Published online May 6, 2017. doi: 10.5527/wjn.v6.i3.123
Peer-review started: October 27, 2016
First decision: December 1, 2016
Revised: December 13, 2016
Accepted: February 28, 2017
Article in press: March 2, 2017
Published online: May 6, 2017
Processing time: 191 Days and 22.7 Hours
Heart failure and kidney disease share common pathophysiological pathways which can lead to mutual dysfunction, known as cardiorenal syndrome. In heart failure patients, renal impairment is related to hemodynamic and non-hemodynamic factors. Both decreased renal blood flow and renal venous congestion due to heart failure could lead to impaired renal function. Kidney disease and worsening renal function are independently associated with poor prognosis in heart failure patients, both in acute and chronic clinical settings. The aim of this review is to assess the role of renal imaging modalities in the evaluation and management of heart failure patients. Renal imaging techniques could complete laboratory data, as estimated glomerular filtration rate, exploring different pathophysiological factors involved in kidney disease and adding valuable information about renal structure and function. In particular, Doppler examination of arterial and venous hemodynamics is a feasible and non invasive technique, which has proven to be a reliable method for prognostic stratification in patients with cardiorenal syndrome. The renal resistance index, a measure related to renal hemodynamics, can be calculated from the Doppler evaluation of arterial flow. Moreover, the analysis of Doppler venous flow patterns can integrate information from the arterial study and evaluate renal congestion. Other imaging modalities are promising, but still confined to research purposes.
Core tip: Kidney disease is a common condition affecting heart failure patients. Cardiorenal syndrome defines the complex interaction with mutual deterioration of these organs. This review aims to describe currently available renal imaging techniques, especially arterial and venous Doppler, and to discuss the potential usefulness of promising research methodologies.
- Citation: Grande D, Terlizzese P, Iacoviello M. Role of imaging in the evaluation of renal dysfunction in heart failure patients. World J Nephrol 2017; 6(3): 123-131
- URL: https://www.wjgnet.com/2220-6124/full/v6/i3/123.htm
- DOI: https://dx.doi.org/10.5527/wjn.v6.i3.123
Over the last few years, there has been growing interest in the interaction between heart and kidney disease, especially in the clinical setting of heart failure (HF). Cardiovascular and renal diseases share common pathophysiological pathways, as the heart function is greatly influenced by fluid balance operated by the kidney. On the other hand, renal function is strictly dependent of blood flow and pressure, both downstream and upstream of the heart[1,2]. This interaction can lead to a mutual deterioration that has been defined as cardiorenal syndrome (CRS), i.e. the complex condition in which the acute or chronic impairment of one organ can lead to the deterioration of the other[3].
The mechanisms involved in the pathogenesis of renal dysfunction in acute and chronic scenarios are primarily hemodynamic. Both decreased renal blood flow and renal venous congestion are independent determinants of worsening renal function (WRF) in patients with heart failure[4]. Impaired cardiac index or intravascular volume depletion caused by aggressive diuretic therapy lead to renal hypoperfusion and eventually to reduction of glomerular filtration rate (GFR). Furthermore, renal venous congestion has an even greater importance in WRF[5]. High venous pressure can increase renal interstitial pressure and glomerular capillary pressure, impairing filtration function and reducing GFR[6]. In addition, non hemodynamic factors are involved in WRF, such as neuro-hormonal hyperactivation, endothelial dysfunction and underlying intrinsic kidney disease. Renal impairment and WRF are independently associated with mortality in HF patients, both in acute and chronic settings[7-10].
Different imaging modalities can explore renal dysfunction, regarding anatomy, perfusion and estimated pressures. The aim of this paper is to analyze the use of current techniques in the diagnosis and management of heart failure patients. Renal imaging techniques are summarized in Table 1, with respective advantages and limitations. In particular, we will focus on renal Doppler techniques used to calculate arterial resistive index and evaluate abnormal spectral venous patterns in order to identify venous congestion.
| Clinical relevance | Advantages | Limitations | |
| Bidimensional ultrasonography | Morphological evaluation (dimensions; echogenicity; corticomedullary ratio) | Fast; highly feasible in clinical practice; excludes obstructive uropathy and some parenchymal abnormalities | Nonspecific |
| Arterial RI (Doppler) | Indirect evaluation of arterial hemodynamics (resistances and vascular compliance) and parenchymal alterations | Fast; highly feasible in clinical practice; prognostic value | Influenced by renal obstructive arterial disease; lower accuracy with irregular rhythms |
| Venous pattern (Doppler) | Indirect evaluation of venous congestion, right atrial function and parenchymal compliance | Fast; highly feasible in clinical practice; prognostic value | Lack of standardization |
| Computed tomography | Evaluation of GFR through measurement of contrast wash-out | High spatial resolution | Nephrotoxicity of contrast media; radiation exposure |
| Nuclear imaging | GFR and RBF estimation through DTPA and MAG3 99mTC labelled uptake intensity measurement | Low radiation dose | Radiation exposure |
| MRI | No radiation exposure | Time consuming; expensive | |
| Phase-contrast MRI | Evaluation of RBF | No use of Gadolinium | Breathing movement artefacts |
| Dynamic MRI | Evaluation of GFR through measurement of signal attenuation | Reliable | Particularly time consuming (> 70 min) |
| BOLD MRI | Evaluation of renal parenchymal oxygenation exploiting superparamagnetic properties of desoxyHb | No use of Gadolinium; unique method to assess this parameter | Lack of standardization; low signal change of BOLD effect; no clinical applications |
| MRI detectable nanoparticles | Evaluation of single-glomerulus volume and structural changes | No use of Gadolinium; unique method to image renal parenchyma at microscopic level | Prone to movement artefacts; possible toxicity of nanoparticles; no clinical applications |
Ultrasound is a safe, low-cost and easily available technique for the morphological evaluation of kidneys. It is especially helpful in differentiating acute from chronic kidney disease and ruling out urinary obstruction as a cause of renal impairment. Normal renal long axis is 10-12 cm and is associated with height[11]. Reduced dimensions are usually seen in chronic kidney disease, although not every chronic alteration may show small kidneys. Increased kidney volume may occur in infiltrative diseases (lymphoma, amyloid nephropathy), parenchymal edema and inflammation, and renal vein thrombosis. Normal parenchyma is isoechoic or hypoechoic compared to the liver. Increased echogenicity is typically associated with chronic disease and is caused by the presence of fibrosis, inflammatory infiltrates and proteinaceous casts[12,13].
In the acute prerenal failure due to HF (i.e., cardiorenal syndrome type 1), there are no specific findings at the conventional ultrasound examination. In the absence of pre-existing abnormalities, renal dimensions and cortical thickness are generally normal. Increased echogenicity may occur in case of long-standing renal hypoperfusion. Furthermore, signs of urinary tract obstruction are absent and the bladder should be empty[14].
In cardiorenal syndrome type 2 nonspecific ultrasonographic parameters of chronicity have been observed. Renal dimensions are reduced, with thinner cortex and reduced cortex-medulla ratio. Hyperechoic cortical parenchyma is commonly caused by interstitial fibrosis. As for kidney disease associated with acute heart failure, no signs of urinary obstruction are expected, provided no renal comorbidity are present[15].
The role of ultrasound in HF patients is greater when the study of renal flow by pulsed Doppler technique is considered. In particular, Doppler evaluation allows the evaluation of both arterial and venous renal blood flow.
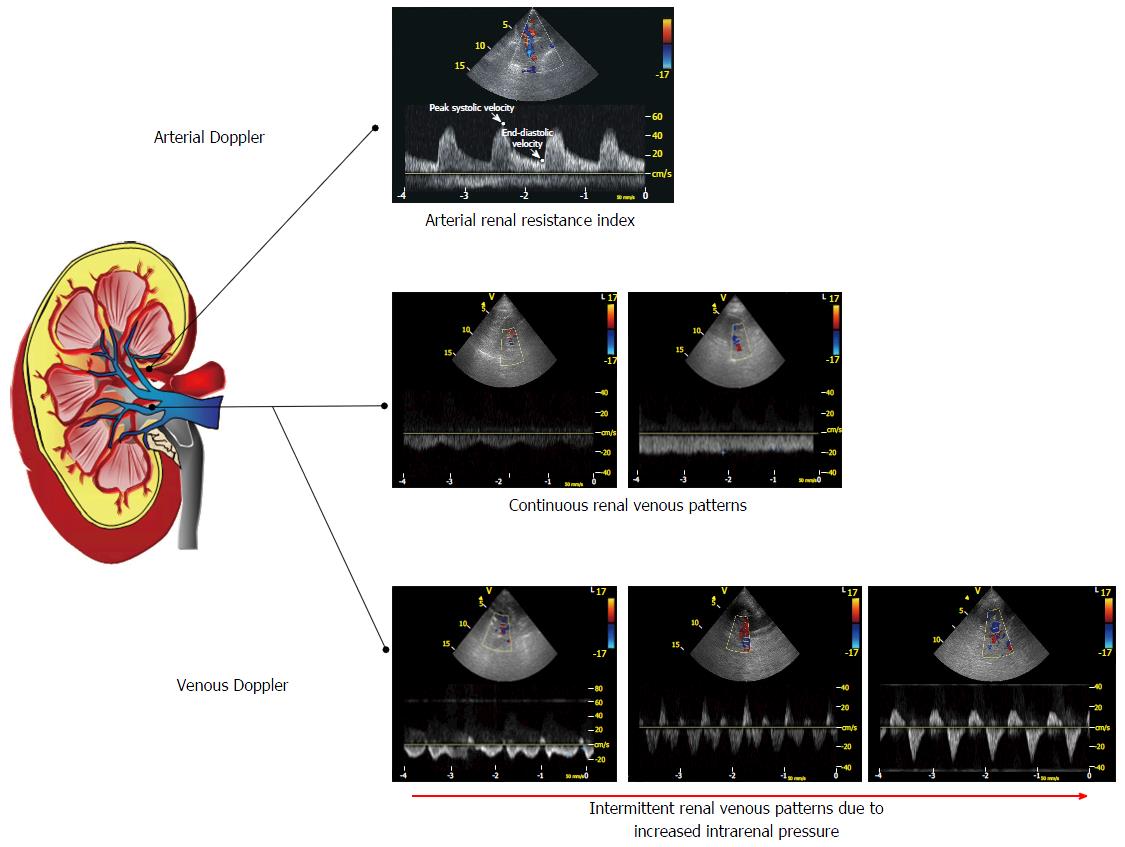
The evaluation of arterial flow provides a measure which reflects the complex pathophysiological mechanisms underlying the progression of renal kidney disease in HF, i.e., the arterial renal resistance index (RRI). The non invasive evaluation of renal arterial hemodynamics is currently allowed by use of Doppler ultrasound. Resistance index is a commonly used variable calculated by the spectral analysis of arterial Doppler waveform using Pourcelot’s formula, i.e., peak systolic velocity minus end-diastolic velocity, divided by peak systolic velocity[16] (Figure 1). In the kidney circulation, RRI is calculated analyzing the intrarenal arterial waveform at the level of segmental arteries[17]. The classical ultrasound technique used in order to evaluate renal arteries is the anterior approach with a convex probe. It is also possible to perform the Doppler analysis during a routine echocardiographic examination with the same probe and the patient in a sitting position, with a posterior approach to the kidney[18].
In healthy adults, normal renal RI values are around 0.60, with a difference between the two kidneys of less than 5%-8%[19]. An RI of 0.70 or higher is considered abnormal and generally predictive of an unfavourable outcome[17,20]. RI reproducibility is considered good in most cases: When performed by experienced investigators, intra-observer variability ranged from 2.07% to 5.1% and inter-observer variability ranged from 3.61% to 6.2%. Differences in RI values between 0.05 and 0.04 are not significant[21].
Glomerular blood pressure, and consequently renal blood flow (RBF), is one of the main driving forces of glomerular filtration. Unlike other organs, RBF is not primarily regulated on the basis of oxygen demand. Reflex (myogenic reflex and tubular-glomerular feedback) and neurohormonal mechanisms are mainly involved in the control of RBF, acting at the level of efferent and afferent arteriolar tone. The autoregulation mechanisms stabilize renal perfusion within a range of arterial pressure between 70 and 180 mmHg. RBF and GFR are adjusted in parallel and fluctuations of arterial pressure, both in physiological and in pathological conditions within the autoregulatory range, will not lead to substantial changes in filtration. Out of autoregulatory range, GFR becomes greatly dependent on RBF[22,23].
In normal conditions, a decrease in cardiac output (CO) will cause the autoregulatory system to reduce renal resistances, in order to keep renal flow (and GFR) within normal range. A depletion or an overrule of these regulatory systems by neurohormonal mechanisms, as in heart failure, could potentially determine a dissociation between CO and RBF, related to an increase of intrarenal resistances. So the increase of arterial resistances, e.g., due to hyperactivation of sympathetic nervous system, could lead to a disproportional decrease in renal perfusion pressure and RBF in the presence of reduced CO, that will result in a deteriorated glomerular filtration and WRF[23]. In renal circulation, the arterial resistances are affected by a number of factors other than the arteriolar tone: Arterial stiffness, atherosclerosis, parenchymal lesions and renal venous congestion.
A sustained increase in renal resistances, due to long-standing vasoconstriction, leads to a decrease in the number of the perfused vessels without anatomical changes in microvascular anatomy. The functional (or structural) loss of vessels has been termed vascular rarefaction[24]. A persistent functional rarefaction, whatever the cause, may lead to progressive vascular changes (i.e., vascular remodelling) caused by tissue ischemia, release of enzymes and growth factors which in turn result in fibrosis. Therefore the functional rarefaction could cause a structural rarefaction, with an actual reduction in renal vasculature and a permanent increase in arterial resistances[25]. The microvascular rarefaction is associated with reduced RBF and, eventually, impaired glomerular function.
RI doesn’t reflect vascular resistances with a totally linear relationship, instead it has a complex interaction with resistances and vascular compliance, as evaluated in experimental in-vitro and ex-vivo models[26-29]. Heart rate and rhythm disturbances can also independently affect RI values, as different diastolic duration in extreme bradycardia and tachycardia can alter end-diastolic velocity[30,31].
A recent study evaluated the physiological and pathological determinants of RI in a population of chronic heart failure patients with depressed ejection fraction. As expected from the results of experimental models, RI was independently correlated with factors commonly associated with renal artery abnormalities and increased arterial stiffness, such as age, diabetes, and pulse pressure. Other significant predictors are systolic pulmonary pressure, GFR, and NYHA class. Furthermore, central venous pressure (CVP) is also a significant determinant of RI, because an increase in CVP and intra-abdominal pressure will lead to an increase in renal interstitial pressure and consequently in vascular resistances[18].
The prognostic role of RI was initially evaluated in patients with renal parenchymal abnormalities. Increased RI values were associated with tubulointerstitial and vascular lesions at the renal biopsy[32]. Moreover, patients with higher values showed poorer renal prognosis, with a greater risk of worsening renal function over time[33].
In the clinical setting of HF, renal RI was first evaluated in a population of patients with HF with preserved ejection fraction. RI was higher in patients with HF, compared to controls affected only by arterial hypertension and it was also associated with poor prognosis[34]. The role of RI in predicting worse outcomes was also studied in the setting of HF with reduced ejection fraction[18]. In this series of CHF outpatients, RI was independently associated with a composite endpoint reflecting HF progression: Hospitalization due to acute decompensated HF, urgent heart transplantation and death due to worsening HF. The prognostic role of RI was independent from GFR, as patients with an RI above 0.75 were at higher risk of events irrespective of GFR values[18]. Subsequently, RI was also associated with all-cause mortality in the same population[35]. In the setting of CHF, RI was independently associated with one year worsening of renal function (increase in creatinine level > 0.3 mg/dL) and with an usage of high doses of loop diuretics[36,37]. Therefore higher values of RI could predict poor diuretic response and identify patients prone to diuretic resistance, possibly because increased renal resistances lead to a reduced delivery of diuretic drugs to the site of action.
Renal RI shows an incremental role compared to GFR in the evaluation of renal function in HF patients. Taking into account different pathophysiological pathways, RI could be used together with estimated GFR in order to better define renal function in this population, considering not only glomerular filtration function, but also hemodynamic and systemic factors eventually related to WRF and prognosis.
In addition to arterial flow profile, echo Doppler techniques allow the analysis of venous flow. The Doppler assessment of renal venous waveform is performed at the middle tract level of an interlobar vein, based on color Doppler signals. Venous waveform is recorded at the end of expiration of suspended respiration with a posterior approach and the patient in a lateral decubitus position or in a sitting position (Figure 1). Renal venous impedance index (VII) is a measure of vein pulsatility and is calculated from Doppler waveform, i.e., maximum velocity minus minimum velocity, divided by maximum velocity.
Besides reduced arterial flow, the role of increased central venous pressure and renal venous congestion is now widely accepted as one of the main pathophysiological mechanisms in impaired renal function both in chronic and acute decompensated heart failure[5,38]. Ex-vivo animal models, as far back as the 1931, have demonstrated the association between renal venous congestion and reduced RBF. In isolated kidneys, increased venous pressure leads to greater reduction in RBF, compared to changes in renal arterial pressure[39]. Subsequently, it has been reported that patients with HF have elevated renal venous pressure compared to subjects with no evidence of cardiovascular or renal disease[40].
The consequences of venous congestion for renal hemodynamics and function are different. A high renal venous pressure would cause congestion of peritubular and glomerular capillaries, reducing the arterio-venous gradient over renal circulation and thereby impairing blood flow. In normal kidneys, the autoregulation will keep GFR within normal range despite the decrease in RBF. If regulation mechanisms are depleted, the reduced pressure gradient could impair glomerular filtration and lead to renal dysfunction. Furthermore, venous hypertension will also be transmitted to the low-compliance interstitium, because of the stiffness of renal capsule, causing an increase in interstitial pressure. The high renal interstitial pressure will increase tubular pressure and, upstream, also the Bowman’s capsule hydrostatic pressure, reducing the gradient between glomerular capillary pressure and capsular pressure, one of the basic forces for filtration function. Decreasing the net filtration pressure, renal congestion will directly affect GFR, independently from RBF[17].
Renal veins have phasic flow associated with respiration and right atrial function. Left vein phasicity is attenuated because of the entrapment in the fork between the abdominal aorta and the superior mesenteric artery. Veins are high capacitance vessels, with negligible resistance to blood flow, so pulsatility is strictly dependent on surrounding tissue compliance. The main determinants of intrarenal vein flow patterns are parenchymal histology, central venous pressure and abdominal pressure[41]. Besides the association with tissue characteristics, renal venous flow is strictly dependent on right heart function. The increase in CVP during end-diastole (corresponding to atrial contraction) can be transmitted to renal veins, especially in patients with congested inferior vena cava and hepatic veins as in HF, causing the damping or eventually the reversal of vein flow.
Renal venous flow was initially evaluated in obstructive kidney disease: Since one of the main determinants of pulsatility is parenchymal compliance, it has been thought that Doppler waveform could be useful to diagnose acute obstructive uropathy before anatomical alterations occur. In fact reduced tissue compliance, caused by uropathy, results in dampening of the renal venous signal[42,43]. Moreover, venous Doppler study showed a greater sensitivity than RI in order to evaluate obstructive uropathy[44]. Venous waveforms have also been evaluated in a population of patients with diabetic nephropathy: Diabetic patients show diminished venous modulation, with significantly lower VII values compared to healthy subjects. This could be due to interstitial fibrosis progressively reducing parenchymal compliance[41].
Iida et al[45] evaluate intrarenal venous flow and VII in a population of outpatients and hospitalized patients affected by heart failure. They found three vein flow patterns: Continuous, biphasic discontinuous and monophasic discontinuous. Patients with monophasic flow were generally older, with worse renal function, elevated right atrial pressure (RAP) and lower hepatic S/D ratio. At the multivariate analysis intermittent patterns were associated with significant hemodynamic factors reflecting venous congestion. In particular, monophasic pattern was associated with right ventricular fractional area change, moderate to severe tricuspid regurgitation, higher RAP and lower hepatic S/D ratio. Furthermore, discontinuous patterns were associated with worse prognosis, independently of CVP and hepatic vein flow[45].
Recently our group evaluated renal venous waveform in a large population of HF outpatients with reduced ejection fraction. Five venous patterns were identified: Flow with normal velocity decrease of telediastolic velocity without interruption (pattern A); continuous flow with reduced modulation (pattern B); forward flow with short presystolic interruption or reversal (pattern C); polyphasic intermittent flow (pattern D); monophasic intermittent flow, with one forward and one reversal wave (pattern E). As in the previous study, patients with intermittent flow patterns (pattern D and E) had worse clinical conditions in our series: They had higher estimated RAP and pulmonary artery systolic pressure, more severe tricuspid regurgitation and worse renal function. Moreover, we confirmed the prognostic role of venous patterns in HF: Pattern C, D and E were associated with events during follow-up at the univariate and multivariate analysis. Patients with intermittent pattern (D and E) showed worse prognosis compared to other groups, while pattern C was associated with an intermediate risk for events. In pattern C we observed the interruption or reversal of flow during atrial contraction, probably related to mild increase in CVP. This pattern may identify patients in the early phase of renal congestion. Differently from the study of Iida et al[45], there were no prognostic differences between polyphasic and monophasic intermittent patterns, they are both related to worse venous congestion and identify patients at higher risk[46].
In conclusion, venous Doppler evaluation shows an incremental prognostic role in the clinical setting of heart failure. Information about renal congestion carried by venous patterns may integrate the arterial study performed with RI in order to better characterize the renal impairment in HF.
Albeit computed tomography (CT) is considered the gold standard in the assessment of renal stones and masses, its role in the evaluation of kidney function, referred as GFR and RBF, is limited in clinical practice. The so-called “functional CT” can provide useful information about GFR, but it is currently only performed in a few centres and its suitability, particularly in patients with impaired renal function, is hampered by the risk of contrast induced nephrotoxicity[47].
In this regard, Hackstein et al[48] have demonstrated a good correlation between GFR, measured with iopromide plasma clearance (considered the gold standard method in this study), and GFR measured by triphasic helical CT of the abdomen (i.e., unenhanced examination followed by two contrast medium enhanced exams in the arterial and portovenous phase). Nevertheless this study highlighted several limitations regarding the routine use of this technique in clinical practice; first of all, patients with acute kidney injury having been excluded from recruitment, they could not be eligible for this techique; secondly, to justify radiation exposure, GFR measurement by functional CT was performed in patients who underwent triphasic helical CT for clinical reasons other than kidney dysfunction[48]. In the setting of heart failure, there is very scarce literature about this topic.
The functional evaluation of kidneys is commonly performed with the use of radioisotopes, with Technetium (99m Tc) preferred above all for its short half-life and low radiation exposure, bound to non-metabolized molecules, namely DTPA for GFR measurement and MAG3 for renal blood flow. This technique can help us to distinguish between the different forms of AKI. In case of prerenal azotemia (as in the setting of cardiorenal syndrome type 1) renal uptake of MAG3 is normal, within 1-2 min following injection, whereas in parenchymal and vascular diseases (as in Acute Tubular Necrosis) it is expected to be reduced. In the late phase, about 20 min after tracer injection, we would expect a rise in renal uptake in patients with pre-renal or renal AKI, and a decrease of it in case of obstructive uropathy[47,49].
In addition to functional data, important structural information can be obtained by performing Positron Emission Tomography (PET). This technique, when combined with CT scanning has the great advantage of combining anatomic and functional data, thus enabling localization of abnormally functioning renal areas. However nowadays its clinical applications are scarce and usually limited to research purposes[47].
Magnetic resonance imaging (MRI) offers the optimal combination between spatial resolution and functional evaluation, in other words between a structural and a functional study[50,51]. Furthermore, it avoids the use of iodinated contrast media and radioisotopes, which is obviously of great advantage in patients suffering from CKD. The main issues with this promising imaging modality are the lack of standardization in its different protocols, with the subsequent inter-observer variability, and the need for interventional studies that should validate this technique showing their clinical value.
MRI provides a pretty wide range of approaches in the assessment of renal function: From the classical parameters GFR and RBF to more innovative ones, such as renal blood oxygenation[52] and single glomerulus volume[53].
Information about RBF is classically obtained by phase-contrast MRI of the renal artery. This method exploits the magnetic properties of the water molecules, located in the blood, when excited by the magnetic fields[54]. This can let us avoid the use of gadolinium and hence, it is particularly useful in those patients, with severely reduced kidney function (GFR < 30 mL/min), in whom the use of gadolinium would expose the risk of SNF (systemic nephrogenic fibrosis).
On the other hand, the determination of GFR is substantially based on the evaluation of the filtration rate of a substance (gadolinium) which is filtered by the glomerular membrane but not reabsorbed from the tubular lumen, just like inulin (used as the gold standard in the assessment of GFR).
In this regard, the most common approaches in clinical practice are two[52]: The first, Patlak-Rutland plot technique, refers to a two compartmental model (only preglomerular and intra-tubular compartments are taken into account) and evaluates the change in signal intensity of a ROI located in the renal cortex; the extent of the signal increase in the cortex after bolus injection is correlated with glomerular filtration rate[55]. The second consists of evaluating the GFR by considering the wash-out of the filtration marker, i.e., gadolinium, from the interstitial space, represented by a ROI in the liver[56]. This method has been adopted in both healthy and CKD affected patients showing reasonably good results[57,58]. The main draw-back is the long time required for acquisition (about 70 min)[52].
Alternatively, GFR can be obtained indirectly from the measurement of renal blood flow (RBF) and renal extraction fraction (REF) which is derived from the intensity gradient of the gadolinium between the renal artery and renal vein. As the REF is equal to the ratio between GFR and RBF, the GFR is determined through the product between RBF and REF[52,59].
Recently, the world of MRI for the study of kidney function has seen a further development towards the microscopic level and molecular imaging, such as with blood oxygen level dependent (BOLD) MRI and MRI detectable nanoparticles. The BOLD MRI offers the unique chance of evaluating renal tissue oxygenation relying on the different magnetic properties of haemoglobin. Oxygenated haemoglobin is diamagnetic, while deoxygenated haemoglobin shows paramagnetic activity, thus yielding a drop in the T2* curve that corresponds to a decrease in image signal intensity[60]. Despite still being in a primordial phase and hampered by the variability of absolute values between the studies, this technique shows indisputable advantages when compared to other gross types of renal function evaluation and could open the door to a possible use in clinical settings not necessarily related only to primitive renal disease. This could be the case, for example, with cardiorenal syndrome type 1, i.e., acute deterioration of kidney function secondary to heart failure. We know from physiology, confirmed by a few studies[52], that renal parenchyma exhibits inhomogeneous oxygenation and that the medulla region is more sensitive to a reduction in blood oxygen supply. We can then postulate that, a drop in renal perfusion, secondary to heart dysfunction, could lead to a rise in deoxygenated blood to the medulla and thus in T2* curve decay well before an increase in serum creatinine would be detectable.
When the limits of BOLD effect are overcome we will be able to use this technique to deeply understand the mechanisms underlying CRS and, possibly, promptly recognize and prevent kidney ischemia at an early stage. Currently there are no studies or trials available about this topic.
Moreover, the technique based on utilization of MRI detectable nanoparticles is also promising.
This revolutionary method would allow the evaluation of the volume and of the structural changes involving the single glomerulus, using an MRI detectable targeted marker. The specific molecule adopted is ferritin, which, with its superparamagnetic properties, can perturbate the magnetic fields of nearby protons and reduce their T2 and T2* relaxation time. This, in turn, will result in a reduction of the MRI signal intensity or, in other words, in a dark spot. Furthermore, ferritin is artificially cationized in order to consent binding with negative charges of proteoglycans located in the basal glomerulus membrane (BGM). In this way, after the systemic injection of the detectable molecule, we are able to image every glomerulus as a dark spot in the renal cortex[53].
The horizons opened by this method are obviously huge but, for now, confined mainly to preclinical studies.
Kidney disease in the setting of heart failure identifies patients prone to adverse events independently from cardiac function. Therefore, the assessment of renal function has a key role in the management of HF patients. Renal imaging could have a complementary role in relation to laboratory data: Imaging techniques explore different pathophysiological pathways involved in renal disease and may identify a worsening of function even before an increase in serum creatinine occurs. In particular, Doppler examination of arterial and venous waveforms provides a feasible and non invasive evaluation of renal hemodynamics, considering systemic and parenchymal factors involved in cardiorenal interaction. With regard to other imaging modalities, even though they seem promising in providing insight to kidney function and microscopic changes, most of them are, so far, still confined to research purposes.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aspromonte N, Iyngkaran P, Prickett TCR S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36:1437-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 371] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 2. | Husain-Syed F, McCullough PA, Birk HW, Renker M, Brocca A, Seeger W, Ronco C. Cardio-Pulmonary-Renal Interactions: A Multidisciplinary Approach. J Am Coll Cardiol. 2015;65:2433-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1271] [Cited by in RCA: 1412] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 4. | Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 340] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 5. | Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1323] [Cited by in RCA: 1175] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 6. | Iacoviello M, Puzzovivo A, Monitillo F, Saulle D, Lattarulo MS, Guida P, Forleo C, Gesualdo L, Favale S. Independent role of high central venous pressure in predicting worsening of renal function in chronic heart failure outpatients. Int J Cardiol. 2013;162:261-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681-689. [PubMed] |
| 8. | Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203-210. [PubMed] |
| 9. | Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 623] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 10. | Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH). Eur J Heart Fail. 2009;11:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Emamian SA, Nielsen MB, Pedersen JF, Ytte L. Kidney dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers. AJR Am J Roentgenol. 1993;160:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 260] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Page JE, Morgan SH, Eastwood JB, Smith SA, Webb DJ, Dilly SA, Chow J, Pottier A, Joseph AE. Ultrasound findings in renal parenchymal disease: comparison with histological appearances. Clin Radiol. 1994;49:867-870. [PubMed] |
| 13. | Nomura G, Kinoshita E, Yamagata Y, Koga N. Usefulness of renal ultrasonography for assessment of severity and course of acute tubular necrosis. J Clin Ultrasound. 1984;12:135-139. [PubMed] |
| 14. | Di Lullo L, Floccari F, Granata A, D’Amelio A, Rivera R, Fiorini F, Malaguti M, Timio M. Ultrasonography: Ariadne’s Thread in the Diagnosis of the Cardiorenal Syndrome. Cardiorenal Med. 2012;2:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Moghazi S, Jones E, Schroepple J, Arya K, McClellan W, Hennigar RA, O’Neill WC. Correlation of renal histopathology with sonographic findings. Kidney Int. 2005;67:1515-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Pourcelot L. Velocimetrie ultrasonore Doppler. Seminaire INSERM. Paris, France: Edition INSERM 1974; 213-240. |
| 17. | Tublin ME, Bude RO, Platt JF. Review. The resistive index in renal Doppler sonography: where do we stand? AJR Am J Roentgenol. 2003;180:885-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 302] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 18. | Ciccone MM, Iacoviello M, Gesualdo L, Puzzovivo A, Antoncecchi V, Doronzo A, Monitillo F, Citarelli G, Paradies V, Favale S. The renal arterial resistance index: a marker of renal function with an independent and incremental role in predicting heart failure progression. Eur J Heart Fail. 2014;16:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Darmon M, Schnell D, Zeni F. Doppler-based renal resistive index: a comprehensive review. Yearbook of intensive care and emergency medicine. Heidelberg: Springer; 2010; 331-338. |
| 20. | Parolini C, Noce A, Staffolani E, Giarrizzo GF, Costanzi S, Splendiani G. Renal resistive index and long-term outcome in chronic nephropathies. Radiology. 2009;252:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Lubas A, Kade G, Niemczyk S. Renal resistive index as a marker of vascular damage in cardiovascular diseases. Int Urol Nephrol. 2014;46:395-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Smilde TD, Damman K, van der Harst P, Navis G, Westenbrink BD, Voors AA, Boomsma F, van Veldhuisen DJ, Hillege HL. Differential associations between renal function and “modifiable” risk factors in patients with chronic heart failure. Clin Res Cardiol. 2009;98:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Braam B, Cupples WA, Joles JA, Gaillard C. Systemic arterial and venous determinants of renal hemodynamics in congestive heart failure. Heart Fail Rev. 2012;17:161-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Chade AR. Renal vascular structure and rarefaction. Compr Physiol. 2013;3:817-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Prewitt RL, Chen II, Dowell R. Development of microvascular rarefaction in the spontaneously hypertensive rat. Am J Physiol. 1982;243:H243-H251. [PubMed] |
| 26. | Gosling RG, Lo PT, Taylor MG. Interpretation of pulsatility index in feeder arteries to low-impedance vascular beds. Ultrasound Obstet Gynecol. 1991;1:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Bude RO, Rubin JM. Relationship between the resistive index and vascular compliance and resistance. Radiology. 1999;211:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 259] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Murphy ME, Tublin ME. Understanding the Doppler RI: impact of renal arterial distensibility on the RI in a hydronephrotic ex vivo rabbit kidney model. J Ultrasound Med. 2000;19:303-314. [PubMed] |
| 29. | Tublin ME, Tessler FN, Murphy ME. Correlation between renal vascular resistance, pulse pressure, and the resistive index in isolated perfused rabbit kidneys. Radiology. 1999;213:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Mostbeck GH, Gössinger HD, Mallek R, Siostrzonek P, Schneider B, Tscholakoff D. Effect of heart rate on Doppler measurements of resistive index in renal arteries. Radiology. 1990;175:511-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Krumme B, Hollenbeck M. Doppler sonography in renal artery stenosis--does the Resistive Index predict the success of intervention? Nephrol Dial Transplant. 2007;22:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Platt JF, Ellis JH, Rubin JM, DiPietro MA, Sedman AB. Intrarenal arterial Doppler sonography in patients with nonobstructive renal disease: correlation of resistive index with biopsy findings. AJR Am J Roentgenol. 1990;154:1223-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 182] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Hanamura K, Tojo A, Kinugasa S, Asaba K, Fujita T. The resistive index is a marker of renal function, pathology, prognosis, and responsiveness to steroid therapy in chronic kidney disease patients. Int J Nephrol. 2012;2012:139565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Ennezat PV, Maréchaux S, Six-Carpentier M, Pinçon C, Sediri I, Delsart P, Gras M, Mounier-Véhier C, Gautier C, Montaigne D. Renal resistance index and its prognostic significance in patients with heart failure with preserved ejection fraction. Nephrol Dial Transplant. 2011;26:3908-3913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Monitillo F, Citarelli G, Antoncecchi V, Doronzo A, Paradies V, Leone M, Puzzovivo A, Iacoviello M, Ciccone MM. Independent and incremental role of renal resistance index in predicting mortality among heart failure outpatients. Eur J Heart Fail. 2014;16:126. |
| 36. | Monitillo F, Citarelli G, Antoncecchi V, Doronzo A, Leone M, Paradies V, Puzzovivo A, Iacoviello M, Ciccone MM. A high renal arterial resistance index is associated to one year worsening of renal function in heart failure outpatients. Eur J Heart Fail. 2014;16:126-127. |
| 37. | Citarelli G, Monitillo F, Leone M, Antoncecchi V, Doronzo A, Paradies V, Puzzovivo A, Iacoviello M, Ciccone MM. The presence of an altered renal arterial resistance index is independently associated with the increase of loop diuretic diuretic dose in heart failure outpatients. Eur J Heart Fail. 2014;16:223. |
| 38. | Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 700] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 39. | Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol. 1931;72:49-61. [PubMed] |
| 40. | Maxwell MH, Breed ES, Schwartz IL. Renal venous pressure in chronic congestive heart failure. J Clin Invest. 1950;29:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Jeong SH, Jung DC, Kim SH, Kim SH. Renal venous doppler ultrasonography in normal subjects and patients with diabetic nephropathy: value of venous impedance index measurements. J Clin Ultrasound. 2011;39:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Bateman GA, Cuganesan R. Renal vein Doppler sonography of obstructive uropathy. AJR Am J Roentgenol. 2002;178:921-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Oktar SO, Yücel C, Ozdemir H, Karaosmanoglu D. Doppler sonography of renal obstruction: value of venous impedance index measurements. J Ultrasound Med. 2004;23:929-936. [PubMed] |
| 44. | Vadana BM, Pasumarthy A, Penumalli N, Bellapa NC. Renal Venous Doppler Study in Obstructive Uropathy. J Clin Diagn Res. 2015;9:TC13-TC15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 45. | Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, Ishizu T, Kawakami Y, Aonuma K. Clinical Implications of Intrarenal Hemodynamic Evaluation by Doppler Ultrasonography in Heart Failure. JACC Heart Fail. 2016;4:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 46. | Puzzovivo A, Iacoviello M, Monitillo F, Leone M, Rizzo C, Lattarulo MS, Grande D, Terlizzese P, Massari F, Ciccone MM. Renal venous pattern: a new parameter for predicting cardiorenal syndrome progression. Eur J Heart Fail. 2016;18:162-163. |
| 47. | Kalantarinia K. Novel imaging techniques in acute kidney injury. Curr Drug Targets. 2009;10:1184-1189. [PubMed] |
| 48. | Hackstein N, Wiegand C, Rau WS, Langheinrich AC. Glomerular filtration rate measured by using triphasic helical CT with a two-point Patlak plot technique. Radiology. 2004;230:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Haufe SE, Riedmüller K, Haberkorn U. Nuclear medicine procedures for the diagnosis of acute and chronic renal failure. Nephron Clin Pract. 2006;103:c77-c84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Michaely HJ, Sourbron S, Dietrich O, Attenberger U, Reiser MF, Schoenberg SO. Functional renal MR imaging: an overview. Abdom Imaging. 2007;32:758-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Laissy JP, Idée JM, Fernandez P, Floquet M, Vrtovsnik F, Schouman-Claeys E. Magnetic resonance imaging in acute and chronic kidney diseases: present status. Nephron Clin Pract. 2006;103:c50-c57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Artunc F, Rossi C, Boss A. MRI to assess renal structure and function. Curr Opin Nephrol Hypertens. 2011;20:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Charlton JR, Beeman SC, Bennett KM. MRI-detectable nanoparticles: the potential role in the diagnosis of and therapy for chronic kidney disease. Adv Chronic Kidney Dis. 2013;20:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Roberts DA, Detre JA, Bolinger L, Insko EK, Lenkinski RE, Pentecost MJ, Leigh JS. Renal perfusion in humans: MR imaging with spin tagging of arterial water. Radiology. 1995;196:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Hackstein N, Heckrodt J, Rau WS. Measurement of single-kidney glomerular filtration rate using a contrast-enhanced dynamic gradient-echo sequence and the Rutland-Patlak plot technique. J Magn Reson Imaging. 2003;18:714-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 56. | Boss A, Martirosian P, Gehrmann M, Artunc F, Risler T, Oesingmann N, Claussen CD, Schick F, Küper K, Schlemmer HP. Quantitative assessment of glomerular filtration rate with MR gadolinium slope clearance measurements: a phase I trial. Radiology. 2007;242:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Artunc F, Yildiz S, Boss A, Frenzel T, Schlemmer HP, Schick F, Risler T, Häring HU, Rossi C. Measurement of glomerular filtration rate using dynamic magnetic resonance imaging in patients with chronic kidney disease. J Nephrol. 2011;24:482-489. [PubMed] |
| 58. | Mendichovszky I, Pedersen M, Frøkiaer J, Dissing T, Grenier N, Anderson P, McHugh K, Yang Q, Gordon I. How accurate is dynamic contrast-enhanced MRI in the assessment of renal glomerular filtration rate? A critical appraisal. J Magn Reson Imaging. 2008;27:925-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 59. | Katzberg RW, Buonocore MH, Low R, Hu B, Jain K, Castillo M, Troxel S, Nguyen MM. MR determination of glomerular filtration rate in subjects with solitary kidneys in comparison to clinical standards of renal function: feasibility and preliminary report. Contrast Media Mol Imaging. 2009;4:51-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Simon-Zoula SC, Hofmann L, Giger A, Vogt B, Vock P, Frey FJ, Boesch C. Non-invasive monitoring of renal oxygenation using BOLD-MRI: a reproducibility study. NMR Biomed. 2006;19:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |