Peer-review started: June 11, 2016
First decision: July 11, 2016
Revised: August 29, 2016
Accepted: October 25, 2016
Article in press: October 27, 2016
Published online: January 6, 2017
Processing time: 200 Days and 4 Hours
To ascertain the frequency of hyponatremic hypertensive syndrome (HHS) in a cohort of children with hypertensive emergency in a tertiary pediatric hospital.
A retrospective review was undertaken among children with hypertensive emergency admitted in our tertiary children hospital between June 2014 and December 2015 with an aim to identify any children with HHS. Three children with HHS were identified during this period.
The 3 patients with HHS presented with hypertensive emergency. They were initially managed with Labetalol infusion and thereafter switched to oral anti-hypertensives (combination of Nifedipine sustained release, Hydralazine and Beta Blocker). All 3 were diagnosed to have unilateral renal artery stenosis. One child was lost to follow up, whereas the other 2 underwent renal angioplasty which was followed with normalization of blood pressure.
Despite activation of renin angiotensin axis secondary to renal artery stenosis, these groups of children have significant hyponatremia. Renal re-vascularisation produces excellent results in most of them.
Core tip: Renovascular hypertension can occur secondary to renal artery stenosis which can sometime present as hyponatremic hypertensive syndrome (HHS). Though deemed a rare disease we identified within a span of 18 mo, 3 children with HHS presentation characterized by hypertension, hyponatremia, hypokalemia and metabolic alkalosis. Despite activation of renin angiotensin axis secondary to renal artery stenosis, they had significant hyponatremia. Renal re-vascularisation produced excellent results in 2 children who underwent surgery whereas another child was lost to follow up.
- Citation: Mukherjee D, Sinha R, Akhtar MS, Saha AS. Hyponatremic hypertensive syndrome - a retrospective cohort study. World J Nephrol 2017; 6(1): 41-44
- URL: https://www.wjgnet.com/2220-6124/full/v6/i1/41.htm
- DOI: https://dx.doi.org/10.5527/wjn.v6.i1.41
Reno vascular hypertension secondary to renal artery stenosis is often a treatable cause of pediatric and accounts for 5%-25% cases of secondary hypertension[1]. Unilateral renal artery stenosis presenting as hypertensive hyponatremic syndrome (HHS) has been infrequently reported in pediatrics, with only three case series till date[2-4]. Our study was performed in order to determine its incidence among children admitted with hypertensive emergency in a tertiary care pediatric hospital.
We undertook a retrospective case notes review of all children admitted with hypertensive emergency within a period of 18 mo from June 2014 to December 2015. Ten such children were identified all of whom had undergone biochemical and radiological investigation as needed for determination of disease etiology. Out of this cohort 3 were diagnosed to have HHS.
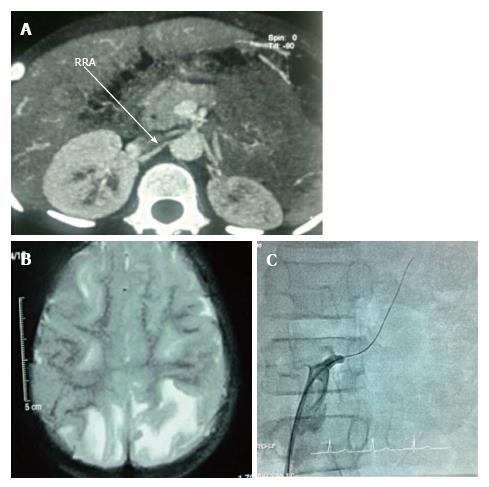
The 5 years old boy presented with convulsions and vomiting for 6 d and obtunded sensorium for 3 d. Examination revealed elevated blood pressure (BP) (236/132 mmHg, 95thP = 108/71), brisk deep tendon reflexes with extensor planters and eye examination consistent with hypertensive retinopathy. He was having feeble peripheral pulses and was hypovolemic. He also had hyponatremic, hypokalemic, metabolic alkalosis with normal creatinine and elevated renin and aldosterone (Table 1). Ultrasonography (USG) showed a small left kidney (5.7 cm) with loss of cortico-medullary distinction and enlarged hyperechogenic right kidney (7.1 cm). Computed tomography angiography (Figure 1A) confirmed occlusion of left renal artery distal to the origin, along with duplication of the artery. Magnetic resonance imaging (MRI) brain showed posterior reversible encephalopathy syndrome (PRES).
| Case 1 | Case 2 | Case3 | |||
| Pre renal vascularisation | Pre renal vascularisation | Post renal vascularisation | Pre renal vascularisation | Post renal vascularisation | |
| Serum sodium (mmol/L) | 112 | 127 | 139 | 126 | 137 |
| Serum potassium (mmol/L) | 3.2 | 3.1 | 3.9 | 3.2 | 4.2 |
| Serum calcium (mg/dL) | 7.9 | 7.5 | 8.8 | 8.4 | 8.7 |
| Serum albumin (mg/dL) | 2.3 | 2.7 | 3.6 | 2.9 | 3.9 |
| Urine protein | 3+ | 2+ | Negative | 2+ | Negative |
| Urine RBCs | Absent | Absent | Absent | Absent | Absent |
| Urine protein/creatinine ratio | 6.847 | 3.912 | < 0.2 | 4.362 | < 0.2 |
| Urine sodium (mmol/24 h) | 115 | 123 | 33 | 109 | 28 |
| Blood pH | 7.448 | 7.532 | 7.422 | 7.605 | 7.392 |
| Blood bicarbonate | 33.4 | 31.8 | 27.2 | 32.2 | 25.4 |
| Serum renin (ng/mL per hour) (normal: 1.31-3.95) | 21.06 | 32.8 | 3.05 | 25.04 | 3.24 |
| Serum aldosterone (ng/dL) (normal: 1-16) | 117.2 | 143.6 | 14.2 | 135.8 | 15.8 |
| Urine osmolality mOsm/kg of water | Not done | Not done | 770 | ||
| MRI brain | PRES | PRES | PRES | ||
Eight years old boy presented with complain of headache for a week with increasing thirst and polyuria. He was having altered sensorium and convulsions prior to admission. He appeared hypovolemic with feeble peripheral pulses, possibly contributed by the polyuria. His BP was 184/110 mmHg (95thP = 114/75). There was left sided hemiparesis along with 6th and 7th nerve palsy. Ophthalmological examination was consistent for hypertensive retinopathy. Investigations revealed hyponatremic, hypokalemic, metabolic alkalosis along with normal creatinine (Table 1). USG with Doppler revealed bilateral hyper-echogenic kidneys with right relatively bigger (7.5 cm) than the left (6.2 cm) and suspected decreased blood flow in the left kidney. Renin and aldosterone was elevated. Digital subtraction angiography (DSA) of kidneys confirmed 90% stenosis of the left renal artery. MRI brain was consistent for PRES (Figure 1B).
The 12 years old boy was admitted with headache for 1 mo, increased thirst, polyuria for 15 d and obtunded sensorium for 4 d. There was a documented decrease in weight of 800 g over the last 2 wk, possibly because of the polyuria. BP was 244/166 mmHg (95thP = 121/79). Ophthalmological examination was consistent with hypertensive retinopathy. Investigation showed hyponatremic, hypokalemic, metabolic alkalosis and normal creatinine (Table 1). USG doppler revealed “parvus- tardus” like flow in left renal artery, with enlarged hyper-echogenic right kidney. MRI brain was consistent with PRES. Digital subtraction angiography confirmed left renal artery stenosis (Figure 1C).
All three cases had high urinary sodium indicating natriuresis as the cause of the hyponatremia (Table 1).
The patients were diagnosed to have HHS presenting as hypertensive emergency. They were initially managed with Labetalol infusion and thereafter switched to oral anti-hypertensives (combination of Nifedipine sustained release, Hydralazine and Beta Blocker). Case 1 and 2 were given 3% NaCl and all required potassium supplements. Electrolyte abnormalities gradually corrected with normalization of BP.
Case 1 was advised PTA but was lost to follow up after 3 mo till when he was hypertensive (132/86 mmHg) despite being on Enalapril, Nifedipine and Atenolol. His electrolytes had normalized but he continued to have significant proteinuria; case 2 underwent percutaneous transluminal balloon angioplasty (PTA), which restored normal blood flow. His sodium normalized in 2 wk and urinalysis by 8 wk. At last follow up (20 mo) he remains off anti hypertensive with normal BP and renal profile; Case 3 underwent a failed balloon angioplasty of left renal artery followed by successful renal artery stenting. At 9 mo follow up, his electrolytes and urinalysis had normalized and he had normal BP (110/70 mmHg) on 1.25 mg of Enalapril. The USG changes in the contra-lateral kidney also normalized by last follow up for Case 2 and 3.
Hilden reported a series of cases in 5 adults associated with hypertensive encephalopathy, hypochloremia and hyponatremia. This was coined as HHS by Seracini et al[1]. It is characterized by severe hypertension along with hyponatremia secondary to natriuresis, making this association unique, as hypertension is usually associated with activation of renin aldosterone axis (RAS), which is characterized with salt retention. Although deemed rare we identified 3 children with HHS in our cohort of 10 children with hypertensive emergency within a span of 18 mo.
Atkinson et al[5] postulated that unilateral decreased renal blood flow results in increased renin secretion which increases the level of angiotensin II (AII) and thereby the level of aldosterone. Increased AII causes arterial hypertension resulting in pressure diuresis and natriuresis from the non-contracted normally functioning contra-lateral kidney, thereby causing polyuria and polydipsia. AII and aldosterone causes potassium loss and ultimately hypokalemia, which further stimulates renin secretion. Hyponatremia has also been known to increase aldosterone secretion[5]. Polyuria causes increasing thirst and volume depletion and thereby causes secretion of anti diuretic hormone, which also contributes to hyponatremia. Thus, activation of RAS in presence of the hypertrophied contra-lateral kidney sets up a vicious cycle leading to natriuresis and hyponatremia along with the usual hypokalemia and metabolic alkalosis[5]. Glycosuria and hypercalciuria suggesting tubule interstitial involvement have also been reported[2,6]. Glomerular hyper-filtration secondary to arterial hypertension results in proteinuria as well as the reversible cortical hyperechogenicity of the normal kidney[1-5,7]. As seen in our series, correcting the stenosis and controlling the BP usually corrects the proteinuria, electrolyte abnormalities and the hyper-echogenecity of the normal kidney[1-4].
Altered sensorium seen in our children was likely secondary to hypertensive encephalopathy and all our cases had MRI brain consistent with PRES[8]. In addition, concomitant hyponatremia might also have been contributing factor.
Management includes initial BP control followed by correction of the underlying etiology which in our cases was the left renal artery stenosis. In presence of unilateral renal artery stenosis, angiotensin converting enzyme inhibitors are the preferred medications but this should be avoided if bilateral stenosis is suspected. Revascularization with PTA is the preferred final intervention[1-3,5,9].
In conclusion, we hope our findings will increase the awareness of this syndrome as this unique combination of hypertension, hyponatremia and nariuresis is strongly suggestive of compromised unilateral renal blood flow. As we identified three such cases over a span of only 18 mo, we do echo statement by other academicians that this may not be as rare as it is thought to be[3].
Renovascular hypertension can occur secondary to renal artery stenosis (RAS), which can sometime present as hyponatremic hypertensive syndrome (HHS). Despite activation of RAS secondary to renal artery stenosis, they had significant hyponatremia.
This study was performed in order to determine its incidence among children admitted with hypertensive emergency in a tertiary care pediatric hospital.
Renal re-vascularisation produced excellent results.
The findings will increase the awareness of this syndrome as this unique combination of hypertension, hyponatremia and nariuresis is strongly suggestive of compromised unilateral renal blood flow.
HHS: High blood pressure with low plasma sodium.
This is a well-written paper.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: India
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aggarwal A, Friedman EA, Pedersen EB S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Seracini D, Pela I, Favilli S, Bini RM. Hyponatraemic-hypertensive syndrome in a 15-month-old child with renal artery stenosis. Pediatr Nephrol. 2006;21:1027-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Trivelli A, Ghiggeri GM, Canepa A, Oddone M, Bava G, Perfumo F. Hyponatremic-hypertensive syndrome with extensive and reversible renal defects. Pediatr Nephrol. 2005;20:102-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Kovalski Y, Cleper R, Krause I, Dekel B, Belenky A, Davidovits M. Hyponatremic hypertensive syndrome in pediatric patients: is it really so rare? Pediatr Nephrol. 2012;27:1037-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Masavkar S, Shanbag P, Belsare N. Hyponatremia in a child with Takayasu arteritis: questions. Pediatr Nephrol. 2013;28:61-62, 63-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Atkinson AB, Morton JJ, Brown JJ, Davies DL, Fraser R, Kelly P, Leckie B, Lever AF, Robertson JI. Captopril in clinical hypertension. Changes in components of renin-angiotensin system and in body composition in relation to fall in blood pressure with a note on measurement of angiotensin II during converting enzyme inhibition. Br Heart J. 1980;44:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Tullus K, Brennan E, Hamilton G, Lord R, McLaren CA, Marks SD, Roebuck DJ. Renovascular hypertension in children. Lancet. 2008;371:1453-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Enríquez G, Castelló F, Sousa P, Aso C, Lucaya J. Increased cortical echogenicity of the normal kidney in infants with unilateral renal artery stenosis: report of two cases. J Ultrasound Med. 1997;16:59-63. [PubMed] |
| 8. | Wright RR, Mathews KD. Hypertensive encephalopathy in childhood. J Child Neurol. 1996;11:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | McLaren CA, Roebuck DJ. Interventional radiology for renovascular hypertension in children. Tech Vasc Interv Radiol. 2003;6:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |