Peer-review started: June 1, 2018
First decision: June 5, 2018
Revised: June 28, 2018
Accepted: June 30, 2018
Article in press: June 30, 2018
Published online: August 7, 2018
Processing time: 67 Days and 17.7 Hours
The incidence of the collapsing variant of focal segmental glomerulosclerosis (FSGS) as a human immunodeficiency virus (HIV)-associated nephropathy has reduced since the introduction of antiretroviral therapy (ART). However, the incidence of other variants of FSGS, except for the collapsing variant, is increasing, and its therapeutic strategies remain uncertain. A 60-year-old HIV infected man in remission with ART was admitted for progressive renal insufficiency and nephrotic-ranged proteinuria. Renal biopsy revealed a tip variant of FSGS and his clinical manifestations resolved with corticosteroid therapy. HIV infected patients might develop non-collapsing FSGS, including tip variant of FSGS and corticosteroid therapy might be effective for them. A renal biopsy might be essential to determine the renal histology and to decide on corticosteroid therapy.
Core tip: Collapsing variant of focal segmental glomerulosclerosis (FSGS) is the most common kidney disease in human immunodeficiency virus (HIV) infected patients. However, the incidence has reduced since the introduction of antiretroviral therapy (ART). Although the incidence of other variants of FSGS, except for the collapsing variant, is increasing, the tip variant of FSGS has been rarely reported. Therefore, we report an HIV infected patient under remission with ART, who presented with a rare tip variant of FSGS, which resolved with corticosteroid therapy. We suggest a renal biopsy might be essential to determine the renal histology and to decide on corticosteroid therapy.
- Citation: Goto D, Ohashi N, Takeda A, Fujigaki Y, Shimizu A, Yasuda H, Ohishi K. Case of human immunodeficiency virus infection presenting as a tip variant of focal segmental glomerulosclerosis: A case report and review of the literature. World J Nephrol 2018; 7(4): 90-95
- URL: https://www.wjgnet.com/2220-6124/full/v7/i4/90.htm
- DOI: https://dx.doi.org/10.5527/wjn.v7.i4.90
Human immunodeficiency virus (HIV) infection as a cause of various kidney diseases is well known. The collapsing variant of focal segmental glomerulosclerosis (FSGS), as an HIV-associated nephropathy (HIVAN), is one of the most frequently occurring kidney diseases[1]. HIVAN was first reported by Rao et al[2] in 1984. It is characterized by tubular abnormalities that include cellular degeneration and necrosis as well as cystic dilatation and interstitial changes that are edematous and often infiltrated by lymphocytes, in addition to the collapsing variant of FSGS[2].
Antiretroviral therapy (ART) is effective for preserving renal function and reversing HIVAN[3]. It is reported that the use of ART slows the progression to renal replacement therapy in patients with HIVAN[4]. However, ART has no beneficial effect on the renal function in patients with lesions other than HIVAN, including the tip variant of FSGS[4]. HIV-immune-complex kidney disease (HIVICK), thrombotic microangiopathy, and nephrotoxicity caused by ART are the other types of renal damage associated with HIV infection[3]. Although the occurrence of other variants of FSGS, except the collapsing variant, is increasing[5], the tip variant of FSGS is still a rare kidney disease in HIV infected patients[6]. We report here, a rare case of HIV associated nephrotic syndrome caused by the tip variant of FSGS, which was resolved with corticosteroid therapy.
A 60-year-old HIV positive Asian male was treated with ART (abacavir, atazanavir, and lamivudine) since the early 2000s. He had been HIV RNA negative since September 2010. There were no urinary abnormalities and his serum creatinine (sCr) level was within normal limits (sCr 0.72 mg/dL) in June 2015. However, he developed edema of the lower limbs beginning in the middle of June 2015 and gained 6 kg in two weeks. No symptoms of heart failure were found during the clinical course. His sCr level increased to 1.0 mg/dL. Atazanavir was changed to dolutegravir at the beginning of July 2015, due to suspicion of atazanavir-induced nephropathy. However, his sCr further increased to 1.99 mg/dL. Hypoalbuminemia (0.8 g/dL) and massive proteinuria (16.96 g/gCr) were observed in late July 2015 at which time he was admitted to our institute.
He had no other significant past history except for the HIV infection. He was on abacavir, lamivudine, and dolutegravir as ART, and azosemid to reduce the edema of the lower limbs. Physical examination on admission was as follows: Height 166 cm, weight 86.2 kg, body mass index (BMI) 31.3 kg/m2, body temperature 36.3 °C, blood pressure 114/89 mmHg, and regular pulse rate 101 beats/min. Extremities had remarkable pitting edema. Laboratory investigations were shown in Table 1.
| Hematology | Blood chemistry | Immunology | Urinalysis | ||||
| WBC | 7720/µL | Na | 133 mEq/L | CRP | 0.27 mg/dL | Protein | 11.28 g/24 h |
| CD4 | 549/µL | K | 4.1 mEq/L | IgG | 426 mg/dL | Sugar | 3+ |
| RBC | 567 × 104/µL | Cl | 106 mEq/L | IgA | 293 mg/dL | Occult blood | 3+ |
| Hb | 18.9 g/dL | Ca | 7.2 mg/dL | IgM | 83 mg/dL | Urinary RBC | 5-9/HPF |
| Hct | 53.20% | Pi | 2.6 mg/dL | CH50 | 48.7 U/mL | β2MG | 65500 g/L |
| Plt | 42.5 × 104/µL | BUN | 20.1 mg/dL | C3 | 159 mg/dL | NAG | 173.2 IU/L |
| sCr | 1.99 mg/dL | C4 | 42 mg/dL | Crystal | (-) | ||
| UA | 4.4 mg/dL | ANA | 40 × | ||||
| LDH | 243 IU/L | MPO-ANCA | < 1.0 U/mL | ||||
| AST | 23 IU/L | PR3-ANCA | < 1.0 U/mL | ||||
| ALT | 20 IU/L | anti-GBM Ab | < 2.0 U/mL | ||||
| TP | 3.9 g/dL | ||||||
| Alb | 0.8 g/dL | ||||||
| LDL-cho | 418 mg/dL | ||||||
| BS | 121 mg/dL | ||||||
| HIV RNA | (-) | ||||||
| HBS Ag | (-) | ||||||
| HCV Ab | (-) | ||||||
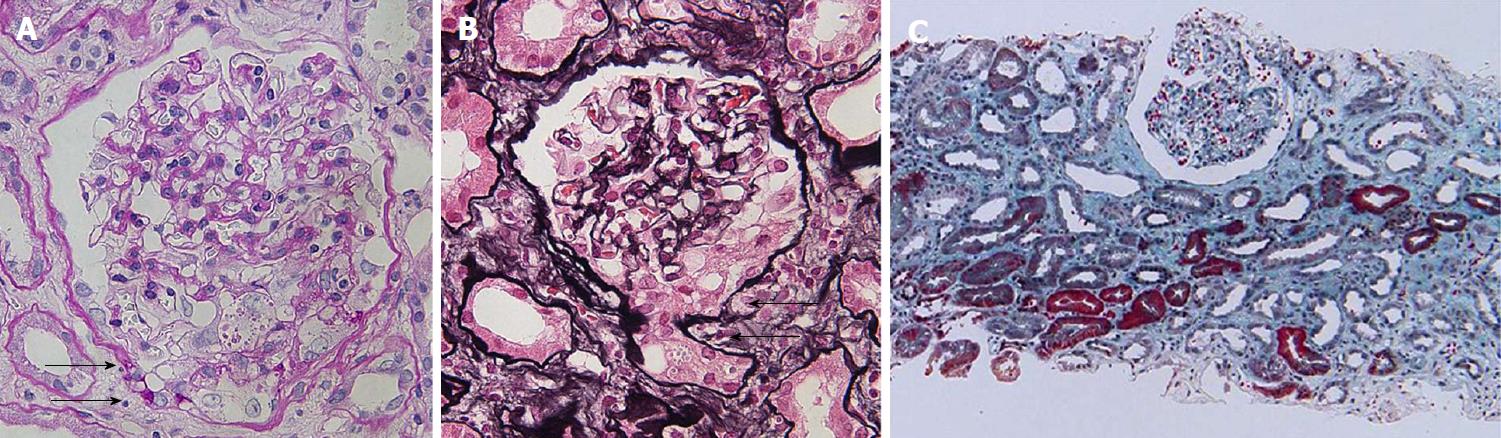
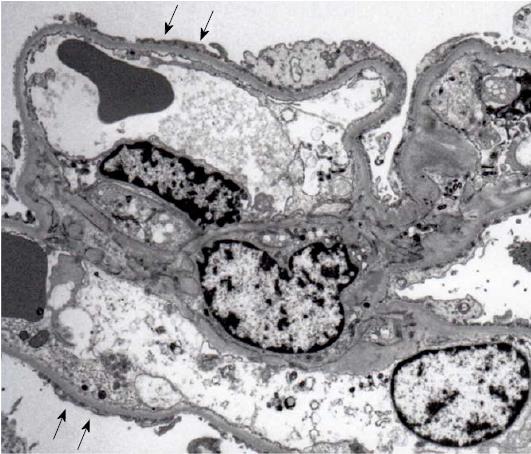
A renal biopsy was performed in late July 2015. The light microscopic findings revealed that 34 out of 35 glomeruli showed minor glomerular abnormalities without glomerular hyperfiltration. In addition, changes of the glomerular capillaries, such as spike formation and bubbling appearance, or glomerular nodular lesions were not found. However, one glomerulus showed epithelial hypercellularity at the tubular pole, where a confluence of the tubular cells at the tubular outlet was observed. No collapse of the glomerular tuft was seen. Although diffuse tubular atrophy and interstitial fibrosis were seen, infiltration of inflammatory cells was sparse and microcystic tubular dilatation associated with HIVAN was absent (Figure 1). Immunofluorescent microscopic examination showed the absence of immunoglobulins and complements (data not shown). Electron microscopy revealed a wide range of foot process effacement. No thickness of glomerular basement membrane was observed. Moreover, no tubulo-reticular inclusions in the glomerular endothelium were found and electron-dense deposits (EDDs) were absent (Figure 2). A diagnosis of tip variant of FSGS was made.
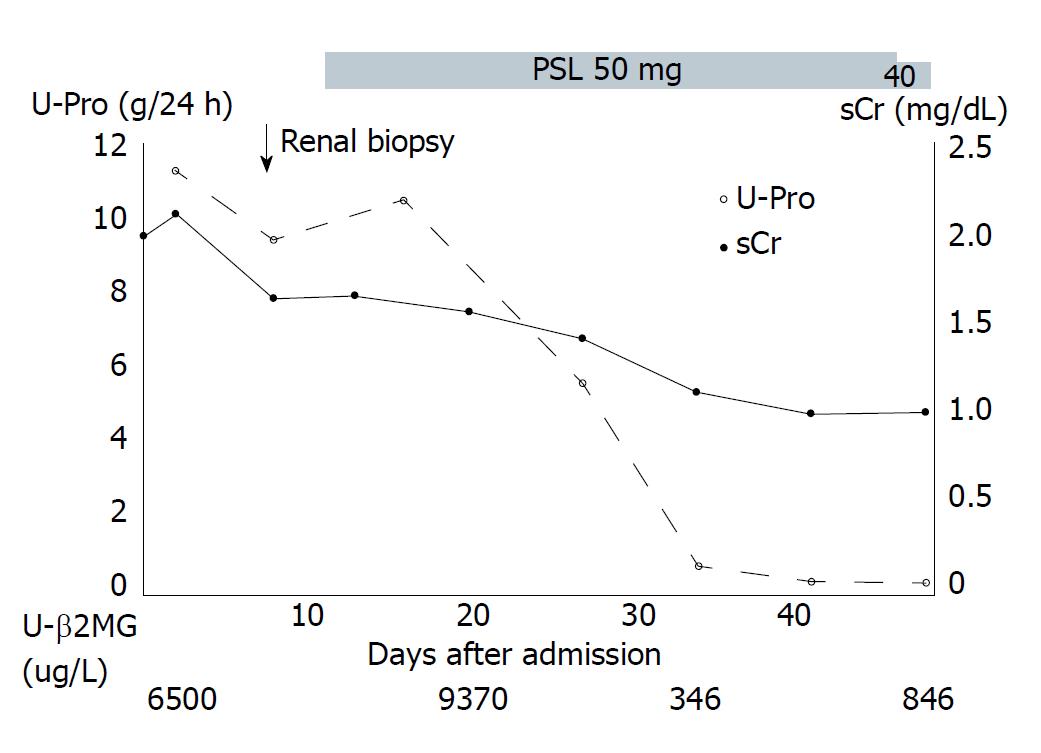
Following renal biopsy, corticosteroid therapy (prednisolone 50 mg/d) and angiotensin II receptor blocker (ARB) were administered to reduce the proteinuria. Three weeks later, proteinuria was absent and the levels of sCr and urinary β2-microglobulin decreased from 1.99 mg/dL to 0.99 mg/dL, and 65500 μg/L to 346 μg/L, respectively. Thereafter, the dose of prednisolone was tapered and there was no recurrence of proteinuria (Figure 3).
An HIV infected patient in remission with ART, was diagnosed with the tip variant of FSGS. Steroid treatment corrected the renal dysfunction and nephrotic-ranged proteinuria.
Although it is generally considered that HIV infection of the renal cells and HIV viral proteins play an important role in the occurrence and the progression of HIVAN[3], HIV RNA was negative in this case. It is known that HIV patients may develop non-collapsing FSGS, even when HIV mRNA levels are negative. Lescure et al[5] reported that FSGS was the primary diagnosis in 21 of the 32 patients (65% of total, HIVAN: 6 patients, non-collapsing FSGS: 15 patients) who underwent a renal biopsy from 2004 to 2007 when ART was the standard therapy for HIVAN. In addition, non-collapsing FSGS accounted for 15 of 26 patients (58%) who underwent renal biopsy for causes other than HIVAN[5]. The high incidence of non-collapsing FSGS in this study may be due to 66% of the included patients identified their race as black. It is known that patients of African descent are susceptible to genetic mutations of APOL1, which is associated with the development of FSGS[7]. Though the incidence of biopsy-proven idiopathic FSGS was reported to be 49% in black patients[8]. Thus, the prevalence of non-collapsing FSGS in patients with HIV infection seemed to be higher compared to that in black patients without HIV infection. Moreover, the patients with HIV infection might be susceptible not only to HIVAN but also to another variant of FSGS.
The tip variant of FSGS is a rare kidney disease in HIV infected patients. Meehan et al reported that only one of twenty-six patients developed a tip variant in a cohort of 46 HIV positive patients[6]. Howie and Breer defined the term glomerular tip lesion (GTL) as hypercellularity at the tubular pole in nephrotic patients for the first time[9]. Since GTLs are observed in some glomerular disorders such as membranous nephropathy, IgA nephropathy, or diabetic nephropathy, it is possible that GTLs are interpreted as nonspecific renal pathological findings[10]. However, since the rate of chronic kidney diseases and end-stage renal failure is higher in patients with GTLs compared to patients with minimal change nephrotic syndrome, GTLs are considered as a variant of FSGS[11]. However, the tip variant of FSGS exhibits unique characteristics among idiopathic FSGS. Several reports mention that the levels of interstitial fibrosis, global sclerosis, and glomeruli with segmental lesions were lower in the tip variant of FSGS and the rate of complete remission of the nephrotic syndrome is higher than that in not otherwise specified (NOS) variants of FSGS[11]. It is difficult to deny that GTLs were completely caused by other diseases. However, in this case no significant findings except for segmental lesions in one glomerulus were found in the light microscopic findings. The absence of immunoglobulins and complements by immunofluorescent microscopic examination and the absence of the thickening of the glomerular basement membrane and EDDs by electron microscopic examination excluded that the GTLs were caused by other diseases and confirmed the diagnosis of the tip variant of FSGS.
Because this patient was remarkably obese (BMI: 31.3 kg/m2), obesity-associated FSGS was suspected as a differential diagnosis. However, this patient’s clinical features were considerably different than those of obesity-associated FSGS based on the following evidence: (1) Although obesity was not improved, urinary protein levels were dramatically decreased by steroid treatment; and (2) Praga et al[12] reported that the levels of proteinuria in obesity-associated FSGS are lower and that the incidences of hypoalbuminemia and edema are less frequent than in patients with idiopathic FSGS. In addition, they indicated that glomerular hyperfiltration is more frequently found in renal biopsy specimens of patients with obesity-associated FSGS than in renal biopsy specimens of patients with idiopathic FSGS. Therefore, this case was unlikely to be of obesity-associated FSGS.
It could not be completely denied that this was a case of a patient with HIV infection and the tip variant of FSGS. However, Lescure et al[5] indicated that HIVAN decreased from 75% in 1995-2000 to 29% in 2004-2007 because of the introduction of ART, and that FSGS other than HIVAN conversely increased from 11.1% in 1995-2000 to 46.9% in the 2004-2007. The incidence of FSGS with HIV infection in both periods is much higher (86.1% in 1995-2000 and 75.9% in 2004-2007) than that of black patients without HIV infection (49%)[8]. Those results indicated that HIV infection is associated with the FSGS pathogenesis with or without the presence of HIV RNA. Additionally, immunodeficiency and dysregulation of immunoglobulin synthetic responses and T-cell function, which can lead to pathogenesis of kidney diseases, are increased in HIV infected patients[13]. It is likely that the corticosteroid therapy corrected the dysregulation of the immune system caused by the HIV infection in this tip variant of FSGS. However, the pathogenesis of FSGS without HIV RNA in HIV infected patients who were effectively treated with ART should be clarified.
At first, atazanavir-induced progressive renal insufficiency and nephrotic-ranged proteinuria were suspected. However, these did not improve after the discontinuation of atazanavir. Moreover, to the best of our knowledge, atazanavir-induced tip variant of FSGS has yet to be reported in literature. Therefore, we believe that atazanavir did not cause the massive progressive renal insufficiency and nephrotic-ranged proteinuria.
In this case, the level of urinary β2MG was markedly elevated at the time of admission and rapidly decreased with corticosteroid therapy. Exposure to tenofovir, indinavir, and atazanavir has been reportedly associated with a higher incidence of chronic kidney disease[14]. Atazanavir is known to cause crystalluria and urolithiasis[15]. In this case, the patient began receiving dolutegravir after it was suspected that the atazanavir was inducing nephropathy. Fujigaki et al[16] reported proximal tubular injuries in adults with minimal change nephrotic syndrome, which was probably due to massive proteinuria. As previously described, early discontinuation of atazanavir, as well as early remission of massive proteinuria, may improve the tubulointerstitial damage and rapidly decrease urinary β2MG excretion levels, as seen in this case.
In conclusion, this was a rare case of a patient in remission from HIV who presented with the tip variant of FSGS. Since the tip variant of FSGS can occur in HIV patients in remission with ART, a renal biopsy might be essential to determine the renal histology, and to decide on corticosteroid therapy. Further research with a larger sample size of patients is necessary to understand the mechanisms and efficacy of corticosteroid therapy for the tip variant of FSGS in HIV-infected patients.
We reported a human immunodeficiency virus (HIV) infected patient in remission with antiretroviral therapy (ART), who presented with a rare tip variant of focal segmental glomerulosclerosis (FSGS), which resolved with corticosteroid therapy.
We diagnosed the patient as a case of HIV infection presenting as a tip variant of FSGS.
HIV-associated nephropathy (HIVAN) or other causes of FSGS have to be differentiated because therapeutic strategies (ART or steroids) are different.
Whether HIV RNA levels are positive or negative are important.
Tip variant of FSGS is needed to diagnose that more than one glomerulus show epithelial hypercellularity at the tubular pole, where a confluence of the tubular cells at the tubular outlet is observed in renal biopsy specimen.
Steroid therapy is considered to administer to other causes of FSGS except for HIVAN including the tip variant.
Lescure et al is important for the readers to understand the changes of HIV-associated kidney glomerular diseases with time and ART.
Tip variant is one of the diagnoses in the Columbia classification of FSGS and is explained as follows: More than one glomerulus shows epithelial hypercellularity at the tubular pole, where a confluence of the tubular cells at the tubular outlet is observed in renal biopsy.
When renal damage is caused in HIV-infected patients, a renal biopsy may be essential to determine the renal histology and to decide on corticosteroid therapy.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): E
P- Reviewer: Chang CC, Pedersen EB, Stavroulopoulos A, Trimarchi H, Trkulja V S- Editor: Ji FF L- Editor: Filipodia E- Editor: Tan WW
| 1. | D’Agati V, Suh JI, Carbone L, Cheng JT, Appel G. Pathology of HIV-associated nephropathy: a detailed morphologic and comparative study. Kidney Int. 1989;35:1358-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 246] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Rao TK, Filippone EJ, Nicastri AD, Landesman SH, Frank E, Chen CK, Friedman EA. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:669-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 376] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Rosenberg AZ, Naicker S, Winkler CA, Kopp JB. HIV-associated nephropathies: epidemiology, pathology, mechanisms and treatment. Nat Rev Nephrol. 2015;11:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Szczech LA, Gupta SK, Habash R, Guasch A, Kalayjian R, Appel R, Fields TA, Svetkey LP, Flanagan KH, Klotman PE. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 247] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 5. | Lescure FX, Flateau C, Pacanowski J, Brocheriou I, Rondeau E, Girard PM, Ronco P, Pialoux G, Plaisier E. HIV-associated kidney glomerular diseases: changes with time and HAART. Nephrol Dial Transplant. 2012;27:2349-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Meehan SM, Kim L, Chang A. A spectrum of morphologic lesions of focal segmental glomerulosclerosis by Columbia criteria in human immunodeficiency virus infection. Virchows Arch. 2012;460:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 656] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 8. | Sim JJ, Batech M, Hever A, Harrison TN, Avelar T, Kanter MH, Jacobsen SJ. Distribution of Biopsy-Proven Presumed Primary Glomerulonephropathies in 2000-2011 Among a Racially and Ethnically Diverse US Population. Am J Kidney Dis. 2016;68:533-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Howie AJ, Brewer DB. The glomerular tip lesion: a previously undescribed type of segmental glomerular abnormality. J Pathol. 1984;142:205-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Howie AJ. Changes at the glomerular tip: a feature of membranous nephropathy and other disorders associated with proteinuria. J Pathol. 1986;150:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Arias LF, Franco-Alzate C, Rojas SL. Tip variant of focal segmental glomerulosclerosis: outcome and comparison to ‘not otherwise specified’ variant. Nephrol Dial Transplant. 2011;26:2215-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Praga M, Hernández E, Morales E, Campos AP, Valero MA, Martínez MA, León M. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2001;16:1790-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 185] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Stokes MB, Markowitz GS, Lin J, Valeri AM, D’Agati VD. Glomerular tip lesion: a distinct entity within the minimal change disease/focal segmental glomerulosclerosis spectrum. Kidney Int. 2004;65:1690-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Mocroft A, Kirk O, Reiss P, De Wit S, Sedlacek D, Beniowski M, Gatell J, Phillips AN, Ledergerber B, Lundgren JD; EuroSIDA Study Group. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24:1667-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 328] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 15. | Hara M, Suganuma A, Yanagisawa N, Imamura A, Hishima T, Ando M. Atazanavir nephrotoxicity. Clin Kidney J. 2015;8:137-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Fujigaki Y, Tamura Y, Nagura M, Arai S, Ota T, Shibata S, Kondo F, Yamaguchi Y, Uchida S. Unique proximal tubular cell injury and the development of acute kidney injury in adult patients with minimal change nephrotic syndrome. BMC Nephrol. 2017;18:339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |