Med Chem Lett. 2009;19:2343-2345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Watanabe T, Abe H, Momose I, Takahashi Y, Ikeda D, Akamatsu Y. Structure-activity relationship of boronic acid derivatives of tyropeptin: proteasome inhibitors. Bioorg Med Chem Lett. 2010;20:5839-5842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Iqbal M, Messina McLaughlin PA, Dunn D, Mallya S, Husten J, Ator MA, Chatterjee S. Proteasome inhibitors for cancer therapy. Bioorg Med Chem. 2012;20:2362-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Dunn D, Iqbal M, Husten J, Ator MA, Chatterjee S. Serendipity in discovery of proteasome inhibitors. Bioorg Med Chem Lett. 2012;22:3503-3505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Micale N, Ettari R, Lavecchia A, Di Giovanni C, Scarbaci K, Troiano V, Grasso S, Novellino E, Schirmeister T, Zappalà M. Development of peptidomimetic boronates as proteasome inhibitors. Eur J Med Chem. 2013;64:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14:1649-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 447] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 13. | Borissenko L, Groll M. 20S proteasome and its inhibitors: crystallographic knowledge for drug development. Chem Rev. 2007;107:687-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 344] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 14. | Milo LJ, Lai JH, Wu W, Liu Y, Maw H, Li Y, Jin Z, Shu Y, Poplawski SE, Wu Y. Chemical and biological evaluation of dipeptidyl boronic acid proteasome inhibitors for use in prodrugs and pro-soft drugs targeting solid tumors. J Med Chem. 2011;54:4365-4377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Cheng JD, Valianou M, Canutescu AA, Jaffe EK, Lee HO, Wang H, Lai JH, Bachovchin WW, Weiner LM. Abrogation of fibroblast activation protein enzymatic activity attenuates tumor growth. Mol Cancer Ther. 2005;4:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Kraus TS, Cohen C, Siddiqui MT. Prostate-specific antigen and hormone receptor expression in male and female breast carcinoma. Diagn Pathol. 2010;5:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Perner S, Hofer MD, Kim R, Shah RB, Li H, Möller P, Hautmann RE, Gschwend JE, Kuefer R, Rubin MA. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 337] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 18. | Poplawski SE, Lai JH, Sanford DG, Sudmeier JL, Wu W, Bachovchin WW. Pro-soft Val-boroPro: a strategy for enhancing in vivo performance of boronic acid inhibitors of serine proteases. J Med Chem. 2011;54:2022-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |