Published online Nov 6, 2016. doi: 10.5527/wjn.v5.i6.551
Peer-review started: April 15, 2016
First decision: May 16, 2016
Revised: July 28, 2016
Accepted: September 21, 2016
Article in press: September 22, 2016
Published online: November 6, 2016
Processing time: 224 Days and 15.5 Hours
Gitelman’s syndrome (GS) is a salt-losing tubulopathy with an autosomal recessive inheritance caused by mutations of SLC12A3, which encodes for the thiazide-sensitive NaCl cotransporter. In this study we report a new mutation of SLC12A3 found in two brothers affected by GS. Hypokalemia, hypocalciuria and hyper-reninemia were present in both patients while hypomagnesemia was detected only in one. Both patients are compound heterozygotes carrying one well known GS associated mutation (c.2581 C > T) and a new one (c.283delC) in SLC12A3 gene. The new mutation results in a possible frame-shift with a premature stop-codon (pGln95ArgfsX19). The parents of the patients, heterozygous carriers of the mutations found in SLC12A3, have no disease associated phenotype. Therefore, the new mutation is causative of GS.
Core tip: Mutations of SLC12A3 gene coding for the thiazide-sensitive NaCl cotrasporter are causative of Gitelman’s syndrome (GS), a tubulopathy transmitted by an autosomal recessive mechanism. This study identifies a new mutation of SLC12A3 in two cases of GS. Genetic evidence that this mutation is a disease causative of GS was also reported.
- Citation: Grillone T, Menniti M, Bombardiere F, Vismara MFM, Belviso S, Fabiani F, Perrotti N, Iuliano R, Colao E. New SLC12A3 disease causative mutation of Gitelman’s syndrome. World J Nephrol 2016; 5(6): 551-555
- URL: https://www.wjgnet.com/2220-6124/full/v5/i6/551.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i6.551
Gitelman’s syndrome (GS) is an autosomal recessive salt-wasting disease prevalently caused by mutations occurring in the SLC12A3 gene coding for the thiazide-sensitive NaCl cotrasporter (NCC)[1]. This disorder is a tubulopathy characterized by hypokalemic metabolic alkalosis, hypocalciuria and hypomagnesemia. Patients affected by GS often experience muscle pain after physical excercise, other clinical signs are weakness, thirst, nocturia, paresthesia, tetany. Symptoms are essentially due to compensation effects determined by sodium salt-wasting that occurs because of inefficient re-absorption of sodium in distal convolute tubule[2,3].
The symptoms of GS are similar but less severe than those characterizing Bartter’s syndrome, a different channelopathy with salt-wasting and hypotension. Differential diagnosis between GS and Bartter’s syndrome, can be made by the demonstration of hypomagnesemia, hypocalciuria and delayed presentation of symptoms in GS[4]. The prevalence of GS is around 1:40000[2].
Although the majority of mutations in GS patients were found in SLC12A3, in a small percentage of subjects mutations of CLCNKB gene, coding for the chloride channel Kb, were demonstrated to be causative of the disease[5]. In addition, a relevant percentage of GS clinical cases are heterozygous carriers or do not show any mutations in SLC12A3[6]. Therefore, the disease causative mutations are not always well characterized and even rare polymorphisms of SLC12A3 have, so far, uncertain classification[6,7]. Mutations of SLC12A3 span along the entire coding sequence[8] and the description of new mutations are extremely useful to assess the risk of GS transmission.
In this study we describe a novel frame-shift mutation in SLC12A3 associated with two cases of GS and we bring genetic evidence that the frame-shift mutation is a novel causative mutation in GS.
Probands are two brothers of 24 and 20 years, the younger one was admitted to the hospital after an episode of paresthesia after intense physical exercise. Laboratory tests showed a severe hypokalemia (1.5 mEq/L normal range 3.5-5.0 mEq/L) that required intravenous potassium supplementation. Subsequent laboratory tests confirmed the persistence of hypokalemia, hypomagnesemia and hypocalciuria (Table 1) that was corrected by therapy based on potassium and magnesium supplements with significant clinical improvements. After this episode, the older brother, although asymptomatic, underwent laboratory tests that showed hypokalemia and hypocalciuria (Table 1), and required potassium and magnesium supplements. Clinical findings were compatible with a diagnosis of GS. Blood samples of the two brothers and their parents were then collected for genetic analysis after informed consent was obtained.
| Variable | Patient 1 | Patient 2 | Reference values |
| Blood tests | |||
| Creatinine (mg/dL) | 1.11 | 0.93 | 0.7-1.2 |
| Glucose (mg/dL) | 104 | 97 | 75-110 |
| Sodium (mEq/L) | 141 | 140 | 135-145 |
| Potassium (mEq/L) | 3.21 | 3.01 | 3.5-5.0 |
| Chloride (mEq/L) | 98 | 951 | 97-108 |
| Calcium (mg/dL) | 9.7 | 10.41 | 8.6-10.2 |
| Phosphate (mg/dL) | 4.1 | 3.4 | 2.5-4.5 |
| Magnesium (mEq/L) | 1.51 | 1.9 | 1.6-2.6 |
| Aldosterone (pg/mL) | 3151 | 271 | 35-300 |
| Renin (pg/mL) | 59.71 | 53.41 | 3.2-32.6 |
| Urine tests | |||
| FEMg (%) | 2.6 | 4.11 | 0.5-4.0 |
| FEK (%) | 23.71 | 17.71 | < 10 |
| Sodium (mEq/24 h) | 200 | 247.51 | 40-220 |
| Potassium (mEq/24 h) | 104.8 | 87.2 | 25-125 |
| Calcium (mg/24 h) | 841 | 85.51 | 100-300 |
| Phosphate (g/24 h) | 1.15 | 1.01 | 0.4-1.3 |
| Magnesium (mg/24 h) | 591 | 128.51 | 73-122 |
| Calcium/creatinine (mg/mg) | 0.055 | 0.056 | < 0.2 |
Total DNA was extracted from whole blood by commercial kit (Nuclear Laser), following manufacturer’s instructions. All the exons and flanking sequences of SLC12A3 gene were amplified by PCR using primers and experimental conditions published by Vargas-Poussou et al[6]. Amplified DNA fragments were checked on agarose gel electrophoresis then purified and bidirectionally sequenced with ABI BigDye terminator cycle sequencing kit on a 310 ABI PRISM Genetic Analyzer (Applied Biosystems). NM_000339.2 was used as reference sequence. The Human Splicing Finder web source was used to predict a possible change in the SLC12A3 splicing[9].
We present a case of two brothers both diagnosed with GS, who were reported for genetic counseling to the unit of medical genetics of our university hospital. Biochemical tests were in agreement with the diagnosis, with the exception of blood magnesium which was in the normal range in one of the patients. Hypomagnesemia is not constant in GS, in fact magnesium levels have been reported normal in some GS patients[10].
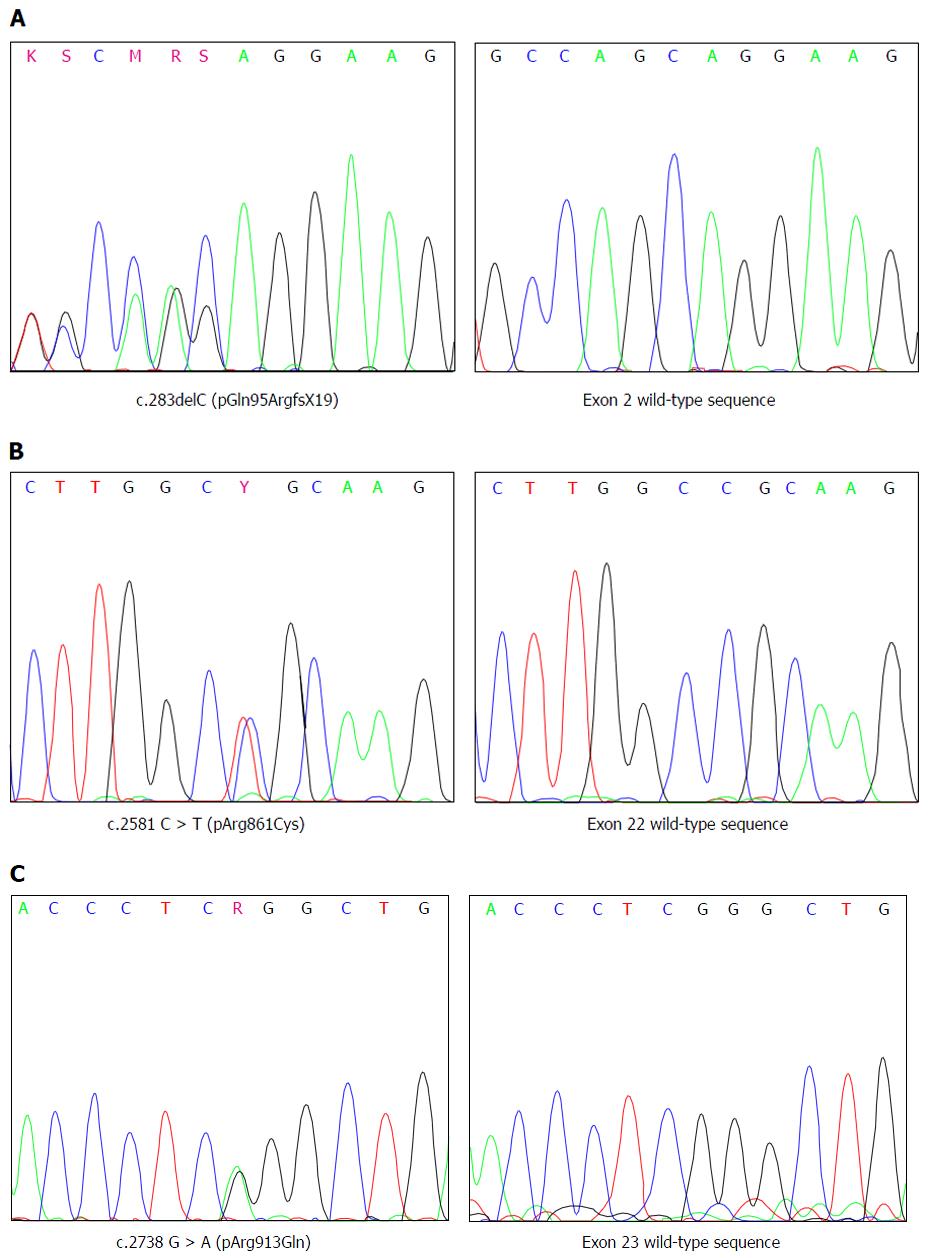
Sequence analysis of SLC12A3 revealed that both the siblings, were compound heterozygotes for a so far undescribed one base-pair deletion c.283delC in exon 2 and for the already known mutation c.2581 C > T (pArg861Cys) in exon 22 associated with GS (Figure 1). In addition, both the siblings were heterozygous for the c.2738 G > A (pArg913Gln) polymorphism which has been associated with GS (Figure 1).
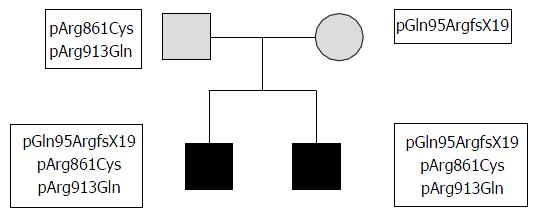
Both the parents of the two siblings were reported normokalemic and had no clinical or biochemical signs of GS suggesting that they could be heterozygous carriers of the GS associated mutations found in their siblings.
To define whether the mutations and the polymorphism detected in the patients were positioned in cis or in trans, exon 2, exon 22 and exon 23 were sequenced in the parents. The analysis revealed that the mother was heterozygous for the frame-shift mutation in exon 2 whereas the father was heterozygous for the mutation in exon 22 and the polymorphism in exon 23, demonstrating that both parents were indeed heterozygous carriers and their siblings are compound heterozygotes for the mutations found in SLC12A3 gene (Figure 2).
Since mutation in exon 2 segregates independently from mutation in exon 22 and polymorphisms in exon 23, we conclude that the mutation in exon 2 is likely to be causative of disease.
The micro-deletion c.283delC affects the first base of exon 2. Human Splicing Finder web source was used to predict the possible consequences of the mutation in the splicing process. The score of mutant acceptor site resulted even higher than that of the wild-type site (82.02 vs 80.82, +1.49%) strongly arguing against a change of splicing site recognition. In fact, the mutation leaves unaltered the last two bases (AG) of intron 1 and modifies the first two bases of exon 2 (AG instead of CA) generating an even better consensus sequence for an acceptor splice site[11]. Thus, c.283delC probably results in a frame-shift that introduces a premature stop-codon nineteen codons downstream of the mutation (pGln95ArgfsX19).
GS is a recessive genetic disorder caused mainly by mutations occurring in SLC12A3 gene. The mutations impair the activity of thiazide-sensitive sodium-chloride cotransporter encoded by SLC12A3 and could be classified in five different classes according to their effect on the protein[12].
The class 1 of mutant impairs protein synthesis. In this class are included mutations that generate a premature termination of the translation (nonsense, frame-shift and splice site mutations) and mutations located in the promoter that may affect gene expression[8]. Large deletions including one or more exons of SLC12A3 have also been detected[6], the majority of these mutations should be included in the class 1. Mutations that generate premature stop codons in the translation produce a truncated inactive protein. In some cases, a nonsense mediated decay mechanism, for the messenger RNA harboring a premature stop-codon, has been suggested[13].
Missense mutations belong to different classes of mutations since they can impair protein processing (class 2), impair the correct positioning into the plasma membrane (class 3), reduce the ion transport activity (class 4) or affect the stability of the protein inducing an accelerated degradation (class 5), of the SLC12A3 protein product.
Class 2 mutants are represented by N-glycosylation deficient proteins such as T392I or R399C[8,13]. N-glycosylation in N404 and N424 residues is required for the correct processing of NCC[14] and it was impaired by this class of mutations. The lack of proper N-glycosylation causes the deficiency of protein into plasma membrane.
Class 3 mutants, such as N442S and Q1030R result in proteins that could be functional but have an impaired insertion into the plasma membrane[8]. In this case, the trafficking of the cotransporter is affected because of the intrinsic nature of the mutant protein itself or as consequence of the loss of interaction with other proteins that have a role in the regulation of NCC trafficking[8].
Class 4 mutants, such as A588V and P751L[8,13], are proteins that show a reduced Na+ uptake. Although these mutant proteins are fully glycosylated and correctly positioned into the plasma membrane, they are deficient in their functional role. Depending on the position of mutated residue in the primary sequence of NCC, decreased ion affinity or impaired regulation was hypothesized as potential mechanisms of reduced cotransporter activity[8,13].
The presence of a class 5 of mutants (accelerated degradation) was postulated but clear examples of this kind of mutants remain to be demonstrated.
We present the case of two siblings, compound heterozygous, carrying mutations of SLC12A3. One of these mutations, inherited from the father, is the missense pArg861Cys which was clearly associated with GS[6]. Although this is a well-known mutation, its effect on the NCCT co-transporter has never been characterized. Moreover, both patients inherited from their father also a polymorphic variation, which has been in some cases associated with GS, although with a less clear involvement in the pathology[6].
The second mutation carried by our patients, inherited from the mother, is a deletion of the first nucleotide of the second exon of SLC12A3. It has never been reported so far. Since the new mutation does not seem to affect the splicing site acceptor recognition, it could result in a frame-shift with a premature stop of the translation and a clear loss-of-function. However, the possibility of skipping of exon 2 cannot be completely discarded, since that mechanism has been experimentally verified for the mutation c.1926delC located at the 5’ end of exon 16 of SLC12A3[15].
A clear genotype/phenotype correlation is not possible in GS probably because many other genetic factors, different from the SLC12A3 causative mutations, can influence the severity of the pathology. Interestingly our patients share an identical SLC12A3 genotype and show a similar disease associated phenotype. The definition of SLC12A3 genotype may be a useful tool in the clinical characterization of GS and in genetic counseling of salt losing tubulopathies.
Two brothers, the youngest one had an episode of paresthesia after intense physical exercise.
Muscular weakness related to a suspected Gitelman’s syndrome (GS), a genetic disease with autosomal recessive inheritance.
Although less severe, GS can be confused with Bartter’s syndrome.
Hypokalemia, hypomagnesemia and hypocalciuria. In both brothers two point mutations of SLC12A3, a gene associated with GS, were detected. One of this mutation was characterized for the first time. Parents, who are healthy, carried just one mutation of SLC12A3.
Supplementation of potassium and magnesium.
GS: GS; NCC: Thiazide-sensitive NaCl cotrasporter.
Although GS could be indolent, symptoms can be experienced after intense physical exercise. Thus, a clinical diagnosis confirmed by a genetic test is very important.
The authors described brothers with Gitelman syndrome who had a new mutation of SLC12A3. The paper is well-written and interesting findings.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lonardo F, Watanabe T, Yorioka N S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 820] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 2. | Knoers NV, Levtchenko EN. Gitelman syndrome. Orphanet J Rare Dis. 2008;3:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Nakhoul F, Nakhoul N, Dorman E, Berger L, Skorecki K, Magen D. Gitelman’s syndrome: a pathophysiological and clinical update. Endocrine. 2012;41:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Al Shibli A, Narchi H. Bartter and Gitelman syndromes: Spectrum of clinical manifestations caused by different mutations. World J Methodol. 2015;5:55-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Jeck N, Konrad M, Peters M, Weber S, Bonzel KE, Seyberth HW. Mutations in the chloride channel gene, CLCNKB, leading to a mixed Bartter-Gitelman phenotype. Pediatr Res. 2000;48:754-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Vargas-Poussou R, Dahan K, Kahila D, Venisse A, Riveira-Munoz E, Debaix H, Grisart B, Bridoux F, Unwin R, Moulin B. Spectrum of mutations in Gitelman syndrome. J Am Soc Nephrol. 2011;22:693-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Tavira B, Gómez J, Santos F, Gil H, Alvarez V, Coto E. A labor- and cost-effective non-optical semiconductor (Ion Torrent) next-generation sequencing of the SLC12A3 and CLCNKA/B genes in Gitelman’s syndrome patients. J Hum Genet. 2014;59:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Glaudemans B, Yntema HG, San-Cristobal P, Schoots J, Pfundt R, Kamsteeg EJ, Bindels RJ, Knoers NV, Hoenderop JG, Hoefsloot LH. Novel NCC mutants and functional analysis in a new cohort of patients with Gitelman syndrome. Eur J Hum Genet. 2012;20:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Desmet FO, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1813] [Cited by in RCA: 2061] [Article Influence: 128.8] [Reference Citation Analysis (0)] |
| 10. | Tosi F, Bianda ND, Truttmann AC, Crosazzo L, Bianchetti MG, Bettinelli A, Ramelli GP. Normal plasma total magnesium in Gitelman syndrome. Am J Med. 2004;116:573-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Padgett RA. New connections between splicing and human disease. Trends Genet. 2012;28:147-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev. 2005;85:423-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Riveira-Munoz E, Chang Q, Godefroid N, Hoenderop JG, Bindels RJ, Dahan K, Devuyst O. Transcriptional and functional analyses of SLC12A3 mutations: new clues for the pathogenesis of Gitelman syndrome. J Am Soc Nephrol. 2007;18:1271-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Hoover RS, Poch E, Monroy A, Vázquez N, Nishio T, Gamba G, Hebert SC. N-Glycosylation at two sites critically alters thiazide binding and activity of the rat thiazide-sensitive Na(+): Cl(-) cotransporter. J Am Soc Nephrol. 2003;14:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Takeuchi Y, Mishima E, Shima H, Akiyama Y, Suzuki C, Suzuki T, Kobayashi T, Suzuki Y, Nakayama T, Takeshima Y. Exonic mutations in the SLC12A3 gene cause exon skipping and premature termination in Gitelman syndrome. J Am Soc Nephrol. 2015;26:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |