Peer-review started: July 1, 2016
First decision: September 5, 2016
Revised: September 22, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: January 6, 2017
Processing time: 181 Days and 15.4 Hours
To evaluate the effects of the non-selective, non-steroidal anti-inflammatory drug (NSAID) acetylsalicylic acid (ASA), on ex vivo embryonic kidney growth and development.
Pairs of fetal mouse kidneys at embryonic day 12.5 were cultured ex vivo in increasing concentrations of ASA (0.04-0.4 mg/mL) for up to 7 d. One organ from each pair was grown in control media and was used as the internal control for the experimental contralateral organ. In some experiments, organs were treated with ASA for 48 h and then transferred either to control media alone or control media containing 10 μmol/L prostaglandin E2 (PGE2) for a further 5 d. Fetal kidneys were additionally obtained from prostaglandin synthase 2 homozygous null or heterozygous (PTGS2-/- and PTGS2-/+) embryos and grown in culture. Kidney cross-sectional area was used to determine treatment effects on kidney growth. Whole-mount labelling to fluorescently detect laminin enabled crude determination of epithelial branching using confocal microscopy.
Increasing ASA concentration (0.1, 0.2 and 0.4 mg/mL) significantly inhibited metanephric growth (P < 0.05). After 7 d of culture, exposure to 0.2 mg/mL and 0.4 mg/mL reduced organ size to 53% and 23% of control organ size respectively (P < 0.01). Addition of 10 μmol/L PGE2 to culture media after exposure to 0.2 mg/mL ASA for 48 h resulted in a return of growth area to control levels. Application of control media alone after cessation of ASA exposure showed no benefit on kidney growth. Despite the apparent recovery of growth area with 10 μmol/L PGE2, no obvious renal tubular structures were formed. The number of epithelial tips generated after 48 h exposure to ASA was reduced by 40% (0.2 mg/mL; P < 0.05) and 47% (0.4 mg/mL; P < 0.01). Finally, growth of PTGS2-/- and PTGS2+/- kidneys in organ culture showed no differences, indicating that PTGS2 derived PGE2 may at best have a minor role.
ASA reduces early renal growth and development but the role of prostaglandins in this may be minor.
Core tip: Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used as painkillers and are available without prescription. They act by inhibiting cyclooxygenase activity thereby preventing prostaglandin synthesis via the prostaglandin synthase enzymes prostaglandin synthase 1 (PTGS1) and PTGS2. Although NSAIDs cross the placenta, they are among the most common drugs prescribed during the first trimester of pregnancy and estimated prevalence of usage is more than 25% of pregnant women. NSAID use during pregnancy has been associated with abnormal renal development and renal failure in the offspring at birth. These may impact the individual throughout their adult life.
- Citation: Welham SJM, Sparrow AJ, Gardner DS, Elmes MJ. Acetylsalicylic acid interferes with embryonic kidney growth and development by a prostaglandin-independent mechanism. World J Nephrol 2017; 6(1): 21-28
- URL: https://www.wjgnet.com/2220-6124/full/v6/i1/21.htm
- DOI: https://dx.doi.org/10.5527/wjn.v6.i1.21
Non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin and ibuprofen are commonly used analgesics for the treatment of low-grade pain and inflammation. NSAIDs are available without prescription and are frequently taken during pregnancy[1]. Up to 25% of women self-prescribe NSAIDs to treat common ailments during pregnancy[2-4]. Although there is clear evidence to suggest that aspirin and other NSAIDs can cross the placenta, recent research on aspirin use in pregnancy has largely been based on the examination of structural birth defects as an outcome[5-7]. The key findings from these studies were that NSAID use during pregnancy does not appear to be a major risk factor for inducing birth defects. However, potentially more subtle effects of maternal NSAID consumption on fetal tissue and organ development have received little attention.
NSAIDs act by inhibition of cyclooxygenase enzymes which catalyse the conversion of arachidonic acid to prostaglandins. Two isoforms exist, prostaglandin synthase 1 (PTGS1) and prostaglandin synthase 2 (PTGS2). Although they share a very similar structure they differ in their pattern of regulation and physiological function[8]. PTGS1 is expressed constitutively in most tissues and is thought to have a cellular “housekeeping” and cytoprotective role[9]. In contrast, PTGS2 is induced as a result of an inflammatory response in most tissues[10], but is constitutively expressed in the fetal and adult kidney[11], highlighting the role for PTGS2 derived prostaglandins in adult kidney function and suggesting a role in kidney development.
Fetal rat kidneys can synthesise prostaglandins prostaglandin E2 (PGE2), PGD2, PGI2, and PGF2α, as well as thromboxanes and leukotrienes[12,13]. This corresponds to intense PTGS2 immunoreactivity and mRNA expression in a subset of thick ascending limb epithelial cells near the macula densa in developing nephrons suggesting a role for PTGS2 derivatives in the maturation and/or early function of nephrons[14]. Disruption of prostaglandin synthesis during early renal development through PTGS2 inhibition suggests that prostaglandins may be required for normal renal development[15-17]. PTGS2 knockout mice exhibit a severe reduction in the number of nephrons[16] and postnatally are observed to possess many immature glomeruli and tubules as well as a thinner outer cortex[18].
PTGS2 inhibitors, such as Aspirin, impair glomerulogenesis during gestation[19]. Clinical studies have revealed various abnormalities such as renal maldevelopment and renal non function syndrome in newborns of mothers treated with NSAIDs during pregnancy[20,21]. Histological analysis of the kidneys revealed crowded glomeruli and few differentiated proximal tubules in the inner cortex[20]. Long term effects of NSAID administration during pregnancy reveal structural and functional abnormalities in the rodent kidney. Ultra-structural changes in fetal glomeruli in rats treated with indomethacin were demonstrated by Sessa et al[22], in the form of enlarged Golgi. A further study provides evidence that chronic administration of indomethacin to pregnant rhesus monkeys also causes neonatal renal hypoplasia decreasing kidney mass by 15%[23].
Evidence from PTGS2-/- mice, however, suggests that the role of prostaglandins in renal development is most likely to be during postnatal maturation in these animals. PTGS2-/- mice have apparently normal kidneys at birth, but subsequently exhibit developmental impairment in the postnatal period immediately prior to the final termination of development. This suggests either that the role for prostaglandins derived from PTGS2 in renal development is only during this short postnatal stage of maturation, or that sufficient prostaglandins are obtained exogenously from the mother prior to birth.
In this study, we examined the requirement for prostaglandin production on early kidney development using global blockade with acetyl-salicylic acid (ASA). We show a significant and terminal detrimental effect on growth of ex-vivo fetal mouse kidneys after exposure to ASA and further that this effect is likely to be independent of the inhibition of prostaglandin generation from the PTGS2 gene due to successful culture of PTGS2-/- organs.
All reagents were purchased from Sigma-Aldrich chemical company (Poole, Dorset, United Kingdom) unless otherwise stated.
The present investigation was performed in accordance with the Home Office Guidance on the operation of the Animals (Scientific Procedures) Act (Great Britain Home Office, 2000). Outbred ICR (Institute for Cancer Research) mice were maintained on a 12-h light-dark cycle with free access to standard laboratory chow and water. Male and female mice were housed together and embryonic day 0.5 (E0.5) was designated upon detection of a vaginal plug, indicating mating, on the floor of the cage. Litters were removed 12 d after plugging. Whole metanephroi were micro-dissected from the E12.5 embryos under sterile conditions, at which point, they were termed Day 0 for ex vivo studies (Barak and Boyle 2011). Ethical approval for this study was provided by the University of Nottingham’s local Animal Welfare and Ethical Review Board.
Fetal kidneys were cultured individually on Millicell inserts (12 mm diameter, 0.4 μm pore; Millipore Corporation, Billerica, MA, United States) in 24-well culture plates with DMEM F-12 media supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), insulin (10 mg/L), sodium selenite (5 g/L), and transferrin (5.5 mg/L) at 37 °C in a humidified atmosphere of air-5% CO2 for up to 7 d. Media was changed every 24-48 h. For experimental NSAID treatment, DMEM media was supplemented with 0.04 mg/mL (0.2 mmol/L), 0.1 mg/mL (0.56 mmol/L), 0.2 mg/mL (1.1 mmol/L) or 0.4 mg/ml (2.2 mmol/L) acetyl salicylic acid (ASA; aspirin) in line with other in vitro studies[24-27].
Recovery experiments included 48 h pre-treatment with 0.2 mg/mL ASA before replacing with control media or media supplemented with 10 μmol/L PGE2 from day 3-7. All metanephroi that were treated with ASA and or PGE2 were compared to their untreated contra-lateral controls for analysis.
During 7 d of culture, metanephros growth was monitored and recorded every 24 h using a Leica DMIL microscope [Leica Microsystems Ltd, Milton Keynes, Bucks, United Kingdom] fitted with a Leica DFC420C digital camera. Cross-sectional areas of whole metanephroi were determined using Image Pro Plus v5.1 (Media Cybernetics, Corporation, Silver Spring, MD, United States). A free-hand drawing tool was used to precisely draw around each metanephros and calculate the total cross-sectional surface area. Cross sectional surface area measurements were converted to μm2 (using micrometer validation) and metanephroi treated with ASA and or PGE2 were compared with their untreated contralateral controls.
Kidneys were fixed in 4% PFA in PBS for 20 min, washed in PBS, permeabilised in 0.2% triton X 100 in PBS overnight at 4 °C. All 4 °C incubation steps were carried out with rolling. Antibodies were diluted in PBS. Organs were washed in PBS incubated with the primary antibody against laminin (L9393, Sigma; incubated at 1:100 dilution) overnight at 4 °C. Kidneys were washed in PBS and incubated with secondary antibody (Anti-Rabbit IgG, FITC F0205, Dako UK Ltd; incubated at 1:20 dilution) overnight at 4 °C. Finally, organs were washed three times in PBS and mounted on slides (pre-prepared with a ring of hardened DPX to preserve the 3D structure of the kidney) in Vectashield Hardset (Vector Laboratories). Organs were imaged using a confocal microscope [Leica Microsystems (UK) Ltd, Milton Keynes, Bucks, United Kingdom].
Statistical analysis was performed using SPSS version 16. Growth data were analysed with day of culture (0 = baseline, day 1 and day 2) as a repeated measure with genotype as a fixed effect in the model. Nested effects in the model, e.g., siblings (males and females) from the same litter and paired organs from the same individual (left kidney = control, right kidney = treated) were accounted for in the model as random effects (litterID/pupID). Analysis of data at single time points was by one-way ANOVA, blocking for individual ID. Results are presented as means ± SEM with aP≤ 0.05 considered statistically significant. Letters are used throughout to represent statistical differences as follows; bP < 0.01 and dP < 0.005.
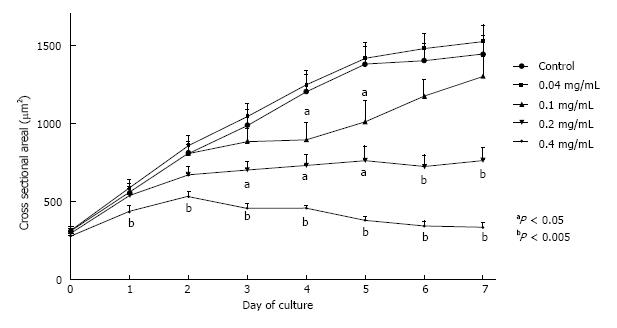
E12.5 mouse kidneys were cultured for seven days in the presence or absence of ASA. Control organs grew robustly over the course of 7 d, increasing in size by more than 3.5 fold (386%; Figure 1). Incubation in ASA at 0.04 mg/mL did not affect organ growth, however incubation in concentrations of ASA above 0.04 mg/mL resulted in increasingly severe growth impairment (Figure 1). Growth of kidneys exposed to 0.2 mg/mL ASA was significantly reduced by day 3 of treatment and organs remained growth impaired for the duration of culture (P < 0.05; Figure 1). Doubling the concentration of ASA to 0.4 mg/mL led to more severe growth restriction by as early as 24 h of culture (P < 0.05; Figure 1). Continued exposure to ASA (> 0.04 mg/mL) beyond three days of culture consistently resulted in organ disintegration by day 7.
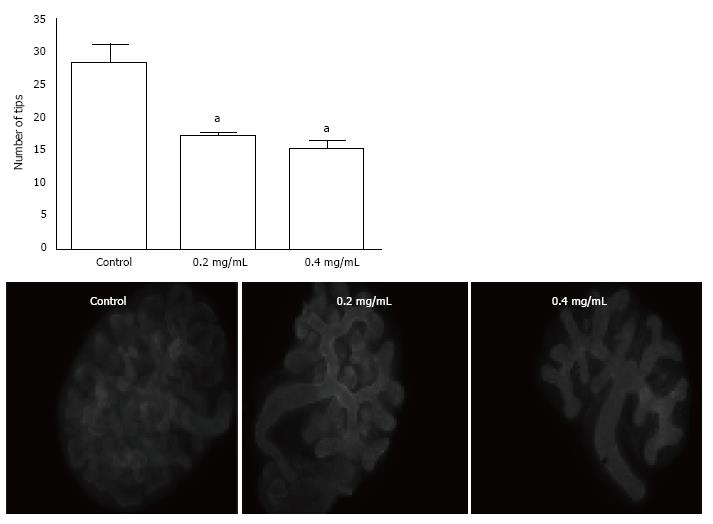
We assessed the influence of ASA on branching morphogenesis by counting the generation of epithelial tips in organs labelled to detect laminin (Figure 2). We found that the number of observable epithelial tips was reduced by 40% and 43% after exposure to ASA for 2 d at concentrations of 0.2 mg/mL and 0.4 mg/mL respectively (P < 0.005).
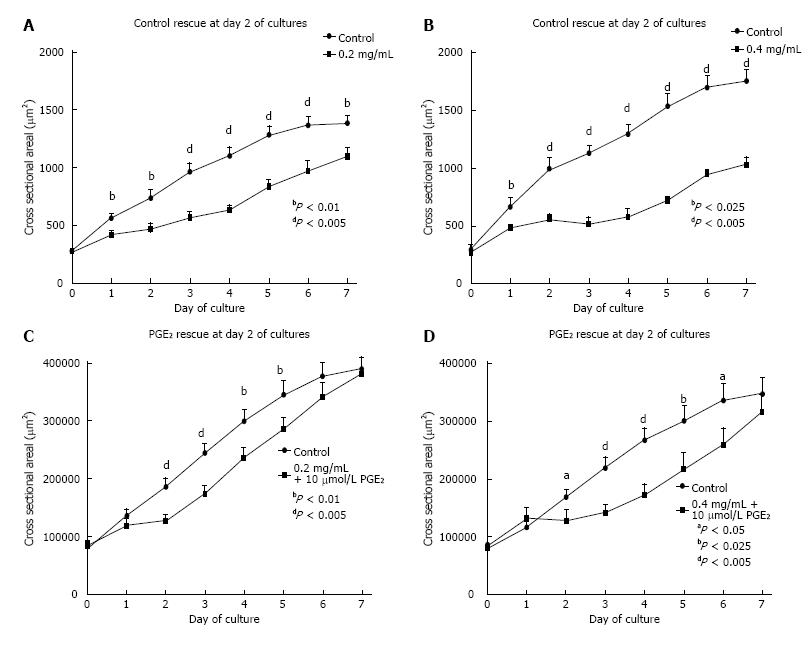
To determine whether the adverse effects of ASA on early renal development could be reversed, embryonic kidneys were cultured in the presence of either 0.2 mg/mL or 0.4 mg/mL ASA for 48 h and subsequently returned to control media or media containing PGE2 (10 μmol/L), or PGE2 combined with 0.2 mg/mL or 0.4 mg/mL ASA during days 2-7 of culture. As expected, exposure to ASA for 48 h (culture days 0-2) blunted embryonic kidney growth compared with their untreated contralateral kidneys (P < 0.05; Figure 3A and B). Replacement of ASA containing media with control culture media (for days 0-2) resulted in a small increase in size. However, kidneys still remained significantly smaller than contralateral controls for each day of culture (days 2-7; Figure 3A and B). Embryonic kidneys exposed to ASA for 48 h and subsequently treated with 10 μmol/L PGE2 in control media (for culture days 2-7) grew more quickly than those in control media alone (Figure 3C and D) such that by culture day 7 their cross-sectional areas matched contra-lateral control kidneys. Nevertheless, despite recovery of growth of kidneys by PGE2 the kidneys remained structurally immature, lacking developing tubules observed in controls. In addition, the blunted growth of ASA treated kidneys grown in culture media containing both PGE2 and ASA from days 2-7, were significantly smaller than their contra-lateral controls and failed to increase in size throughout the culture period (P < 0.05). These experiments suggested that blockade of prostaglandins by ASA may have permanent detrimental consequences for fetal kidney growth.
Therefore, to determine if the growth impairment seen in the presence of ASA might indicate a requirement for PTGS2 derived prostanoids, we cultured kidneys from PTGS2 null (PTGS2-/-) or PTGS2 heterozygous (PTGS2-/+) mice. Growth of organs from PTGS2-/- and PTGS2-/+ mice (n = 3 for each group including contralaterals as internal controls) were unaffected by genotype, increasing by 74% ± 11% vs 62% ± 13% respectively after 48 h (P = 0.13). Size of organs was comparable at all time points with those from wild type ICR mice.
In this study we have demonstrated that exposure of the developing kidney to aspirin impairs overall growth. Such impairment of growth suggested at best a minor role for prostaglandins, as organs examined ex-vivo from mice genetically manipulated to prevent prostaglandin production (PTGS2-/-) were able to grow similarly to kidneys from control mice.
PTGS1-/- mice exhibit no renal phenotype[28] and PTGS2-/- mice only appear to show evidence of renal malformation during postnatal life[19]. This has been suggested to indicate that there may be little or no requirement for prostaglandins in the early (fetal) stages of kidney development, however this does not preclude the possibility that maternally derived prostanoids replace those otherwise acquired from endogenous production.
The role PTGS derived products play in controlling embryonic and organ growth has been previously explored. Incubation of cultured whole rat embryos with the PTGS1/2 inhibitor ASA or Indomethacin for 48 h exhibited dysmorphogenesis. Furthermore, the teratogenic effects of each inhibitor were diminished following PGE2 supplementation[29]. Mice and rats treated with PTGS2 selective inhibitors throughout pregnancy produce offspring with kidneys which exhibit impaired development similar to that observed in PTGS2 knockout mice, including reduced glomerular diameter and the presence of numerous immature glomeruli[19]. However, this was only apparent when administration was continued during the postnatal period until weaning at P21. When organs were examined at P0, there was no difference between any of the groups. In addition, the authors found no effect of administration of the PTGS1 selective inhibitor SC58560 at any stage in development.
A reduction in the nephrogenic zone following PTGS2 inhibition was observed in the study by Komhoff[19] and by PTGS1 and 2 inhibition in preterm baboons treated with ibuprofen to pharmacologically close the ductus arteriosus[30]. A reduction in nephrogenic zone growth is suggestive of either an early cessation of nephrogenesis and or premature renal maturation. These adverse effects of PTGS1/2 inhibition may result in a nephron deficit, and have detrimental consequences on renal health in later life[31].
We did not determine the identity of the cells which were most affected in our experiments, so are unable to make a specific statement about the population of nephrogenic precursors. However, we would speculate that reduction in the population of nephron progenitors would significantly hamper further development[32]. An important factor with regard to the phenotype most normally observed after PTGS inhibition is that the final glomeruli to be formed are immature and very closely apposed to the underside of the renal capsule. This is not necessarily something that would be observed as a result of a reduced population of nephron precursors. It rather argues in favour of an impairment of maturation of the vasculature. This might not be all that surprising considering the final location of the PTGS2 in the adult kidney and its requirement for vasodilation[33].
Long term use of NSAIDS can restrict blood flow to such an extent in the kidney that these organs exhibit phenotypic changes reminiscent of ischemic damage[33,34]. It seems possible that the ability to vasodilate the developing afferent arterioles is potentially a requirement for the final stages of glomerular maturation. Assessment of this role may require grafting of developing organs onto an external vasculature or the use of a carefully targeted mouse mutant possessing loci enabling deletion of PTGS2 within the afferent arteriole which may be regulated by exogenous means (i.e., temporal application of tamoxifen). This would potentially enable deletion of PTGS2 from some groups of arterioles and not others, labelling of those arterioles and examination of the influence of the loss of PTGS2 on the development of their associated glomeruli.
Whilst we show there to be no influence of genotype on the ability to grow in organ culture, we did not include wild-type litter mates in the culture system. Instead we used heterozygotes as controls. Recently published work[35] has demonstrated that there is in fact an influence of the reduction of gene dosage on renal structural and functional outcome. However, the differences compared with wild-type, whilst significant were relatively small and differed greatly with those seen in homozygotes. Therefore, although we cannot definitively state that our experiments show there to be no influence of a loss of PGTS2 on fetal renal growth in ex-vivo culture, we are reasonably confident that this is indeed the case.
As has been mentioned above, we were unable to perform detailed analysis of the cell types most influenced by exposure to ASA. Such information would be particularly helpful in establishing the specific mechanism by which ASA impairs early organogenesis, particularly since this appears to be prostaglandin independent and therefore occurs via a process distinct from organ disruption seen by PTGS2 deletion. Additionally, the data presented here are exclusively derived from organ explant culture experiments and therefore may not be wholly reflective of the process of renal developmental impairment seen in vivo after overconsumption of ASA or similar NSAIDs. Future studies will be required to address these issues in order to clarify the precise pathways of damage and the specific relevance to humans.
In conclusion, the work we describe in this study adds to the wider data which suggests that, for early kidney development, there is no specific requirement for prostaglandins. Kidneys from animals lacking PTGS2 grew entirely normally in ex vivo culture without the requirement for exogenous prostaglandins. This strongly suggests that fetal renal development does not require inducible prostaglandin production. We cannot entirely rule out a requirement for other prostaglandins in these studies since the PTGS2-/- organs will still have retained PTGS1 expression, however, since no demonstration of its requirement for renal development has been identified[28] we would suggest that our data argue against a role for prostaglandins in mediating nephrogenesis in the fetus. Our data further suggests that the impairment of growth induced by exposure of organs to ASA in ex vivo culture is likely due to factors independent of their ability to produce prostaglandins.
The authors acknowledge the expert technical assistance of Mrs Armett C. This work was supported by a Kids Kidney Fund Research Project grant (KKR 2012/2).
Exposure to non-steroidal anti-inflammatory drugs (NSAIDs) is not uncommon for unborn fetuses and also for premature infants. There are clear nephrotoxic effects of NSAIDs during specific stages of kidney development. Considerable work has shown the requirement for inducible prostaglandin synthase 2 (PTGS2) on the final maturation of the kidney but the literature suggests that this may not be required for the earlier stages of kidney development despite the apparent expression of this gene and expression of relevant PG receptors.
It is unclear whether the late malformation due to pharmacological PG blockade is exclusively due to the lack of prostaglandins, or whether alternative pathways of damage might be active. The apparent requirement for PGs during final maturation may suggest that PG mediated vasodilation within the final stages of tubular development is needed to permit glomerular maturation.
In this work, the authors show that the structural deficit induced by exposure of early developing kidneys to NSAIDs is likely independent of PG production. The authors also show that there is no specific requirement of PGs derived from the inducible PTGS2 for early kidney development and clarify with organ culture that maternally derived PGs probably don’t replace any required PGs generated from within the organ.
Work needs to be conducted to determine the precise requirement for PG production in the final maturation of the renal vasculature and whether this indicates a specific need for blood flow to be maintained to facilitate maturation. In addition, the work here clarifies the need to establish the non-PG effects of NSAIDs on renal maturation. By understanding these two aspects of NSAID treatment/loss of PG production or signalling, may help to modify treatments particularly for premature infants faced with a requirement to receive NSAIDs.
The study is relevant for research field and the experiments are clear and result in consistent findings.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Olowu WA, Silva ACSE S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Olesen C, Steffensen FH, Nielsen GL, de Jong-van den Berg L, Olsen J, Sørensen HT. Drug use in first pregnancy and lactation: a population-based survey among Danish women. The EUROMAP group. Eur J Clin Pharmacol. 1999;55:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Cleves MA, Savell VH, Raj S, Zhao W, Correa A, Werler MM, Hobbs CA. Maternal use of acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), and muscular ventricular septal defects. Birth Defects Res A Clin Mol Teratol. 2004;70:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Glover DD, Amonkar M, Rybeck BF, Tracy TS. Prescription, over-the-counter, and herbal medicine use in a rural, obstetric population. Am J Obstet Gynecol. 2003;188:1039-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol. 2005;193:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 268] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Hernandez RK, Werler MM, Romitti P, Sun L, Anderka M. Nonsteroidal antiinflammatory drug use among women and the risk of birth defects. Am J Obstet Gynecol. 2012;206:228.e1-228.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Kozer E, Nikfar S, Costei A, Boskovic R, Nulman I, Koren G. Aspirin consumption during the first trimester of pregnancy and congenital anomalies: a meta-analysis. Am J Obstet Gynecol. 2002;187:1623-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 156] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | van Gelder MM, Roeleveld N, Nordeng H. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and the risk of selected birth defects: a prospective cohort study. PLoS One. 2011;6:e22174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 8. | Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol. 1996;62:167-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 515] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 9. | Wang LH, Hajibeigi A, Xu XM, Loose-Mitchell D, Wu KK. Characterization of the promoter of human prostaglandin H synthase-1 gene. Biochem Biophys Res Commun. 1993;190:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | DeWitt DL. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochim Biophys Acta. 1991;1083:121-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 468] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Kömhoff M, Grone HJ, Klein T, Seyberth HW, Nüsing RM. Localization of cyclooxygenase-1 and -2 in adult and fetal human kidney: implication for renal function. Am J Physiol. 1997;272:F460-F468. [PubMed] |
| 12. | Day NA, Attallah AA, Lee JB. Letter: Presence of prostaglandin A and F in fetal kidney. Prostaglandins. 1974;5:491-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Gleason CA. Prostaglandins and the developing kidney. Semin Perinatol. 1987;11:12-21. [PubMed] |
| 14. | Zhang MZ, Wang JL, Cheng HF, Harris RC, McKanna JA. Cyclooxygenase-2 in rat nephron development. Am J Physiol. 1997;273:F994-1002. [PubMed] |
| 15. | van der Heijden BJ, Carlus C, Narcy F, Bavoux F, Delezoide AL, Gubler MC. Persistent anuria, neonatal death, and renal microcystic lesions after prenatal exposure to indomethacin. Am J Obstet Gynecol. 1994;171:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 687] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 17. | Stevenson KM, Lumbers ER. Effects of indomethacin on fetal renal function, renal and umbilicoplacental blood flow and lung liquid production. J Dev Physiol. 1992;17:257-264. [PubMed] |
| 18. | Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 801] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 19. | Kömhoff M, Wang JL, Cheng HF, Langenbach R, McKanna JA, Harris RC, Breyer MD. Cyclooxygenase-2-selective inhibitors impair glomerulogenesis and renal cortical development. Kidney Int. 2000;57:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Voyer LE, Drut R, Méndez JH. Fetal renal maldevelopment with oligohydramnios following maternal use of piroxicam. Pediatr Nephrol. 1994;8:592-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Veersema D, de Jong PA, van Wijck JA. Indomethacin and the fetal renal nonfunction syndrome. Eur J Obstet Gynecol Reprod Biol. 1983;16:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Sessa A, Allaria PM, Conte F, Cioffi A, D’Amico G. Ultrastructural changes of the glomeruli of the rat induced by indomethacin. Nephron. 1973;10:238-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Novy MJ. Effects of indomethacin on labor, fetal oxygenation, and fetal development in rhesus monkeys. Adv Prostaglandin Thromboxane Res. 1978;4:285-300. [PubMed] |
| 24. | Borthwick GM, Johnson AS, Partington M, Burn J, Wilson R, Arthur HM. Therapeutic levels of aspirin and salicylate directly inhibit a model of angiogenesis through a Cox-independent mechanism. FASEB J. 2006;20:2009-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Goel A, Chang DK, Ricciardiello L, Gasche C, Boland CR. A novel mechanism for aspirin-mediated growth inhibition of human colon cancer cells. Clin Cancer Res. 2003;9:383-390. [PubMed] |
| 26. | Yao JC, Duan WG, Yun Y, Liu DQ, Yan M, Jiang ZZ, Zhang LY. Screening method for nonsteroidal antiinflammatory drugs based on the cyclooxygenase 2 pathway activated by serum-free stimulation in A549 cells. Yakugaku Zasshi. 2007;127:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Lu L, Liu H, Peng J, Gan L, Shen L, Zhang Q, Li L, Zhang L, Su C, Jiang Y. Regulations of the key mediators in inflammation and atherosclerosis by aspirin in human macrophages. Lipids Health Dis. 2010;9:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 812] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 29. | Wentzel P, Eriksson UJ. Antioxidants diminish developmental damage induced by high glucose and cyclooxygenase inhibitors in rat embryos in vitro. Diabetes. 1998;47:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Sutherland MR, Yoder BA, McCurnin D, Seidner S, Gubhaju L, Clyman RI, Black MJ. Effects of ibuprofen treatment on the developing preterm baboon kidney. Am J Physiol Renal Physiol. 2012;302:F1286-F1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Gubhaju L, Sutherland MR, Black MJ. Preterm birth and the kidney: implications for long-term renal health. Reprod Sci. 2011;18:322-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Welham SJ, Wade A, Woolf AS. Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the start of rat metanephrogenesis. Kidney Int. 2002;61:1231-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Harris RC. Physiologic and pathophysiologic roles of cyclooxygenase-2 in the kidney. Trans Am Clin Climatol Assoc. 2013;124:139-151. [PubMed] |
| 34. | Pérez Gutthann S, García Rodríguez LA, Raiford DS, Duque Oliart A, Ris Romeu J. Nonsteroidal anti-inflammatory drugs and the risk of hospitalization for acute renal failure. Arch Intern Med. 1996;156:2433-2439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Slattery P, Frölich S, Schreiber Y, Nüsing RM. COX-2 gene dosage-dependent defects in kidney development. Am J Physiol Renal Physiol. 2016;310:F1113-F1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |