Published online Nov 6, 2016. doi: 10.5527/wjn.v5.i6.517
Peer-review started: June 20, 2016
First decision: July 11, 2016
Revised: August 29, 2016
Accepted: September 13, 2016
Article in press: September 18, 2016
Published online: November 6, 2016
Processing time: 144 Days and 3 Hours
To compare the effects of renal transplantation on cardiac functions in children and adults.
One hundred and ten patients attending the nephrology outpatient clinic were enrolled in this study and were divided into six groups. The first two groups consisted each of 30 renal transplant patients who had a successful renal transplantation more than six months, but less than one year. Group I were less than 18 years and group II were more than 18 years. The third and fourth groups, each were 20 chronic renal failure patients on regular hemodialysis. Again, group III were less than 18 years and group IV were more than 18 years. Group V and VI (The control Groups) consisted each of 5 subjects below and above 18 years of age, respectively with normal kidney functions. All patients were subjected to history and examination. The kidney functions and the hemoglobin were analyzed. After obtaining informed consent, echocardiography was done to all patients.
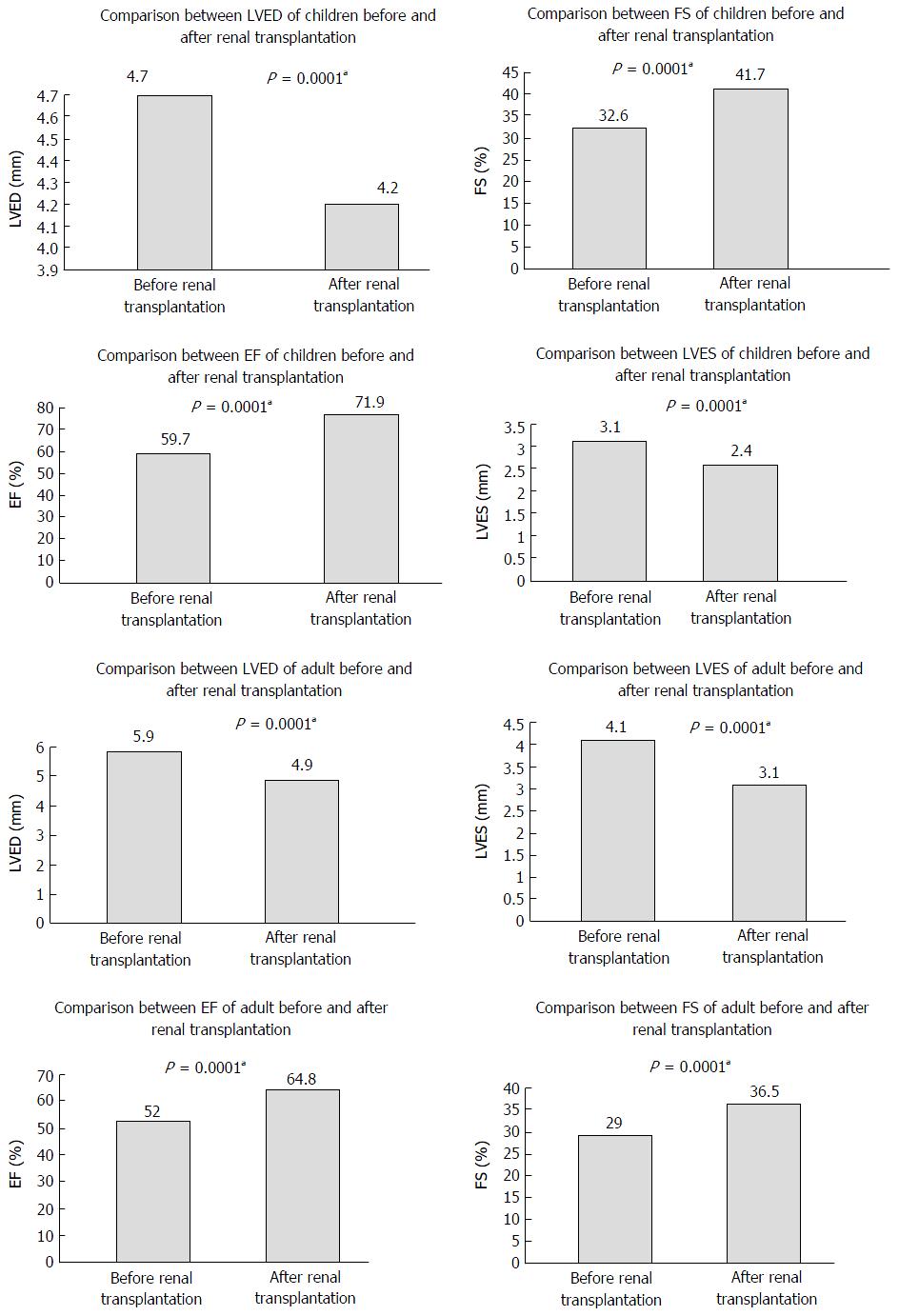
There was a statistically significant improvement (P < 0.0001) in all cardiac parameters. A regression in left ventricular end diastolic volume (LVED) both in children (4.7 ± 0.8 to 4.2 ± 0.5) and in adults (5.9 ± 0.7 to 4.9 ± 0.6) were found. There was a regression in left ventricular end systolic volume (LVES) both in children (3.1 ± 0.6 to 2.4 ± 0.4) and in adults (4.1 ± 0.9 to 3.1 ± 0.5). Fractional shortening improves both in children (32.6 ± 5.3 to 41.7 ± 7.6) and in adults (29.0 ± 6.6 to 36.5 ± 4.1). The improvement in ejection fraction (EF) was higher in children (59.7 ± 7.0 to 71.9 ± 6.1) than in adults (52.0 ± 12.5 to 64.8 ± 5.9). However, this degree of improvement (in children: 12.2 ± 5.1) did not show statistical difference (P-value 0.8), when compared to adults (12.7 ± 9.8).
After renal transplantation cardiac functions and morphology (EF/LVED/LVES) do improve markedly and rapidly in both children and adults.
Core tip: Cardiac functions do improve in chronic kidney disease patients after renal transplantation. This improvement is evident even in the early post-transplant period. In our study, we concluded that this improvement is even more marked in children. Renal transplantation in children with end-stage rend disease should, therefore, be encouraged.
- Citation: El-Khashab SO, Mohamed EES, Soliman MA, Kassem HH, Soliman AR. Impact of renal transplantation on cardiac morphological and functional characteristics in children and adults. World J Nephrol 2016; 5(6): 517-523
- URL: https://www.wjgnet.com/2220-6124/full/v5/i6/517.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i6.517
Chronic kidney disease (CKD) affects almost 13% of the Unites States citizens and is definitely related to increased risk of cardiovascular (CV) diseases. After renal replacement therapy (RRT) became available, it became evident that the cause of death of patients with advanced CKD was more likely related to CV compromise[1].
In hemodialysis (HD) or peritoneal dialysis patients, Coronary artery disease (CAD) prevalence is estimated at 40% with a 9% annual CV mortality[2]. Renal transplant recipients (RTRs) have a lower CAD prevalence (15%) with an annual CV mortality of 0.54%[3]. Left ventricular hypertrophy (LVH) is an independent risk factor for cardiovascular disease (CVD) related to death in patients on RRT[4]. In a prospective echocardiographic study, patients with renal insufficiency and clearances of 25 to 50 mL/min and less than 25 mL/min, the prevalence of LVH was 31% and 45% respectively[5]. Echocardiographic studies of patients on dialysis have revealed severe systolic dysfunction [left ventricular (LV) ejection fraction (EF) of ≤ 25%] in 15% of patients; with 74% of patients having LVH and 32% of patients demonstrating LV dilatation[6]. Although increased awareness of CVD has resulted in a reduction in related deaths over time, yet still they remain the leading mortality cause of in RTRs[7]. The American Society for Transplantation guidelines has recommended pretransplant risk stratification and noninvasive stress testing for candidates at high cardiac risk[8].
Although cardiovascular problems in adults were thoroughly investigated, yet much less work has been done in children and adolescents with end-stage rend disease (ESRD) whether before or after renal transplantation. Cardiac changes are believed to be less prevalent in children compared to adults with chronic renal failure (CRF). However, no recent studies discussing their frequency are available. Long-term prognosis of cardiac morphological changes in children with CRF and after renal transplantation is largely unknown. When the pediatric mortality in RRT patients in Europe between 1987 and 1990 was investigated, a CV cause of death was identified in 51% of dialysis and in 37% of transplanted subjects[9]. Cardiac disease is known to be the second most common cause of mortality in children after infection and is the leading cause of death in young adults who have undergone renal transplantation[10]. Although CV mortality is relatively high in pediatric RTRs, it does not always follow that the same risk factors as in adults[11]. According to Johnstone et al[12] the most common echocardiographic abnormality in ESRD before renal transplantation is LVH (47.9%). This prevalence is higher in comparison to other studies, e.g., in the only large study performed by European Dialysis Transplant Association in children, an incidence of 22% of post-transplant LVH only was found. Mitsnefes et al[13] found that the prevalence of LVH was 56% among children and adolescents after renal transplantation. Johnstone and his colleagues indicated that LVH was found to be more frequent and severe in children after transplantation; when compared to those on HD or with advanced renal failure[12]. Alvares et al[14] found that pretransplant HD resulted in an increased left ventricular mass index (LVMI) in children, especially if dialysis lasted for more than two years. They concluded that although this cardiac hypertrophy was reversible after renal transplantation, children may benefit from an earlier transplantation. Mitsnefes et al[13] also found that hypertension was a predictor of increased LVMI after transplantation in both children and adolescents and that control of blood pressure might help in preventing LVH progression in RTRs.
To evaluate the impact of renal transplantation on the cardiac morphology and functional characteristics and to study its clinical correlation with renal graft function in children and adults.
Our work was carried out in the Nephrology department at Cairo University. One hundred and ten patients attending the nephrology outpatient clinic were enrolled in this study and were divided into six groups. The first two groups consisted each of 30 renal transplant patients who had a successful renal transplantation in a period more than six months, but less than one year. Group 1 was less than 18 years, and group 2 was more than 18 years. The third and fourth groups, each were 20 CRF patients on regular hemodialysis (RHD). Group 3 were less than 18 years, and group 4 was more than 18 years. Group 5 and 6 consisted each of 5 subjects below and above 18 years of age, respectively with normal kidney functions.
All patients underwent full medical history taking and clinical examination. In all cases, we recorded age, body mass index (BMI) and a complete blood count. Patients in group 1 and 2 had their hemoglobin (Hb) measured before and six months after transplantation. Trans-thoracic echocardiography (TTE) was done to all our patients. Again, patients in group 1 and 2 had their TTE done before and six months after transplantation (Table 1).
| Age in years | Male/female | BMI (kg/m2) | Hb pre Tx | Creatinine ne post Tx (mg/dL) | Hb post Tx (g/dL) | Duration of dialysis in years | |
| Group 1 | 9.8 ± 3.7 | 18/12 | 25.8 ± 4.4 | 6.9 ± 1.0 | 0.9 ± 0.3 | 13.9 ± 0.9 | 0.6 ± 0.2 |
| Group 2 | 26.8 ± 5.6 | 25/5 | 24.7 ± 3.1 | 6.9 ± 1.2 | 1.2 ± 0.2 | 13.3 ± 1.9 | 0.9 ± 0.4 |
| Group 3 | 10.7 ± 2.4 | 12/8 | 28.2 ± 9.0 | 8.7 ± 1.5 | 3.3 ± 1.2 | ||
| Group 4 | 43.9 ± 14.1 | 8/12 | 22.7 ± 5.2 | 9.7 ± 1.9 | 8.5 ± 4.7 | ||
| Group 5 | 12.0 ± 1.5 | 5 males | 18.0 ± 1.5 | 14.2 ± 0.8 | |||
| Group 6 | 30.2 ± 3.7 | 5 males | 25.7 ± 3.8 | 13.2 ± 1.3 |
We excluded patients older than sixty years, patients with ischemic heart diseases, diabetics, liver cirrhosis, chronic obstructive pulmonary disease and those with a high serum creatinine(> 1.5 mg/dL) after renal transplantation.
SPSS version 9.0 was used to analyze the data. Data was summarized as mean, SD. t-test for dependent and independent variables were used for analysis of two quantitative data. One Way ANOVA test was done for analysis of more than two variables, followed by post HOCC test for detection of significance. Pearson correlation was also done. Value was considered significant if < 0.05.
The improvement in all cardiac parameters in the renal transplant groups was statistically significant. Left ventricular end diastolic volume (LVED) decreased in children (4.7 ± 0.8 to 4.2 ± 0.5) and in adults (5.9 ± 0.7 to 4.9 ± 0.6) after renal transplantation. There was a regression in left ventricular end systolic volume (LVES) both in children (3.1 ± 0.6 to 2.4 ± 0.4) and in adults (4.1 ± 0.9 to 3.1 ± 0.5). There was an improvement in fractional shortening (FS) both in children (32.6 ± 5.3 to 41.7 ± 7.6) and adults (29.0 ± 6.6 to 36.5 ± 4.1) (Figure 1). The improvement in EF was higher in children (59.7 ± 7.0 to 71.9 ± 6.1) than in adults (52.0 ± 12.5 to 64.8 ± 5.9). The degree of improvement in pediatrics (12.2 ± 5.1), when compared to adults (12.7 ± 9.8) did not show statistical difference (Table 2).
| LVED pre Tx | LVES pre Tx | FS pre Tx | EF pre Tx | LVED post Tx | LVES post Tx | FS post Tx | EF post Tx | |
| Group 1 | 4.7 ± 0.8 | 3.1 mm ± 0.6 mm | 32.6% ± 5.3% | 59.7% ± 7.0% | 4.2 mm ± 0.5 mm | 2.4 mm ± 0.4 mm | 41.7% ± 7.6% | 71.9 ± 6.1 |
| Group 2 | 5.9 ± 0.7 | 4.1 ± 0.9 | 29.0 ± 6.6 | 52.0 ± 12.5 | 4.9 ± 0.6 | 3.1 ± 0.5 | 36.5 ± 4.1 | 64.8 ± 5.9 |
| Group 3 | 4.7 ± 0.8 | 3.2 ± 0.6 | 30.9 ± 5.0 | 56.5 ± 6.3 | ||||
| Group 4 | 5.6 ± 0.8 | 4.0 ± 1.0 | 29.2 ± 4.3 | 50.2 ± 7.4 | ||||
| Group 5 | 4.2 ± 0.4 | LVES 3.0 ± 0.5 | 41.9 ± 2.3 | 71.6 ± 4.0 | ||||
| Group 6 | 5.0 ± 0.3 | 3.1 ± 0.6 | 38.0 ± 1.9 | 64.8 ± 3.4 |
When the data in post-transplant children were compared to those on RHD and normal children, there were statistically significant differences, regarding Hb levels (P-value 0.0001), LVED (P-value 0.02), LVES (P-value 0.0001), FS (P-value 0.0001) and EF (P-value 0.0001) (Table 3).
| Variables | Before renal transplantation(mean ± SD) | After renal transplantation(mean ± SD) | P-value(P-value is significant if < 0.05) |
| Hemoglobin n (g/dL) | 6.9 ± 1.0 | 13.9 ± 0.9 | 0.0001 |
| LVED (mm) | 4.7 ± 0.8 | 4.2 ± 0.5 | 0.0001 |
| LVES (mm) | 3.1 ± 0.6 | 2.4 ± 0.4 | 0.0001 |
| FS (%) | 32.6 ± 5.3 | 41.7 ± 7.6 | 0.0001 |
| EF (%) | 59.7 ± 7.0 | 71.9 ± 6.1 | 0.0001 |
When comparing adults’ data post renal transplantation to data of adults on RHD and normal adults, there was a statistically significant difference regarding Hb level, LVED, LVES, FS and EF% (P-value 0.0001 in all cases) (Table 4). When correlating the degree of improvement in EF after transplantation to other parameters in both children and adults, there was no statistically significant correlation as regards Hb levels (P-value 0.4 in children and 0.1 in adults), age (P-value 0.5 vs 0.1), duration of dialysis (P-value 0.3 vs 0.4), BMI (P-value 0.8 vs 0.7). Comparison of the EF between children and adults showed there was a statistically significant difference, whether before P-value 0.005 or after renal transplantation P-value 0.0001).
| Variables | Before renal transplantation(mean ± SD) | After renal transplantation(mean ± SD) | P-value(P-value is significant if < 0.05) |
| Hemoglobin (g/dL) | 6.9 ± 1.2 | 13.3 ± 1.9 | 0.0001 |
| LVED (mm) | 5.9 ± 0.7 | 4.9 ± 0.6 | 0.0001 |
| LVES (mm) | 4.1 ± 0.9 | 3.1 ± 0.5 | 0.0001 |
| FS (%) | 29.0 ± 6.6 | 36.5 ± 4.1 | 0.0001 |
| EF (%) | 52.0 ± 12.5 | 64.8 ± 5.9 | 0.0001 |
Compared with the general population, (RTRs) are at a higher risk for morbidity and mortality, largely as a result of (CVD)[15]. Marked improvements in all cardiac functions are evident after successful renal transplantation. The changes are apparent in the early post-transplant period and continue over time, depending on BP control and renal functions[16]. The aim of our work is to evaluate the impact of renal transplantation on the cardiac morphological and functional characteristics in children and adults who had renal transplantation and to compare the degree of improvement in children to adults. The study showed that a statistically significant improvement in LVED, LVES and FS and EF occured 6 mo after renal transplantation in the pediatric population. This goes in agreement with the work previously published by El-Husseini et al[17] where they reported a 56% increase in FS after transplantation; larger than that expected with correction of anemia. Few studies used EF to reflect improvement in cardiac characteristics after renal transplantation, although many used LVH and LVMI.
In our study, the adult group of renal transplant recipients also showed a statistically significant improvement in LVED, LVES, FS and EF, six months after renal transplantation. Montanaro used LVM and LVMI left compared to the pre-transplantation period to assess cardiac status amelioration. He observed that the prevalence of LVH significantly decreased (78% vs 44%, P < 0.03). Systolic 24-h BP was the only predictor of LVM and LVMI at two years after transplantation. He concluded that successful renal transplantation produced a regression in LVH and that this beneficial effect depended on a decrease in systolic pressure levels.
In our work, we attributed the marked improvement in cardiac morphological and functional characteristics after renal transplantation. In to correction of anemia, control of blood pressure, normalization kidney function and reduction of volume overload.
When comparing pediatric RTRs to those on RHD, there was a statistically significant difference in EF between the two groups (group 1 and 3); with improvement in EF after renal transplantation. These parameters reflect improvement in the degree of left ventricular dilatation and systolic dysfunction in RTR than those on HD. This was in agreement with Chinali et al[18] Children with CRF have a reduced EF; this was found to be in agreement with work published by Colan et al[19] who found a markedly reduced EF in children on HD; though many other former studies showed that predialysis or dialysis-dependent CRF children may have a normal or further more a supernormal EF at rest[20].
Schrier[21] reported that in ESRD patients the most common CV abnormalities are systolic dysfunction (30%-60%), diastolic dysfunction (17%), LVH (up to 93%) and LV dilatation (27%). Iqbal et al[22] 2006 stated that marked beneficial alterations in cardiac function and morphology become apparent as early as three months post renal transplantation.
Correction of anemia and proper control of BP contribute to the reduction in cardiac ventricular diameters. Long term maintenance of these changes are definitely more in patients with functioning renal graft. Systolic or diastolic hypertension is risk factors for patients and graft survival after renal transplantation. The effect of hypertension on kidney grafts has been attributed to amplification of vascular injury[23]. Salvatierra et al[24] reported that in CRF, cardiomyopathy presents by systolic dysfunction, concentric LVH or LVD. Renal transplantation leads to normalization of left ventricular contractility, regression of LVH and improvement of cavity volume.
A statistically significant increase in Hb level was noted after transplantation in both children and adults; however, when correlating the degree of improvement in Hb with the degree of improvement in EF after transplantation, no statistically significant correlation was found. This was considered to be contrary to Iqbal et al[25] in 2008, who showed that the reduction in BP with correction of anemia and a decreased in creatinine level influenced the improvements in LV parameters. Foley et al[6] reported that after renal transplantation, a 17% increase in LVMI (similar to the degree of regression of hypertrophy found on partial correction of anemia by erythropoietin) occurred. LVH regression may be compromised by hypertension, as a clear association was seen between the fall in blood pressure and the fall in LVMI.
Correction of anemia in CRF patients and RTRs is a an important issue as shown by Walker et al[26] 2006 who reported that patients with anemia and CRF had elevated risks for CVD. Risks for hospitalization with myocardial infarction was found to be 2-5 times higher in anemic (Hb < 12 g/dL) patients[27]. The risks for hospitalization for congestive heart failure reduced from a doubling risk at Hb < 10% to a 61% decrease at Hb 15 g/dL after increasing Hb[28].
In our study, the comparison of the EF between children and adults showed there was a statistically significant difference, whether before or after renal transplantation. Although the improvement in EF was higher in children than in adults, yet the degree of improvement in children, when compared to the degree of improvement in adults did not show a statistically significant difference. As stated by Salvatierra et al[29] function graft after renal transplant remains the most beneficial RRT for children with ESRD (Figure 1).
Transplanting children is very challenging. Graft and patient survival were often reported to be less promising in young patients compared with older children and adults, yet the results have recently improved significantly[30].
In conclusion, marked improvement in cardiac morphological and functional characteristics occurs after renal transplantation. These findings were found to be more significant in the pediatric population. Renal transplantation is therefore expected to reduce mortality and cardiovascular deaths than dialysis in CRF patients, although, in both groups, survival remains worse than in the general population. Renal transplantation is the treatment of choice for CRF, especially in children and adolescence. A larger number of patients need to be studied with a longer follow-up period up to five years after transplantation with frequent cardiological assessment and proper correlation with anemia correction, BP control, lipid profile correction and detection and management of new-onset diabetes.
Cardiovascular diseases are the principal causes of morbidity and mortality among adults and children with chronic kidney disease (CKD). After renal transplantation, there is a substantial improvement in cardiac morphology and functions. The early and rapid improvement in cardiac status is even more marked in children.
In Egypt, the number of children with CKD having a renal transplant is increasing. The cardiac problems in children with CKD are among the most common reasons for not choosing renal transplantation for those children. There is a very few English literature documenting the marked improvement in the ejection fraction of Egyptian children especially in comparison to adults. The study hotspot is to emphasize not only the rapid (as early as six months post-transplant) but also the marked improvement in the ejection fraction (EF) in children when compared to adults after have a renal transplant.
In recent years the list of contraindications and relative contraindications to renal transplant has been changing. The impaired cardiac function was among the relative contraindications to renal transplantation. The present study shows that there is a marked improvement in EF after renal transplantation both in adults and in children with CKD. Although the improvement is marked and significant in both adults and children yet, the authors’ study also shows that the improvement in children is even more.
The data in their work suggested that the EF of patients with ESRD improves after renal transplantation, this improvement is marked in children.
EF is the amount of blood pumped from the heart by heartbeat. It is low in systolic congestive heart failure. It is a similar but different mathematically when compared to stroke volume, which (together with heart rate) measures cardiac output. EF is often determined by echocardiography, in which the volumes of the heart's chambers are measured during the cardiac cycle. EF can then be calculated by dividing the volume ejected by the heart (stroke volume) by the volume of the filled heart (end-diastolic volume). It can also be measured by computed tomogrophy scan, magnetic resonance imaging, ventriculography, gated SPECT and radionuclide angiography scanning.
Available papers concerning the comparison between the improvements in cardiac function after renal transplantation in children and adults are rare.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ananthasubramaniam K, Nechifor G, Said SAM S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Hage FG, Venkataraman R, Zoghbi GJ, Perry GJ, DeMattos AM, Iskandrian AE. The scope of coronary heart disease in patients with chronic kidney disease. J Am Coll Cardiol. 2009;53:2129-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Perkovic V, Ninomiya T, Arima H, Gallagher M, Jardine M, Cass A, Neal B, Macmahon S, Chalmers J. Chronic kidney disease, cardiovascular events, and the effects of perindopril-based blood pressure lowering: data from the PROGRESS study. J Am Soc Nephrol. 2007;18:2766-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Mark PB, Johnston N, Groenning BA, Foster JE, Blyth KG, Martin TN, Steedman T, Dargie HJ, Jardine AG. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int. 2006;69:1839-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, Drury PL, Esmatjes E, Hricik D, Pohl M. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol. 2005;16:2170-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Levin A. Clinical epidemiology of cardiovascular disease in chronic kidney disease prior to dialysis. Semin Dial. 2003;16:101-105. [PubMed] |
| 6. | Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 845] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 7. | Meier-Kriesche HU, Kaplan B. Death after graft loss: a novel endpoint for renal transplantation. Transplant Proc. 2001;33:3405-3406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2429] [Cited by in RCA: 2578] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 9. | Ehrich JH, Loirat C, Brunner FP, Geerlings W, Landais P, Mallick NP, Margreiter R, Raine AE, Selwood NH, Tufveson G. Report on management of renal failure in children in Europe, XXII, 1991. Nephrol Dial Transplant. 1992;7 Suppl 2:36-48. [PubMed] |
| 10. | McDonald SP, Craig JC. Long-term survival of children with end-stage renal disease. N Engl J Med. 2004;350:2654-2662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 571] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 11. | Jardine AG. Assessing the relative risk of cardiovascular disease among renal transplant patients receiving tacrolimus or cyclosporine. Transpl Int. 2005;18:379-384. [PubMed] |
| 12. | Johnstone LM, Jones CL, Grigg LE, Wilkinson JL, Walker RG, Powell HR. Left ventricular abnormalities in children, adolescents and young adults with renal disease. Kidney Int. 1996;50:998-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 111] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Mitsnefes MM, Schwartz SM, Daniels SR, Kimball TR, Khoury P, Strife CF. Changes in left ventricular mass index in children and adolescents after renal transplantation. Pediatr Transplant. 2001;5:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Alvares S, Mota C, Soares L, Henriques C, Pereira E, Sarmento AM, Lima CA. Cardiac consequences of renal transplantation changes in left ventricular morphology. Rev Port Cardiol. 1998;17:145-152. [PubMed] |
| 15. | Shirali AC, Bia MJ. Management of cardiovascular disease in renal transplant recipients. Clin J Am Soc Nephrol. 2008;3:491-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Peteiro J, Alvarez N, Calviño R, Penas M, Ribera F, Castro Beiras A. Changes in left ventricular mass and filling after renal transplantation are related to changes in blood pressure: an echocardiographic and pulsed Doppler study. Cardiology. 1994;85:273-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | El-Husseini AA, Sheashaa HA, Hassan NA, El-Demerdash FM, Sobh MA, Ghoneim MA. Echocardiographic changes and risk factors for left ventricular hypertrophy in children and adolescents after renal transplantation. Pediatr Transplant. 2004;8:249-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Chinali M, de Simone G, Matteucci MC, Picca S, Mastrostefano A, Anarat A, Caliskan S, Jeck N, Neuhaus TJ, Peco-Antic A. Reduced systolic myocardial function in children with chronic renal insufficiency. J Am Soc Nephrol. 2007;18:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Colan SD, Sanders SP, Ingelfinger JR, Harmon W. Left ventricular mechanics and contractile state in children and young adults with end-stage renal disease: effect of dialysis and renal transplantation. J Am Coll Cardiol. 1987;10:1085-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Palcoux JB, Palcoux MC, Jouan JP, Gourgand JM, Cassagnes J, Malpuech G. Echocardiographic patterns in infants and children with chronic renal failure. Int J Pediatr Nephrol. 1982;3:311-314. [PubMed] |
| 21. | Schrier RW. Cardiorenal versus renocardiac syndrome: is there a difference? Nat Clin Pract Nephrol. 2007;3:637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Iqbal MM, Banerjee SK, Rahman MH, Rashid HU. Cardiac functional and morphologic changes of renal allograft recipients in the early posttransplant period. Transplant Proc. 2006;38:3527-3529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Schwenger V, Zeier M, Ritz E. Hypertension after renal transplantation. Curr Hypertens Rep. 2001;3:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Salvatierra O, Alfrey E, Tanney DC, Mak R, Hammer GB, Krane EJ, So SK, Lemley K, Orlandi PD, Conley SB. Superior outcomes in pediatric renal transplantation. Arch Surg. 1997;132:842-847; discussion 847-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Iqbal MM, Rashid HU, Banerjee SK, Rahman MH, Mohsin M. Changes in cardiac parameters of renal allograft recipients: a compilation of clinical, laboratory, and echocardiographic observations. Transplant Proc. 2008;40:2327-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Walker AM, Schneider G, Yeaw J, Nordstrom B, Robbins S, Pettitt D. Anemia as a predictor of cardiovascular events in patients with elevated serum creatinine. J Am Soc Nephrol. 2006;17:2293-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Al-Ahmad A, Rand WM, Manjunath G, Konstam MA, Salem DN, Levey AS, Sarnak MJ. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;38:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 499] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 28. | Pereira AA, Sarnak MJ. Anemia as a risk factor for cardiovascular disease. Kidney Int Suppl. 2003;S32-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Salvatierra O, Tanney D, Mak R, Alfrey E, Lemley K, Mackie F, So S, Hammer GB, Krane EJ, Conley SB. Pediatric renal transplantation and its challenges. Transpl Rev. 1997;11:51-69. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Ettenger RB. Children are different: the challenges of pediatric renal transplantation. Am J Kidney Dis. 1992;20:668-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |