Published online Nov 6, 2016. doi: 10.5527/wjn.v5.i6.507
Peer-review started: July 1, 2016
First decision: August 5, 2016
Revised: August 31, 2016
Accepted: September 21, 2016
Article in press: September 22, 2016
Published online: November 6, 2016
Processing time: 127 Days and 3.5 Hours
To investigate the prevalence of nutritional parameters of risk for cardiovascular disease (CVD) and kidney diseases in healthy preschool children.
This is an observational cross-sectional study with 60 healthy children, of both genders, aged two to six years old and 56 mothers, in Belo Horizonte, Minas Gerais, Brazil. Preschool children and their families with regular activities at public schools were invited to paticipate in the study. The following characteristics were assessed: Socio-demographic condictions, clinical health, anthropometric, biochemical, lifestyle and data on food consumption. The 56 healthy children were divided into two groups, overweight (C1) and non-overweight (C2), as well as their mothers, respectively, in overweight (M1) and non-overweight (M2). Nutritional status was defined according to results obtained through the Anthro® Software for nutritional analysis.
Thirty-five children were male, with mean age of 4.44 ± 1.0 years old. Eighty-nine percent of them were eutrophic, 86.7% were sedentary and they had five meals a day. Body mass index (BMI) for age and total cholesterol (TC) was higher on C1 (P = 0.0001) and high density lipoprotein cholesterol (HDL-c) was higher on C2. Mothers were 32.5 ± 7.1 years old, mostly married and employed. Eighty-six percent of them were sedentary and 62.5% were overweight with BMI = 26.38 ± 5.07 kg/m2. Eighteen percent of the overweight mothers had isolated total hypercholesterolemia (TC levels elevated) and 12.5% had low HDL-c levels. The present study showed an association between overweight and obesity during the preschool years and the correspondent mothers’ nutritional status of overweight and obesity (OR = 4.96; 95%CI: 0.558-44.17). There was a positive correlation between the food risk associated with CVD by children and mothers when their consumption was 4 times/wk (P = 0.049; r = 0.516) or daily (P = 0.000008; r = 0.892).
Analyzed children showed high rates of physical inactivity, high serum cholesterol levels and high consumption of food associated with risk for CVD and renal disease. Changes in habits should be encouraged early in kindergarten.
Core tip: This is an observational cross-sectional study with 60 healthy preschool children and 56 mothers. Children were divided in overweight and non-overweight groups, as well as their mothers. There were 35 male children, mean age 4.44 ± 1.0 years old, 89% of all children were eutrophic, 87% sedentary. Body mass index/age and total cholesterol were higher in the overweight group. Mother’s age was 32.5 ± 7.1 years old, mostly married and employed, 86% of them were sedentary, 63% overweight. There was an association between overweight and obesity during preschool years and the correspondent mothers’ nutritional status of overweight and obesity. There was a positive correlation between food risk consumption associated with cardiovascular disease by children and mothers.
- Citation: da Silva AC, de Sousa Tavares M, Penido MGMG. Prevalence of risk factors for cardiovascular and kidney disease in Brazilian healthy preschool children. World J Nephrol 2016; 5(6): 507-516
- URL: https://www.wjgnet.com/2220-6124/full/v5/i6/507.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i6.507
Child growth is not limited to increased weight and height, but is characterized by a complex process involving body size and number of cells influenced by genetic, environmental and psychological factors[1,2].
The first process is the formation, when tissues are organized and cellular and tissue functions are defined[2]. Subsequently, acquisition and improvement of more refined functional abilities arise, depending on the interaction with external stimuli. The progressive development of motor coordination increases physical activity and proportionally energy needs[3]. Most children are picky about the food and the consumption of inadequate or unbalanced amount of food can interfere with their development[4]. The formation of eating habits takes place gradually during infancy. There are genetic predispositions to like or dislike certain foods and differences in sensitivity to some tastes and flavors inherited from parents. These genetic influences will be increasingly shaped by the experiences along the person’s life and will be combined with cultural, social, affective or emotional and behavioral values.
Brazil is experiencing a period of epidemiological and nutritional transition with increasing prevalence of chronic non-communicable diseases (CND) in all age groups. According to the World Health Organization (WHO), the CND are the leading causes of death and disability worldwide. Obesity, high blood pressure (BP) and hypertension, type 2 diabetes mellitus and dyslipidemias feature a metabolic profile that creates favorable conditions for the development of cardiovascular disease (CVD) and progressive renal disease (RD)[5-7]. This metabolic profile or risk factors for development of CVD and RD was not important in children in the past. However, profound changes in lifestyle have favored the emergence of these risk factors in pediatric patients.
Studies that focus on risk factors for the development of CVD and RD in children, especially in preschool age, are scarce. Such information is important, since they allow estimating the possible factors associated with worsening health and determine the nutritional inadequacies in individuals early on. Such information allows a better planning of targeted interventions, which may provide better fitness levels of clinical and metabolic control since childhood. In this scope, this study aimed to investigate the relationship between nutritional parameters and risk factors for CVD and RD in healthy preschool children, and whether a combination of these factors and the biological inheritance between mother and child may occur.
The study was approved by the institutional review board of the Federal University of Minas Gerais, Brazil (ETIC 0030.0.410.203-09) and by the Secretaria Municipal de Saúde de Belo Horizonte (0030.0.410.410-09 A). It was performed in accordance with the ethical standards laid down in 1964 by Declaration of Helsinki. The participants and/or their guardians signed informed consent forms.
This is a cross-sectional, observational, epidemiological school-based survey. Children and their mothers were selected from a total of 80, but 20 participants were excluded due to non-accordance to participation. The sample consisted of 60 healthy preschool children (75% of the original population), from 2 to 6 years old, both gender; and 56 mothers (some had more children in the study) recruited from March to September 2010 in kindergartens and public schools in the Northeast region of the city of Belo Horizonte, capital of the state of Minas Gerais, Brazil, which had 2258096 inhabitants[8].
Exclusion criteria were with any of the following conditions: Those under 2 years old or over 6 years old, those with chronic degenerative diseases, acute infectious and febrile diseases, with co-morbidities or use of drugs that could interfere with the biochemical analysis of blood and urine. The recruited children and their mothers attended the Primary Care Unit in a pre-scheduled date for obtaining measurements and blood and urine collection.
We used a semi-structured questionnaire previously tested and adapted for the studied population. This questionnaire contained social, demographic, economic, clinical data and family history. Anthropometric measurements (weight and height) were performed according to the techniques recommended by Jelliffe (1968)[9].
The body mass index for age (BMI/A) was calculated for the purpose of evaluating the adequacy of weight for height and age. This index was obtained by dividing the current weight (kg) by height squared (m2) and through the calculation performed by the WHO Anthro v3.0.1 program for children up to 5 years old, and WHO Anthro Plus v1.0.2, for children older than 5 years old. For the analysis of BMI/A in children we used the cutoffs of WHO (2006) for children between 0 and 5 years old and WHO in 2007 for children between 5 and 10 years old[10]. Overweight or obesity were characterized as z-score for BMI/A higher than +2 and called C1, with the purpose of comparison to children with z-score for BMI/A below this limit, called C2.
BMI of mothers was also calculated to assess nutritional status. This index was obtained by dividing the current weight (kg) by height squared (m2)[11]. For the analysis of BMI of the mothers, the nutritional diagnostic criteria were used as recommended for adults by the WHO (1995 and 1997)[12]. Mothers were divided into 2 groups, mothers with overweight or obesity (M1) and those with other nutritional classification (M2). Children of mothers with overweight and obesity (O1) were compared with children whose mothers with other nutritional state (O2).
BP was measured according to the recommendations of the VI Brazilian Guidelines on Arterial Hypertension (2010). The cut-off points of systolic blood pressure (SBP) and diastolic blood pressure (DBP) of the children were analyzed according to the percentiles of height for age obtained by the statistical program Epi Info Windows version 3.5.1. It was considered as “BP above the normal values” when the SBP or DBP were above the 90th percentile of the reference population, as recommended by the fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents. For mothers, the cut-offs of SBP and DBP were also analyzed according to the values described by the VI Brazilian Guidelines on Arterial Hypertension (2010), where BP high values are referred as equal to or above 140 mmHg × 90 mmHg. For blood sampling, mothers and children were instructed to fast for 12 h and to have at least 6 h sleep the night before. Mothers should not have consumed alcohol in the 48 h prior to the examination, they had not made use of diuretics at least 7 d in advance and should report the use of medications that may interfere with test results[13].
We evaluated the total cholesterol (TC) and its fractions such as high density lipoprotein cholesterol (HDL-c), low density lipoprotein cholesterol (LDL-c) and very low density lipoprotein cholesterol (VLDL-c), triglycerides (TG), glucose (GL), albumin (ALB), uric acid, hemoglobin (HG) and creatinine (CR) to calculate creatinine clearance by the formula of Schwartz[14] for children and the formula of Cockcroft Gault for mothers[15]. Analysis of TC and TG levels was made by an enzymatic method. The colorimetric method without precipitation was used for HDL-c and obtained by calculation of the LDL-c fraction and VLDL-c from the Friedwald formula. The cut-off points for children followed the recommendation of the American Academy of Pediatrics[16]. For mothers, the adopted cut-off points were the IV Brazilian Directive on Dyslipidemia and Prevention of Atherosclerosis (2007)[17].
The classification of GL levels was performed according to the American Diabetes Association (2006)[18] and the method of analysis was the enzymatic. The cut-off point used for the classification of ALB values was 3.5 to 5.0 g/dL. The analysis method used was the colorimetric. For checking the AU was used the colorimetric method, being considered as altered, values above 7.0[19].
The CR serum level was used as a risk marker for the development of RD and the method of analysis was kinetic. For children, we used the criteria proposed by The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative[20] and for mothers the reference values was from 0.7 to 1.5 mg/dL[21].
The concentration of HG was obtained by the colorimetric method and the considered reference values were 11.5 to 13.5 g/dL for children and 12.2 to 18.1 g/dL for mothers.
A random urine sample was collected from children and their mothers after 12 h of fasting for albuminuria detection and normal values were considered below 30 mcg/g creatinine, according to Cari Guidelines (2004)[22]. The analysis of ALB was made by the immunoturbidimetry and creatinine by colorimetry.
The food intake of children and their mothers was assessed by a food frequency questionnaire (FFQ) validated and adapted to the studied population. The list of foods in this questionnaire was built considering the foods most commonly consumed by children in Belo Horizonte, based on data retrieving the use of food recall 24 h in 10 children and their mothers.
After setting all parameters, the developed instrument presented 63 food items for group I, classified as risk foods or promoters of CVD, and 39 food items for group II, classified as protectors of CVD food. The foods were divided into 7 time units according to frequency of consumption: Not consuming, 1 time/mo, 2-3 times/mo, 1-2 times/wk, 3-4 times/wk, 5-6 times/wk or consume daily.
The FFQ was answered individually, and the mothers were asked to report the frequency of consumption of foods listed and to add the foods consumed regularly, but not included in the questionnaire. For each item of the questionnaire it was also informed the average frequency of food consumption for the last 6 mo and the respective time unit (if daily, weekly, monthly or not consumed). To minimize the interviewer’s information bias, training sessions were conducted to standardize the procedure and school meetings were scheduled with the mothers to demonstrate how to fill the FFQ correctly. Each item of the FFQ was entered in a spreadsheet (Excel, 2007), each time unit converted into scores and obtained the sum of consumption of each food, the average and the frequency of consumption of risk food and protection for CV diseases. To qualify the usual food consumption, a minimal consumption once a week was considered, usual consumption when frequency was ≥ 4 times/wk and daily consumption, with the usual consumption of food ≥ 4 times/wk chosen as ideal considered by approaching the median of week days.
The statistical analysis and review of the study was performed by a biomedical statistician. A descriptive analysis of the data and the values were expressed as mean (and SD) or median, according to normal/non-normal distribution; first and third quartiles, otherwise. GraphPad Prism® v.5.0 statistical software was used and GraphPad Software (San Diego, California, United States), adopting a significance level of 5% (P < 0.05) for all analyzes. For comparison and correlation purposes, we used the student t test and Pearson’s coefficient. For the variables without normal distribution the Mann-Whitney test and the Spearman coefficient was used to measure correlation. The association test, when using contingency tables, was the Fisher’s exact test. To estimate the risk odds ratio and 95%CI were chosen (OR)[23,24].
Children: There were 35 (58.3%) male children, with a mean age of 4.44 ± 1.0 years old. The average birth weight was 3.1 kg and height was 49.8 cm. The duration of breastfeeding was 8.3 mo, excluding exclusivity. The BMI/A z-score was 0.30 reflecting adequacy of current weight and height for age. Elevated levels of SBP were found in 18.3% of children and 35% of them had high levels. Most children were eutrophic, despite sedentary.
In the sample, 86.7% had 5 meals a day and between the 3 main meals (breakfast, lunch and dinner) 28.3% skipped dinner. HDL-c values were reduced in 15% of the children (Table 1). When comparing children with overweight (C1) and without (C2) no difference was found except in BMI/A z-score (P < 0.0001).
| Children (n = 60) | Mothers (n = 56) | |
| Clinical and demographic characteristic | ||
| Male | 58.33% | |
| Age (yr) | 4.44 ± 1.01 | 32.5 ± 7.11 |
| Gestational age at birth (wk) | 38.72 ± 1.831 | |
| Birth weight (kg) | 3.12 ± 0.601 | |
| Length at birth (cm) | 49.83 ± 3.611 | |
| Breastfeeding duration (mo) | 8.25 (4-21.50) | |
| BMI/A z-score (children) and BMI (mothers) | 0.30 (-0.55-1.33) | 26.38 ± 5.07 |
| Prehypertension systolic n (%) | 11 (18.34) | 1 (1.79) |
| Prehypertension diastolic n (%) | 21 (35) | 8 (14.29) |
| Overweight and obesity n (%) | 7 (11.67) | 35 (62.5) |
| Physical activity n (%) | 8 (13.33) | 8 (14.29) |
| Familial history of cardiovascular event n (%) | 32 (57.14) | |
| Marital status n (%) | ||
| Married or cohabiting | 41 (73.21) | |
| Divorced, single or widowed | 15 (26.79) | |
| Education (> 8 yr of schooling) | 38 (67.86) | |
| Occupation (working outside the home) | 42 (75) | |
| Income per capita (> 1 minimum wage) | 13 (23.21) | |
| Cardiovascular and renal risk factors n (%) | ||
| Total cholesterol > 200 mg/dL | 2 (3.33) | 10 (17.86) |
| LDL-c > 130 mg/dL | 3 (5) | 8 (14.29) |
| HDL-c < 35 mg/dL | 9 (15) | 7 (12.5) |
| Triglycerides > 150 mg/dL | 2 (3.33) | 6 (10.71) |
| Uric acid > 7 mg/dL | 0 (0) | 0 (0) |
| Albuminuria > 30 mcg/mg of creatinine | 2 (3.33) | 2 (3.57) |
The comparison between C1 and C2 is shown in Table 2 and a difference was observed only for HG, being higher in C1 group (P = 0.038). The values of TC, LDL-c, VLDL-c and TG were higher in C1, although not statistically significant. The value of HDL-c was higher in the C2 group, also not statistically significant.
| Children (n = 60) | Mothers (n = 56) | |||||
| C1 | C2 | P | M1 | M2 | P | |
| Uric acid (mg/dL) | 2.8 (2.0-3.2) | 3 (2.5-3.0) | NS | 4.8 (3.1-4.9) | 3.7 (3.0-4.3) | NS |
| Total cholesterol (mg/dL) | 166 (119-167) | 155 (136-169) | NS | 169 (145-189) | 163 (133.5-190.8) | NS |
| HDL-c (mg/dL) | 40 (37-45) | 49 (42.5-57.5) | NS | 52 (45-58) | 49.5 (43-57.5) | NS |
| LDL-c (mg/dL) | 92 (57-116) | 87 (76-103) | NS | 103 (81-123) | 94 (73.5-114) | NS |
| VLDL-c (mg/dL) | 14 (9-18) | 13 (9.5-18) | NS | 14 (11-19) | 16 (10-24) | NS |
| Triglycerides (mg/dL) | 72 (45-91) | 65 (47.5-92) | NS | 69 (53-97) | 83 (50-120.5) | NS |
| Albuminuria1 | 5.0 (2-10.37) | 5.0 (3.17-10.56) | NS | 3 (1.9-3.9) | 4.01 (2.99-6.0) | NS |
| Estimated creatinine clearance2 (mL/min per 1.73 m2) | 143.7 (123.2-151.9) | 129.8 (118-144.4) | NS | - | - | - |
| CrCl3 | - | - | - | 111.4 (105.1-131.2) | 109.9 (93.5-147.3) | NS |
| Glucose (mg/dL) | 75 (70-85) | 82 (77-85) | NS | 84 (79-93) | 84 (79-89) | NS |
| Albumin (g/dL) | 4.3 (4.2-4.5) | 4.4 (4.3-4.7) | NS | 4.3 (3.9-4.4) | 4.4 (4.3-4.5) | NS |
| Hemoglobin (mg/dL) | 13.1 (12.8-14.25) | 12.7 (12.15-13.1) | 0.03 | 13.6 (13-14.7) | 13.4 (12.7-13.9) | NS |
Mothers: The average age of mothers was 32.5 years old and most were married or cohabitating. The educational level ranged from 0 to 15 years of formal study and approximately 2/3 of them had a minimum of 8 years of study. Most of them worked and per capita income ranged from BRL $85.80 to BRL $2000.00. Only 23.2% had monthly income above one minimum wage per person (BRL$510.00) at the time of the survey (Table 1).
We found a high rate of overweight (BMI > 25 kg/m2) mothers and most were sedentary. Consumption was no more than 4 meals/d and among the top 3 (breakfast, lunch and dinner) 53.6% skipped dinner.
The mean SBP was 111 mmHg and for DBP was 75 mmHg, values considered suitable for adults. Just over half of the mothers had a family history of CV event. Laboratory evaluations of M1 and M2 groups are presented in Table 2 and there was no difference. Only 2 mothers had high values of ALB in random urine sample (3.33%), a result that may have been found by chance.
The children of mothers with overweight/obesity (O1) were compared to those of mothers without overweight/obesity O2 (Table 3). The values for LDL-c and TG were lower in the O2 group, although not statistically significant. The O1 group had higher ALB values but all results within the range considered normal. The likelihood of a child being overweight when this child was the mother’s child with the same nutritional status was 85.7%, positive predictive value (PPV) of 0.8571 (95%CI: 0.4213-0.9964). The proportion of children without overweight whose caregiver also showed that nutritional classification was 96%, with a specificity of 0.96 (95%CI: 0.7965-0.9990); negative predictive value (NPV) of 0.4528 (95%CI: 0.3156-0.5955) and 0.1714 sensitivity (95%CI: 0.06562-0.3365). The analysis of the mother’s weight relative to the weight of the child, particularly with regard to the presence of excess weight in the mother, is a predictor of overweight indicator in children. Although the test is very sensitive, it appears to be very specific for predicting normal weight in children.
| Variables | P | r | |
| Foods associated with risk | |||
| Frequency consumption | 1/wk | 0.079 | 0.516 |
| 4 times/wk | 0.049 | 0.516 | |
| Daily | 0.000008 | 0.892 | |
| Foods associated with protection | |||
| Frequency consumption | 1/wk | 0.218 | 0.429 |
| 4 times/wk | 0.946 | -0.031 | |
| Daily | 0.009 | 0.796 |
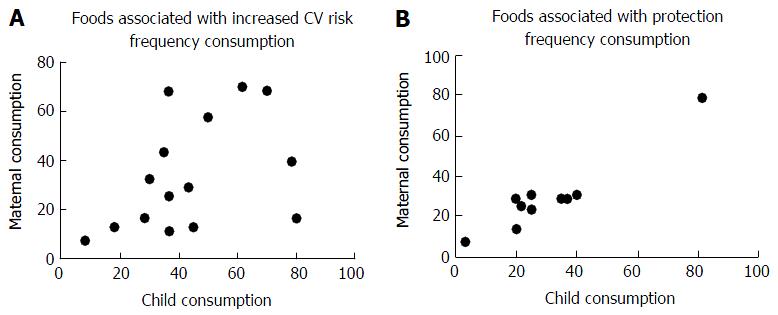
The analysis of consumption of each food by risk group or protection of children and their mothers showed a positive correlation, especially for foods that pose a risk for the development of CVD (Table 3).
The main risk for CVD food most consumed by children and mothers were: Whole milk, coffee, spaghetti, French bread, margarine, sweets like candies, lollipops and chewing gum; broth, artificial juices and soft drinks. As protectors against CVD a higher frequency of consumption of beans, banana, carrots, orange and tomato was described.
Figure 1 demonstrated the positive correlation between the daily consumption of food associated with risk and protective ones, respectively, for CVD between mothers and preschool children.
Previous research has shown that the first nutritional experience of a person may influence susceptibility to certain chronic diseases in school age children and adulthood, especially obesity. Even children with an appropriate BMI may have asymptomatic clinical and metabolic changes that contribute to the development of CND[25]. Physical inactivity in childhood tends to perpetuate in adulthood and physical activity in pediatric patients significantly declined in the last two decades, favoring the onset of risk factors for CVD and RD[25,26]. The increasing prevalence of overweight reflects the positive energy balance caused by excessive energy intake and/or decrease in physical activity in children and adolescents in the last 3 decades of life[27].
The world scenario of NCD is a new challenge for public health[28,29]. The possibility of preschool children to become obese adults, sedentary and with metabolic changes that will culminate in CVD or RD is worrisome to health managers. There are few studies about the prevalence of risk factors for these conditions in preschool children, making it difficult to properly approach and elaborate actions that could change the course and the deleterious effects of these diseases. Most likely, kindergartens and schools would be the most supportive environments to promote healthy lifestyles. This study aimed to assess the prevalence of risk factors for CVD and RD in preschool children from public schools in Belo Horizonte, Brazil, and the influence of lifestyle habits of their mothers on them.
Clinical and demographic characteristics of the children point to some health concerns on them. Elevated systolic and diastolyc BP levels were observed in 18.3% and 35% of the children, respectively. These pressure levels were higher than a previous studies conducted in Southern Brazil published only locally[30], which suggested that the best age to intervene in children is before 6 years of age when high BMI/A z-score are diagnosed.
It was also observed that overweight and obesity were independent predictors for arterial hypertension[31,32]. In the present study the BMI/A z-score was within the normal range for most children and cannot be considered responsible for the higher BP levels observed. Probably, there was influence of other behavioral factors such as lifestyle (sedentary lifestyle) and food (high intake of fat and salt). In agreement with other studies, no difference in DBP between genders was found. The elevated SBP levels (18.3%) and high DBP levels (35%) observed in the study may be related to food intake considered as high risk for CVD (sodium), dinner substitution snacks in 28.3% of the cases (11.7% overweight), and prevalence of physical inactivity (86.7%). Rodríguez-Moran et al[33] identified hyperglycemia (0.3%), hypertension (3.4%), metabolic syndrome (10.1%) and hyperinsulinemia (13.4%) in 358 schoolchildren. Hyperglycemia and hyperinsulinemia in children with maternal history of hypertension were also observed, suggesting familial inheritance for the increased risk to develop this comorbidity[33].
Studies have shown that the reduction in the number of nephrons is associated with development of primary hypertension. According to the “Brenner-Barker Hypothesis”, the chronic degenerative diseases of the adult may result from environmental conditions experienced during fetal life. Birth weight was associated with nephronic mass reduction and thus this hypothesis synthesizes the interaction gene-environment-disease in the causation of hypertension[34,35]. Low birth weight was associated with a reduced number of glomeruli and with increased volume of them[36].
Hughson et al[37] found that birth weight is a determining factor in the number of nephrons and consequently postnatal renal size. These findings support the hypothesis that low birth weight is a risk factor for hypertension and chronic kidney disease (CKD)[37]. In the study of patients with hypertension, Keller et al[38] found fewer glomeruli per kidney when compared to normotensive patients and the hypertensive patients had a higher glomerular volume than the control group. It is suggested that intrauterine growth would have regulatory influence on the formation of nephrons and renal function in humans, which extends beyond the neonatal period[39]. In our study, weight and gestational age at birth were suitable for all children and there were no differences when we compared obese children and children with other nutritional status.
Prospective epidemiological studies have shown that active lifestyle and aerobic fitness are independently associated with reduced incidence of NCDs and overall mortality and CVD. This practice is also an important protective factor against obesity, type 2 diabetes, some cancers and some mental disorders[27]. However, this is not the reality of children in our study. Only 13.3% of the sample performed some kind of physical activity outside the school. The prevalence of childhood obesity has grown 10% to 40% in the last 10 years in most European countries and occurs in the first years of life, between 5 and 6 years old and adolescence[40]. In the United States it affects between 20% to 27% of children and adolescents[41]. In Brazil, obesity is more prevalent in developed regions, where the industrial modernization process is advanced, in children during the first years of life and is associated with early weaning practices and dissemination of incorrect dietary instructions that encourage overfeeding[42]. This study with supposedly healthy children found 11.7% of overweight. This percentage is high, but it is close to that shown above by Abrantes et al[43] in children in northeast and southeast regions of Brazil.
The comparison between children classified as overweight and those with normal weight showed no differences in gestational age and birth weight, duration of breastfeeding and current age. However, the BMI/A z-score showed a significant difference (P < 0.0001), suggesting that other factors could be involved in the determination of this nutritional status, such as diet, lifestyle and genetic inheritance[44]. Studies correlating genetic factors and obesity demonstrated interference of these aspects in up to 25% of obese children, suggesting that the accumulation of excessive body fat, in most cases, would be triggered by social and environmental aspects[6].
According to Barja et al[45], the prevalence of obesity in families of obese adolescents is related to familiy history of obesity and possibly by the combined effect of genetic factors and lifestyle habits. The mothers, in our study, were overweight (62.5%), sedentary (85.7%) and with high BMI. Twelve percent of children were overweight, suggesting influence of life and eating habits of mothers on their children. Guo et al[46], studying obese children and adolescents showed that 33% of boys and 50% girls remained obese in adulthood.
Breastfeeding has been considered protective against obesity, however, there is controversy[41]. O’Callaghan et al[47] did not observe association between duration of breastfeeding and the prevalence of obesity at 5 years of age in 4062 children in Australia. In this study, overweight and obesity children had shorter breastfeeding when compared to those with normal weight, although not statistically significant.
Dyslipidemia may start in childhood and perpetuate. Some children have a metabolic profile characterized by decreased HDL-c, increased LDL-c and TG, reduced activity of the enzyme lipoprotein lipase and increased insulin resistance, favorable to the development of CVD and progressive RD[48]. A previous study with Brazilian children and adolescents (2 to 12 years old, 12 to 19 years old) found a correlation between dyslipidemia and obesity and overweight[49].
The Bogalusa Heart Study correlated the finding of atherosclerosis in autopsies of children with risk factors detected before death (elevated serum levels of TC, LDL-c and low HDL-c) and concluded that these alterations were related to atherosclerotic lesions present from the earlier stages in childhood[50]. In this study 3.3% and 5% of children had serum levels of TC and TG and LDL-c, respectively, above the desirable values and 15% with HDL-c below. It is known that low HDL-c accelerates progression of atherogenesis[47].
There is evidence that peripheral endothelial cells have modulating effects on vascular reactivity. This condition may disrupt homeostasis, can lead to endothelial dysfunction and contribute to atherosclerosis and CVD[51]. According to de Oliveira et al[42], studies have demonstrated the presence of at least one risk factor (hypertension, hyperlipidemia or hyperinsulinemia) for CVD in 60% of children and adolescents overweight, and 20% had two or more risk factors.
Ribeiro et al[32] evaluated CV risk in children and adolescents aged 6 to 18 and found that 32.9% and 25.1% had TC and LDL-c above normal values, respectively, and 17% had HDL-c below the recommended values. The children in the study were separated into 2 groups according to overweight and higher values for TC, LDL-c and TG and lower values for HDL-c were observed in those classified as overweight compared with eutrophic ones, although not statistically significant. This suggests a positive association between excess weight and metabolic changes.
Obesity, dyslipidemia and hypertension are increasingly prevalent in the pediatric population and are considered risk factors for KD[20,52]. A cross-sectional study with 274 healthy school children was conducted to identify risk factors for developing CKD and found 8.1% of low birth weight; 23.6% of obesity in grandparents, 6.3% in parents and in 13.8% of the mothers; and 7.1% of them were hypertensive. There was also a positive correlation between SBP/DBP and BMI as well as with waist circumference[53].
Excess body weight was associated with the presence of proteinuria and obese individuals had a higher risk for developing glomerulopathy[54]. Obesity in a parent leads to increased risk of obesity in children and can be almost 2 times higher for individuals with obese father and mother[55]. In this study, there was a tendency of mothers with overweight towards having children also overweight and obesity in the future (OR = 4.96, 95%CI: 0.56-44.17). The PPV of the test was 85.7% and the NPV was 45.3% (probability of a child to be eutrophic when she or he is mother’s daugther without overweight). For the specificity of the same test, the proportion of children without overweight whose caregiver was also classified with the same nutritional status was 96%. The presence of excess weight in the mother was a very sensitive indicator (17.1%) in predicting overweight in children but very specific in predicting normal weight in this population.
Children have their food style heavily influenced by family, friends and media[4]. Most children in the study had at least 5 meals/d and 51.8% of mothers 4 meals/d. The fact of not having dinner was identified in 28.3% of children and 53.6% of mothers with replacement of that meal for food associated with CVD and RD risk. This finding reinforces the mother’s influence on the food quality of their children and the damage on the nutritional status of the individual. Another important finding of this study was the correlation between the consumption of certain food by children and their mothers and the protection for CVD. It was found a close relationship between the profiles of food consumed by mothers and their children’s. The risk of developing CVD was higher when specific food consumption (those associated with increased CV risk) was 4 times/wk (P = 0.049, r = 0.516) or daily (P = 0.000008, r = 0.892). It was necessary a daily intake of protective foods for adequate protection for CVD (P = 0.009, r = 0.796).
There are limitations in the present study. The small number of participants, the difficulty of collection of laboratory samples and to perform physical examination at kindergarten/school are some of these limitations. The small number of participants could be justified because we have difficulty to recruit them due to age and because the parents’ refusal to accept the study. Data regarding this age group are scarce on cardiovascular risks factors and KD risk factors. In the same way, it is very difficult to collect blood and urine samples from preschool children as well as it is difficult to perform physical examination without to cause some disconfort to the child.
The results of BP measurements in children is frequenyly influenced by environmental aspects, such as technical acceptance by the child and difficulty and lack of time of mothers to repeat the measurement, preventing reliable correlations between variables and inferences. Thus, we have to be careful with the interpretation of these results.
Nevertheless, the study is valueable due to assess-ment of CVD and KD risk in a population of preschool children. Data regarding this age group are few. Day care and pre-school are friendly environments to promote proper lifestyle habits. Programs that provide healthy foods and preparations, the creation of support groups for women who are breastfeeding and the viability of public safe spaces for regular physical activity are essential for prevention of these diseases.
Most children were sedentary, although eutrophic. The group of overweight children had high TC and BMI/A z-score. Most mothers were also sedentary and overweight. The possibility of a child being overweight was 4.96 times higher in cases of being born from obese mothers. There was a positive correlation to the risk of food consumption and protectors for CVD among children and mothers. Considering the importance of maintaining adequate metabolic clinical control and prevention of the increased risk of CVD and RD development, it is necessary the proper guidance of parents, as well as the need for adequacy of life and eating habits of these. For the implementation of such a strategy it is necessary for the nutritional and educational intervention programs to be introduced into the routine of children, their families and educators.
The authors thank the Cachoeirinha Primary Care Unit and São Marcos Primary Care Unit for their valuable technical laboratorial assistance and personal support; Igor L Araújo, Lucas Lage Marinho and Luciana M Caetano for samples collections.
The first nutritional experience of a person may influence susceptibility to certain chronic diseases in school age children and adulthood, especially obesity. Physical inactivity in childhood tends to perpetuate in adulthood and physical activity in pediatric patients significantly declined in the last two decades, favoring the onset of risk factors for cardiovascular diseases (CVDs) and renal diseases. The prevalence of risk factors for these conditions in preschool children are not well known making it difficult to properly approach and elaborate actions that could change the course and the deleterious effects of these diseases. Such information allows a better planning of targeted interventions, which may provide better fitness levels of clinical and metabolic control since childhood.
This study aimed to investigate the prevalence of nutritional parameters of risk for CVD and renal diseases in healthy preschool children as well as their relationship with food profiles consumed by mothers and their children.
It is important to know the metabolic profile or risk factors for development of CVDs and renal diseases in order to maintain adequate metabolic clinical control and prevention of these diseases. A proper guidance of parents is necessary, as well as the need for adequacy of life and eating habits.
This study confirms that the nutritional parameters of risk for cardiovascular and renal diseases are already present in preschool children. It also confirms that there is a relationship between the profiles of food consumed by mothers and their children.
Traditional risk factors for cardiovascular and renal diseases are high blood pressure, dyslipidemia, overweight/obesity, diabetes mellitus and some habits related to lifestyle (diet high in calories, saturated fats, increased cholesterol, salt consumption and sedentary lifestyle). The study was done based on these risk factors.
This is a a well written observational cross-sectional study with 60 healthy pre-school children and 56 mothers. The results showed an association between overweight/obesity during preschool years and the correspondent mothers. The design and results are novel.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Wang F, Zhang ZH S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | World Health Organization. Nutrition in adolescence - issues and challenges for the health sector. 2005; Available from: http://www.who.int. |
| 2. | Gandra YR. [Preschool children of 2 to 6 years of age and the assistance given them]. Rev Saude Publica. 1981;15 Suppl:3-8. [PubMed] |
| 3. | Birch LL, Johnson SL, Andresen G, Peters JC, Schulte MC. The variability of young children’s energy intake. N Engl J Med. 1991;324:232-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 229] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Sixsmith R, Furnham A. A content analysis of British food advertisements aimed at children and adults. Health Promot Int. 2010;25:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Hansen SE, Hasselstrøm H, Grønfeldt V, Froberg K, Andersen LB. Cardiovascular disease risk factors in 6-7-year-old Danish children: the Copenhagen School Child Intervention Study. Prev Med. 2005;40:740-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Muennig P, Sohler N, Mahato B. Socioeconomic status as an independent predictor of physiological biomarkers of cardiovascular disease: evidence from NHANES. Prev Med. 2007;45:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Franco MC, Akamine EH, Rebouças N, Carvalho MH, Tostes RC, Nigro D, Fortes ZB. Long-term effects of intrauterine malnutrition on vascular function in female offspring: implications of oxidative stress. Life Sci. 2007;80:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Instituto Brasileiro de Geografia e Estatística. Resultados da amostra do censo demográfico 2010. Available from: http://www.ibge.gov.br. |
| 9. | Jelliffe DB. Weight scales for developing regions. Lancet. 1968;2:359-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | World Health Organization. WHO Child Growth Standards. 2007. Available from: http://www.who.int/childgrowth. |
| 11. | Bray GA, Gray DS. Obesity. Part I--Pathogenesis. West J Med. 1988;149:429-441. [PubMed] |
| 12. | World Health Organization. WHO. 1997. Available from: http://www.who.int. |
| 13. | Slinde F, Rossander-Hulthén L. Bioelectrical impedance: effect of 3 identical meals on diurnal impedance variation and calculation of body composition. Am J Clin Nutr. 2001;74:474-478. [PubMed] |
| 14. | Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2233] [Cited by in RCA: 2766] [Article Influence: 172.9] [Reference Citation Analysis (0)] |
| 15. | Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10672] [Cited by in RCA: 11004] [Article Influence: 224.6] [Reference Citation Analysis (1)] |
| 16. | Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 780] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 17. | Sposito AC, Caramelli B, Fonseca FA, Bertolami MC, Afiune Neto A, Souza AD, Lottenberg AM, Chacra AP, Faludi AA, Loures-Vale AA. [IV Brazilian Guideline for Dyslipidemia and Atherosclerosis prevention: Department of Atherosclerosis of Brazilian Society of Cardiology]. Arq Bras Cardiol. 2007;88 Suppl 1:2-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 18. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29 Suppl 1:S43-S48. [PubMed] |
| 19. | Alper AB, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension. 2005;45:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 226] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, Balk E, Lau J, Levin A, Kausz AT. National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics. 2003;111:1416-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 410] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 21. | Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3156] [Cited by in RCA: 3210] [Article Influence: 145.9] [Reference Citation Analysis (0)] |
| 22. | Caring for Australians with Renal Impairment (CARI). The CARI guidelines. Urine protein as diagnostic test: evaluation of proteinuria in children. Nephrology (Carlton). 2004;9 Suppl 3:S15-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Zhang Z. Model building strategy for logistic regression: purposeful selection. Ann Transl Med. 2016;4:111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 290] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 24. | Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med. 2016;4:91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 25. | Fisberg M, Baur L, Chen W, Hoppin A, Koletzko B, Lau D, Moreno L, Nelson T, Strauss R, Uauy R. Obesity in children and adolescents: Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39 Suppl 2:S678-S687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Loucaides CA, Jago R, Charalambous I. Promoting physical activity during school break times: piloting a simple, low cost intervention. Prev Med. 2009;48:332-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 368] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 28. | Baker S, Barlow S, Cochran W, Fuchs G, Klish W, Krebs N, Strauss R, Tershakovec A, Udall J. Overweight children and adolescents: a clinical report of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child. 2004;89:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 30. | Goldraich NP, Biernat MS, Pilla C. Pressão arterial sistólica e diastólica em crianças saudáveis de 24 a 87 meses: influência do índice de massa corporal das crianças e dos pais e da história familiar de hipertensão arterial em pais e avós. J Bras Nefrol. 2008;30 Supl 3:23. |
| 31. | Oliveira AM, Oliveira AC, Almeida MS, Almeida FS, Ferreira JB, Silva CE, Adan LF. [Environmental and anthropometric factors associated with childhood arterial hypertension]. Arq Bras Endocrinol Metabol. 2004;48:849-854. [PubMed] |
| 32. | Ribeiro RQ, Lotufo PA, Lamounier JA, Oliveira RG, Soares JF, Botter DA. [Additional cardiovascular risk factors associated with excess weight in children and adolescents: the Belo Horizonte heart study]. Arq Bras Cardiol. 2006;86:408-418. [PubMed] |
| 33. | Rodríguez-Moran M, Aradillas-García C, Simental-Mendia LE, Monreal-Escalante E, de la Cruz Mendoza E, Dávila Esqueda ME, Guerrero-Romero F. Family history of hypertension and cardiovascular risk factors in prepubertal children. Am J Hypertens. 2010;23:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1559] [Cited by in RCA: 1582] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 35. | Brenner BM. Remission of renal disease: recounting the challenge, acquiring the goal. J Clin Invest. 2002;110:1753-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Schreuder MF, Nauta J. Prenatal programming of nephron number and blood pressure. Kidney Int. 2007;72:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Hughson M, Farris AB, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63:2113-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 546] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 38. | Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 729] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 39. | Patel UD. Fetal origins of renal disparities. Semin Nephrol. 2010;30:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 40. | Mello ED, Luft VC, Meyer F. [Childhood obesity--towards effectiveness]. J Pediatr (Rio J). 2004;80:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Balaban G, Silva GA. [Protective effect of breastfeeding against childhood obesity]. J Pediatr (Rio J). 2004;80:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | de Oliveira AM, Cerqueira Ede M, de Oliveira AC. [Prevalence of overweight and childhood obesity in Feira de Santana-BA: family detection vs. clinical diagnosis]. J Pediatr (Rio J). 2003;79:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Abrantes MM, Lamounier JA, Colosimo EA. [Overweight and obesity prevalence in Northeast and Southeast Regions of Brazil]. Rev Assoc Med Bras (1992). 2003;49:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Hughes AR, Sherriff A, Lawlor DA, Ness AR, Reilly JJ. Incidence of obesity during childhood and adolescence in a large contemporary cohort. Prev Med. 2011;52:300-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Barja S, Arteaga A, Acosta AM, Hodgson MI. [Insulin resistance and other expressions of metabolic syndrome in obese Chilean children]. Rev Med Chil. 2003;131:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653-658. [PubMed] |
| 47. | O'Callaghan MJ, Williams GM, Andersen MJ, Bor W, Najman JM. Prediction of obesity in children at 5 years: a cohort study. J Paediatr Child Health. 1997;33:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Cherian MA, Santoro TJ. The role of saturation of fat depots in the pathogenesis of insulin resistance. Med Hypotheses. 2006;66:763-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Gerber ZR, Zielinsky P. [Risk factors for atherosclerosis in children: an epidemiologic study]. Arq Bras Cardiol. 1997;69:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Webber LS, Voors AW, Srinivasan SR, Frerichs RR, Berenson GS. Occurrence in children of multiple risk factors for coronary artery disease: the Bogalusa heart study. Prev Med. 1979;8:407-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Gonzalez MA, Selwyn AP. Endothelial function, inflammation, and prognosis in cardiovascular disease. Am J Med. 2003;115 Suppl 8A:99S-106S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 52. | McClellan WM. Epidemiology and risk factors for chronic kidney disease. Med Clin North Am. 2005;89:419-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Naghettini AV, Salgado CM, Freitas JS, Salgado LM. [Identification of risk factors for chronic kidney disease among schoolchildren]. J Bras Nefrol. 2010;34:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 54. | Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 983] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 55. | Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature. Prev Med. 1993;22:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1023] [Article Influence: 32.0] [Reference Citation Analysis (0)] |