Published online Sep 6, 2016. doi: 10.5527/wjn.v5.i5.471
Peer-review started: May 11, 2016
First decision: June 14, 2016
Revised: June 22, 2016
Accepted: August 11, 2016
Article in press: August 13, 2016
Published online: September 6, 2016
Processing time: 115 Days and 7.9 Hours
To evaluate thresholds for serum 25(OH)D concentrations in relation to death, kidney progression and hospitalization in non-dialysis chronic kidney disease (CKD) population.
Four hundred and seventy non-dialysis 3-5 stage CKD patients participating in OSERCE-2 study, a prospective, multicenter, cohort study, were prospectively evaluated and categorized into 3 groups according to 25(OH)D levels at enrollment (less than 20 ng/mL, between 20 and 29 ng/mL, and at or above 30 ng/mL), considering 25(OH)D between 20 and 29 ng/mL as reference group. Association between 25(OH)D levels and death (primary outcome), and time to first hospitalization and renal progression (secondary outcomes) over a 3-year follow-up, were assessed by Kaplan-Meier survival curves and Cox-proportional hazard models. To identify 25(OH)D levels at highest risk for outcomes, receiver operating characteristic (ROC) curves were performed.
Over 29 ± 12 mo of follow-up, 46 (10%) patients dead, 156 (33%) showed kidney progression, and 126 (27%) were hospitalized. After multivariate adjustment, 25(OH)D < 20 ng/mL was an independent predictor of all-cause mortality (HR = 2.33; 95%CI: 1.10-4.91; P = 0.027) and kidney progression (HR = 2.46; 95%CI: 1.63-3.71; P < 0.001), whereas the group with 25(OH)D at or above 30 ng/mL did not have a different hazard for outcomes from the reference group. Hospitalization outcomes were predicted by 25(OH) levels (HR = 0.98; 95%CI: 0.96-1.00; P = 0.027) in the unadjusted Cox proportional hazards model, but not after multivariate adjusting. ROC curves identified 25(OH)D levels at highest risk for death, kidney progression, and hospitalization, at 17.4 ng/mL [area under the curve (AUC) = 0.60; 95%CI: 0.52-0.69; P = 0.027], 18.6 ng/mL (AUC = 0.65; 95%CI: 0.60-0.71; P < 0.001), and 19.0 ng/mL (AUC = 0.56; 95%CI: 0.50-0.62; P = 0.048), respectively.
25(OH)D < 20 ng/mL was an independent predictor of death and progression in patients with stage 3-5 CKD, with no additional benefits when patients reached the levels at or above 30 ng/mL suggested as optimal by CKD guidelines.
Core tip: This study examines the prognosis value of 25(OH)D levels on death, chronic kidney disease (CKD) progression, and hospitalization in a cohort of 3-5 stage CKD subjects not on dialysis. The main findings were the predictor value of vitamin D deficiency (< 20 ng/mL), but not insufficiency (< 30 ng/mL), for the 3-year incidence of death and CKD progression, which remained significant after multivariate adjustments. These results could highlight the need for a revision of the current guidelines, which have defined optimal vitamin D status at ≥ 30 ng/mL based on levels required to suppress parathyroid hormone, as opposed to our study, which evaluates thresholds for serum 25(OH)D concentrations in relation to “hard” endpoints.
- Citation: Molina P, Górriz JL, Molina MD, Beltrán S, Vizcaíno B, Escudero V, Kanter J, Ávila AI, Bover J, Fernández E, Nieto J, Cigarrán S, Gruss E, Fernández-Juárez G, Martínez-Castelao A, Navarro-González JF, Romero R, Pallardó LM. What is the optimal level of vitamin D in non-dialysis chronic kidney disease population? World J Nephrol 2016; 5(5): 471-481
- URL: https://www.wjgnet.com/2220-6124/full/v5/i5/471.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i5.471
There is a high prevalence of vitamin D (VD) deficiency in all stages of chronic kidney disease (CKD)[1-5]. Observational studies in this population have shown that VD levels correlated with cardiovascular disease and markers of renal injury, including albuminuria[1,6], renal progression[4,6-8], vascular calcification[9,10], left ventricular hypertrophy[9] and mortality[8,11-13]. Moreover, growing evidence supports a potential role for VD receptor activation in suppressing the renin-angiotensin system, reducing proteinuria and ameliorating kidney dysfunction[14-16], showing 25-hydroxyvitamin D [25(OH)D] as an attractive, cheap and feasible treatment target[17]. As a result of these findings, current guidelines have suggested VD supplementation in CKD patients[18-21], increasing VD supplementation rates among this population[22].
Nevertheless, these recommendations are opinion based and the optimal VD levels as well as the upper safe limit of VD intakes remains controversial[23,24]. Based on the inverse relationship between serum concentrations of 25(OH)D and parathyroid hormone (PTH), most current guidelines have defined VD deficiency and insufficiency, as a serum 25(OH)D level of < 20 ng/mL (50 nmol/L) and 20-29 ng/mL (52-72 nmol/L) respectively[18,19], suggesting a serum concentration of 25(OH)D above 30-40 ng/mL (75-100 nmol/L) to be desirable, levels at which PTH is suppressed to a minimum in its relation to 25(OH)D[25,26]. By contrast, the Institute of Medicine advocates VD repletion as a level of 20 ng/mL [27]. Determining the 25(OH)D target level for optimal health is especially important in CKD population, where overuse of VD leads to hypercalcemia, hypercalciuria and hyperphosphatemia, which could predispose to vascular calcification, nephrolithiasis and reduced glomerular filtration rate[28-30]. All these data suggest an optimal level of VD exists that is neither too high nor too low[31].
Aware of the lack of evidence behind guidelines recommendations, and our concerns about VD over-supplementation, encouraged us to investigate the optimal VD status in non-dialysis CKD patients. The aim of our study was to evaluate thresholds for serum 25(OH)D concentrations in relation to hard end-points such as death, kidney progression and hospitalization in this population.
OSERCE-2 was a 3-year follow-up prospective, observational, study which enrolled 742 adults with 3 to 5-stage CKD not on dialysis subjects attending 39 centres in Spain, to evaluate the effects of vascular calcifications and CKD-mineral bone disorders on mortality, hospitalization and kidney progression[32]. Inclusion criteria were age ≥ 18 years and CKD Stages 3-5. Exclusion criteria were acute kidney injury, transplantation, hospitalization in the month previous to the enrollment, and severe comorbidity. In this post-hoc analysis of the OSERCE-2 study, patients on current treatment with active VD (calcitriol, α-calcidol or paricalcitol) were also excluded, so 25(OH)D levels reflected the effect of the exposure to VD.
The study was reviewed and approved by the Dr Peset Hospital Research Ethics Committee. All study participants provided informed written consent prior to study enrollment.
The study protocol of the OSERCE-2 study has been previously reported[32]. All patients were assessed at baseline for blood pressure measurement, lateral lumbar, pelvis and hands X-ray, an ankle brachial pressure index (ABPI) determination and laboratory blood sampling. All blood samples were analyzed in a central laboratory, including 25(OH)D, 1,25(OH)2 vitamin D, creatinine, calcium, phosphorus, intact PTH, albumin, and high-sensitive C-reactive protein. 25(OH)D levels were assessed by radioimmunoassay (Biosource), which were transformed to the usual method of reference (DiaSorin Liaison chemiluminescent radioimmunoassay) for improving the comparability of the results, as previously described[32]. To study the renal progression, blood samples for determination of serum creatinine levels were obtained every 12 mo. Estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in renal disease (MDRD) formula[33].
Deaths episodes (primary outcome), time to first hospital admission and the appearance of a combined renal end-point, defined as a drop > 30% in eGFR, or beginning of renal replacement therapy (secondary outcomes), were prospectively gathered over a 3-year period[32].
Summary statistics were reported as frequencies or percentages, and as mean ± SD, for categorical and quantitative variables, respectively. Skewed quantitative variables were expressed as geometric mean (95%CI), after log transformation. Presence or absence of prominent calcification for Adragao (AS) and Kauppila scores (KS) was reported as AS ≥ 3 and KS > 6, respectively.
Patients were classified further into 3 groups by 25(OH)D level: < 20 ng/mL (deficiency), 20-29 ng/mL (insufficiency) and ≥ 30 ng/mL. Comparison of baseline characteristics in these 3 groups was assessed using one-way analysis of variance (ANOVA) for continuous variables, and χ2 test for trend, for categorical variables. Analysis of variables independently related to 25(OH)D levels was assessed by lineal regression model. To assess the relationship between the odds of VD deficiency and clinical and laboratory baseline characteristics, a stepwise binary logistic regression was performed between 25(OH)D level < 20 or ≥ 20 ng/mL as dependent variables. PTH and 1,25(OH)D levels were considered as posterior variables to 25(OH)D levels and then they were not introduced in the models, to avoid an overadjustment bias. Twenty-four hours urine proteinuria was not included either because it was available in only 50% of the patients.
Kaplan-Meier analysis and log-rank tests were used to estimate the effects of VD status on all-cause mortality, appearance of the composite renal endpoint, and hospitalizations. We then used univariate and multivariate Cox proportional hazard regression models to determine the association of VD levels with various pre-specified outcomes. Patients with 25(OH)D levels between 20 to 29 ng/mL were considered as reference group. Covariates significantly associated in the univariate analysis were entered (forward selection: Likelihood ratio) into the models. The relatively small number of deaths limited the list of adjustment variables that were included in the regression analyses. To identify VD levels at highest risk for outcomes, we performed a receiver operating characteristic (ROC) curve. The value associated with the highest accuracy was considered as the cut-off point for defining an increased risk of death, appearance of the composite renal endpoint, and hospitalization.
The literature indicates that annual mortality in patients with stage 3 to 5 CKD (not on dialysis), is between 3% and 9%. Previous studies have shown a 35% prevalence of VD deficiency in this population[3]. Compared with the group with VD deficiency, the group with VD insufficiency shows a 57% decrease in mortality[8]. With 470 patients included, a minimum follow-up of three years, and considering an error of alfa = 0.05, the power estimation of the study is 0.754. The statistical methods of this study were reviewed by MD Molina, from the Department of Mathematics, Universidad de Alicante, Spain, who was included as a co-author. All data analyses were conducted using SPSS, version 15.0 (SPSS Inc., Chicago, IL). A P-value < 0.05 was considered statistically significant.
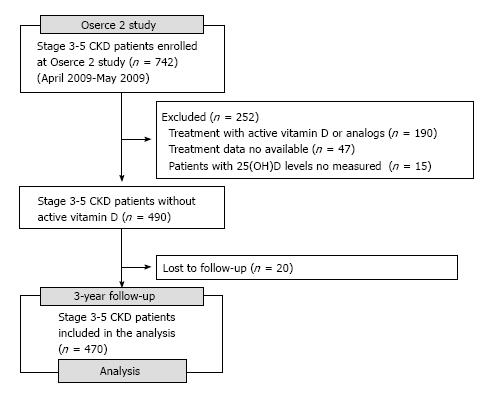
From the 742 subjects enrolled at OSERCE-2 Study, 252 were excluded and 20 were lost to follow-up, leaving 470 patients in the final analysis (Figure 1). Tables 1 and 2 show the patient characteristics and laboratory values, respectively, as a function of vitamin D status. According to 25(OH)D levels, the proportion of patients with deficiency or insufficiency was 53% and 33%, respectively. At baseline, the proportion of patients with 5-stage CKD, diabetes mellitus, diabetic nephropathy and chronic heart failure was higher in the group with less 25(OH)D levels. ABPI, eGFR, PTH, 1,25(OH)2 vitamin D and albumin levels were increased in groups with better VD status, which showed lower degree of proteinuria. The group with 25(OH)D less than 20 ng/mL was prescribed more frequently treatment with diuretics and erythropoietin-stimulating agents, with a lower proportion of patients under native VD treatment.
| All | 25(OH)D < 20 ng/mL | 25(OH)D 20-29 ng/mL | 25(OH)D ≥ 30 ng/mL | P | |
| n | 470 | 252 (53%) | 154 (33%) | 64 (14%) | |
| Age (yr) | 66.1 ± 12.9 | 65.8 ± 13.1 | 65.9 ± 11.9 | 68.1 ± 12.1 | 0.421 |
| Male sex (%) | 309 (66%) | 162 (64%) | 101 (66%) | 46 (72%) | 0.303 |
| High blood pressure (%) | 444 (95%) | 242 (96%) | 144 (94%) | 58 (91%) | 0.072 |
| Dyslipidemia (%) | 311 (66%) | 168 (68%) | 101 (66%) | 42 (66%) | 0.646 |
| Diabetes mellitus (%) | 183 (39%) | 114 (45%) | 53 (34%) | 16 (25%) | 0.001 |
| Ischemic heart disease (%) | 104 (22%) | 60 (24%) | 33 (22%) | 11 (17%) | 0.224 |
| Chronic heart failure (%) | 43 (9%) | 33 (13%) | 7 (5%) | 3 (5%) | 0.005 |
| Stroke (%) | 52 (11%) | 30 (12%) | 15 (10%) | 7 (11%) | 0.668 |
| Peripheral arterial disease (%) | 93 (20%) | 59 (24%) | 22 (14%) | 12 (19%) | 0.117 |
| Stage of CKD (%) | |||||
| 3 (eGFR = 30-59 mL/min per 1.73 m2) | 221 (47%) | 103 (41%) | 84 (54%) | 34 (53%) | 0.002 |
| 4 (eGFR = 15-29 mL/min per 1.73 m2) | 205 (44%) | 105 (46%) | 64 (42%) | 26 (41%) | |
| 5 (eGFR < 15 mL/min per 1.73 m2) | 44 (9%) | 34 (13%) | 6 (4%) | 4 (6%) | |
| Etiology of CKD (%) | |||||
| Hypertension | 108 (23%) | 54 (21%) | 40 (26%) | 14 (22%) | 0.039 |
| Diabetes mellitus | 108 (23%) | 72 (29%) | 29 (19%) | 7 (11%) | |
| Tubulointerstitial disease | 65 (14%) | 24 (10%) | 25 (16%) | 16 (25%) | |
| Glomerulonephritis | 47 (10%) | 26 (10%) | 15 (10%) | 6 (10%) | |
| Unknown/others | 142 (30%) | 75 (30%) | 44 (29%) | 20 (32%) | |
| Smoking (%)1 | |||||
| Never | 231 (53%) | 124 (52%) | 82 (58%) | 25 (44%) | 0.494 |
| Ex-smoker | 144 (33%) | 81 (34%) | 44 (31%) | 19 (33%) | |
| Active | 64 (14%) | 35 (14%) | 16 (11%) | 13 (23%) | |
| Blood pressure (kPa) | |||||
| Systolic | 19.0 ± 2.9 | 19.3 ± 2.9 | 18.6 ± 2.8 | 19.0 ± 3.1 | 0.085 |
| Diastolic | 10.2 ± 1.5 | 10.2 ± 1.6 | 10.1 ± 1.4 | 10.3 ± 1.7 | 0.617 |
| Pulse pressure (kPa) | 8.8 ± 2.5 | 9.1 ± 2.5 | 8.5 ± 2.5 | 8.7 ± 2.5 | 0.098 |
| Body mass index (kg/m2) | 28.6 ± 5.1 | 28.8 ± 5.5 | 28.6 ± 4.6 | 27.7 ± 4.4 | 0.294 |
| Underweight ( ≤ 18.5) | 6 (1%) | 4 (2%) | 1 (1%) | 1 (2%) | 0.353 |
| Normal (18.6-24.9) | 96 (20%) | 50 (20%) | 30 (19%) | 16 (25%) | |
| Overweight (25.0-29.9) | 210 (45%) | 111 (44%) | 68 (44%) | 31 (48%) | |
| Obesity (> 29.9) | 158 (34%) | 87 (34%) | 55 (36%) | 16 (25%) | |
| Waist (cm) | |||||
| Males | 102.2 ± 12.0 | 102.1 ± 13.0 | 102.2 ± 10.6 | 102.4 ± 11.5 | 0.989 |
| Females | 97.8 ± 13.5 | 98.3 ± 14.7 | 97.7 ± 12.2 | 95.7 ± 11.4 | 0.760 |
| ABPI | 1.01 ± 0.21 | 0.98 ± 0.20 | 1.04 ± 0.21 | 1.05 ± 0.22 | 0.013 |
| Abnormal ABPI2 | 194 (41%) | 100 (41%) | 66 (44%) | 28 (44%) | 0.539 |
| Abnormal Kauppila score3 | 107 (29%) | 52 (27%) | 35 (29%) | 20 (36%) | 0.183 |
| Abnormal Adragao score4 | 121 (32%) | 66 (33%) | 38 (30%) | 17 (29%) | 0.474 |
| Vitamin D supplementation (%) | 43 (9%) | 16 (6%) | 17 (11%) | 10 (16%) | 0.012 |
| Use of phosphate binders (%) | 72 (15%) | 47 (19%) | 16 (11%) | 9 (14%) | 0.105 |
| Use of ACEI/ARB (%) | 365 (78%) | 196 (79%) | 121 (82%) | 48 (76%) | 0.947 |
| Use of diuretic (%) | 287 (61%) | 173 (70%) | 88 (58%) | 26 (42%) | < 0.001 |
| Use of ESA (%) | 124 (26%) | 77 (31%) | 31 (20%) | 16 (25%) | 0.015 |
| All (n = 470) | 25(OH)D < 20 ng/mL (n = 252) | 25(OH)D 20-29 ng/mL (n = 154) | 25(OH)D ≥ 30 ng/mL (n = 64) | P | |
| 25-hydroxivitamin D (nmol/L) | 52 ± 21 | 36 ± 9 | 61 ± 7 | 90 ± 16 | < 0.001 |
| 1,25(OH)2 vitamin D (pmol/L) | 103 ± 28 | 97 ± 27 | 111 ± 28 | 107 ± 23 | < 0.001 |
| Caalb (mmol/L) | 2.40 ± 0.20 | 2.40 ± 0.15 | 2.42 ± 0.23 | 2.45 ± 0.23 | 0.163 |
| P (mmol/L) | 1.10 ± 0.26 | 1.10 ± 0.26 | 1.10 ± 0.26 | 1.07 ± 0.26 | 0.517 |
| iPTH (ng/L)1 | 91 (85-97) | 106 (96-116) | 81 (73-91) | 64 (55-74) | < 0.001 |
| Creatinine (μmol/L) | 221 ± 97 | 239 ± 106 | 212 ± 88 | 212 ± 88 | 0.017 |
| eGFR (MDRD, mL/min per 1.73 m2) | 29.4 ± 11.5 | 28.1 ± 11.9 | 30.8 ± 10.8 | 30.5 ± 11.1 | 0.049 |
| Urine protein excretion (g/24 h)12 | 0.592 (0.502-0.697) | 0.699 (0.573-0.853) | 0.448 (0.321-0.626) | 0.448 (0.271-0.742) | 0.034 |
| hsCRP (nmol/L)1 | 36.2 (29.5-39.1) | 37.1 (33.3-41.0) | 36.2 (31.4-41.0) | 32.4 (26.7-39.1) | 0.506 |
| Albumin (g/L) | 40 ± 5 | 39 ± 5 | 41 ± 5 | 40 ± 5 | 0.011 |
| Total proteins (g/L) | 77 ± 12 | 77 ± 11 | 77 ± 13 | 76 ± 14 | 0.877 |
| Total cholesterol (mmol/L) | 4.7 ± 1.1 | 4.7 ± 1.1 | 4.7 ± 1.0 | 4.8 ± 1.1 | 0.603 |
| HDL cholesterol (mmol/L) | 1.3 ± 0.4 | 1.3 ± 0.3 | 1.3 ± 0.4 | 1.3 ± 0.4 | 0.973 |
| LDL cholesterol (mmol/L) | 2.7 ± 0.9 | 2.7 ± 0.9 | 2.7 ± 0.9 | 2.9 ± 0.8 | 0.344 |
| Hemoglobin (g/L) | 130 ± 16 | 129 ± 16 | 132 ± 16 | 132 ± 18 | 0.058 |
| Ferritin (pmol/L)1 | 225 (207-245) | 227 (202-252) | 216 (187-252) | 247 (191-319) | 0.635 |
| Transferrin (μmol/L) | 3.0 ± 1.2 | 2.9 ± 1.2 | 3.0 ± 1.2 | 3.1 ± 1.3 | 0.289 |
| Glucose (mmol/L) | 6.3 ± 2.2 | 6.3 ± 2.3 | 6.4 ± 2.4 | 5.9 ± 1.5 | 0.241 |
Linear correlation analysis showed significant correlation between 25(OH)D levels and eGFR (R = 0.10; P = 0.027), body mass index (R = -0.10; P = 0.046), serum levels of albumin (R = 0.10; P = 0.027), calcium (R = 0.12; P = 0.013), 1,25(OH)2 vitamin D (R = 0.20; P < 0.001), PTH (R = -0.26; P < 0.001), and hemoglobin (R = 0.13; P = 0.005), proteinuria (log transformed, R = -0.19; P = 0.004) and ABPI (R = 0.15; P = 0.002). Multivariate binary logistic regression analysis showed as independent predictors of 25(OH) < 20 ng/mL the albumin levels (OR = 0.61; 95%CI: 0.40-0.92; P = 0.018), the ABPI (OR = 0.28; 95%CI: 0.11-0.73; P = 0.010), and treatment with native VD (OR = 0.35; 95%CI: 0.17-0.73; P = 0.005), and diuretics (OR = 2.03; 95%CI: 1.35-3.06; P = 0.001).
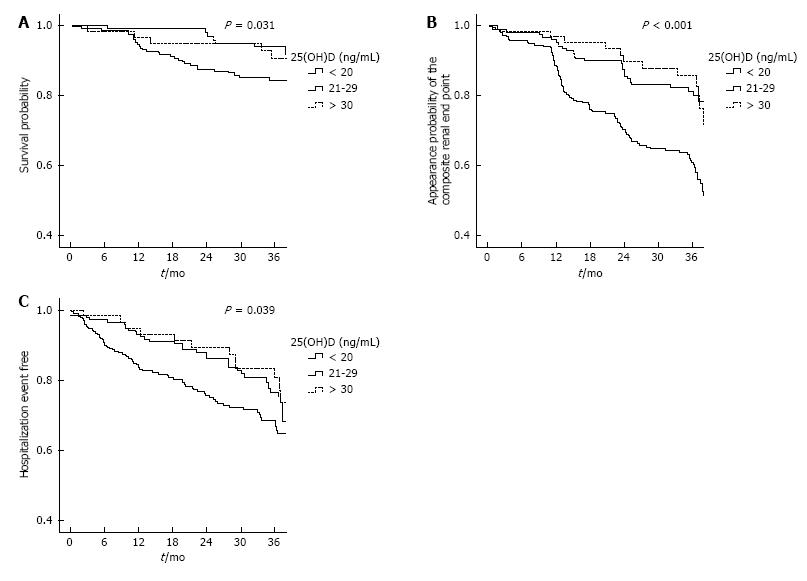
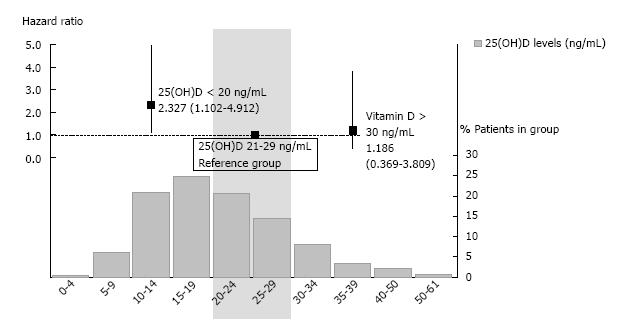
Forty-six (10%) patients died after a mean follow-up of 29 ± 12 mo. Cardiovascular disease (n = 16, 35%) and infections (n = 8, 17%) were the most common causes of death. Tumors and others accounted for 11% (n = 5) and 13% (n = 6) of deaths, respectively. In 11 cases (24%) the cause of death was not identified. The Kaplan-Meier survival analysis (Figure 2A) suggested that patients with 25(OH)D less than 20 ng/mL had significantly higher mortality than the other two groups (log rank test, P = 0.031). Univariate Cox regression found a more than twice higher risk of death in the group with the 25(OH)D level less than 20 ng/mL compared with the reference group (HR = 2.47; 95%CI: 1.18-5.18; P = 0.017), whereas the group with 25(OH)D at or above 30 ng/mL was not significantly different from that with the 25(OH)D between 20 to 29 ng/mL (HR = 0.78; 95%CI: 0.26-2.32; P = 0.650). Multivariate analysis showed the predictive value of 25(OH)D levels as a continuous variable for preventing death when adjusted for multiple covariates in different models (Table 3). Adjusted for age, comorbidity, diabetes mellitus, eGFR and phosphorous and albumin levels, the HR for all-cause mortality for 25(OH)D < 20 vs 20-29 was 2.33 (95%CI: 1.10-4.91; P = 0.027; Figure 3). The 25(OH)D ≥ 30 group did not have a significantly different mortality hazard from the reference group (HR = 1.19; 95%CI: 0.37-3.81; P = 0.775).
| Model | Covariates controlled for | Adjusted HR (95%CI) | P |
| 0 (Unadjusted) | 25-hydroxivitamin D levels (mg/dL) | 0.95 (0.91-0.99) | 0.009 |
| 1 | 25-hydroxivitamin D levels (mg/dL) + age | 0.95 (0.91-0.99) | 0.009 |
| 2 | Model 1 + diabetes mellitus, ischemic heart disease, chronic heart failure | 0.96 (0.92-0.99) | 0.028 |
| 3 | Model 1 + peripheral arterial disease, abnormal ABPI1, phosphorous (mg/dL) | 0.95 (0.92-0.99) | 0.023 |
| 4 | Model 1 + DBP (mm Hg), 1,25(OH)2 vitamin D (pg/mL), estimated GFR (mL/min per 1.73 m2) | 0.96 (0.92-0.99) | 0.020 |
| 5 | Model 1 + vascular calcification [Kauppila score (log), Adragao score (log)], CKD stage 5 | 0.95 (0.91-1.00) | 0.050 |
| 6 | Model 1 + obesity, hemoglobin (g/L), albumin (g/dL) | 0.95 (0.92-0.99) | 0.019 |
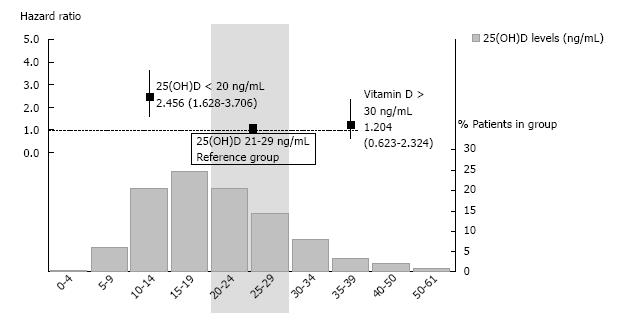
During the follow-up, 81 (17%) patients started renal replacement therapy and 156 (33%) patients showed the composite renal end-point. Kaplan-Meier analysis (Figure 2B) showed that the 25(OH)D < 20 group had significantly more risk than the other two groups (log rank test, P < 0.001). Univariate Cox regression found again higher risk of the renal end-point with 25(OH)D level less than 20 ng/mL compared with 20 to 29 ng/mL (HR = 2.78; 95%CI: 1.84-4.16; P < 0.001), whereas the group with 25(OH)D above 30 ng/mL did not show different risk from reference group (HR = 1.13; 95%CI: 0.59-2.13; P = 0.717). Multivariate analysis showed the predictive value of VD levels as a continuous variable for preventing appearance of renal end point when adjusted for multiple covariates (Table 4). Adjusted for age, gender, diabetes mellitus, eGFR, and phosphorous levels, the HR for the composite renal end-point for the 25(OH)D < 20 group compared to the reference group was 2.46 (95%CI: 1.63-3.71; P < 0.001; Figure 4). The 25(OH)D ≥ 30 group did not have a significantly different hazard for kidney progression from the reference group (HR = 1.20; 95%CI: 0.62-2.32; P = 0.581).
| HR (95%CI) | P value | |
| 25-hydroxivitamin D (ng/mL) | 0.97 (0.95-0.99) | 0.004 |
| Age (yr) | 0.99 (0.97-1.00) | 0.044 |
| Male sex | 2.20 (1.47-3.30) | < 0.001 |
| Estimated GFR (mL/min per 1.73 m2) | 0.93 (0.91-0.95) | < 0.001 |
| ABPI (mmHg) | 0.23 (0.10-0.53) | 0.001 |
| Hemoglobin (g/L) | 0.84 (0.78-0.94) | 0.001 |
During the follow-up, 126 (27%) patients were admitted for hospitalization, cardiovascular (49%) and infections (20%) being the most common causes. Kaplan-Meier analysis (Figure 2C) indicated that crude hospitalization event-free period was different between the VD groups (log rank test, P = 0.039). Univariate Cox regression found a shorter hospitalization event-free period in patients with 25(OH)D level less than 20 ng/mL compared with 20-29 ng/mL (HR = 1.58; 95%CI: 1.05-2.36; P = 0.027), with no difference between the 25(OH)D ≥ 30 and the reference groups (P = 0.861). Hospitalization outcomes were predicted by 25(OH) levels (HR = 0.98; 95%CI: 0.96-1.00; P = 0.027) in the unadjusted Cox proportional hazards model, but not after adjusting for age, eGFR, diabetes and comorbidity.
ROC curves identified VD levels at highest risk for death, the composite renal endpoint, and hospitalization, at 17.4 ng/mL [area under the curve (AUC) = 0.60; 95%CI: 0.52-0.69; P = 0.027], 18.6 (AUC = 0.65; 95%CI: 0.60-0.71; P < 0.001), and 19.0 (AUC = 0.56; 95%CI: 0.50-0.62; P = 0.048), respectively.
One of the main limitations for the development of evidence-based clinical recommendations for VD supplementation lies in the discrepancies in the criteria for defining VD deficiency and insufficiency, which can explain conflicting results from meta-analysis addressing vitamin D levels and outcomes[24,34]. These criteria vary among authors and societies, including 25(OH)D levels below which osteomalacia [10 ng/mL (25 nmol/L)] or secondary hyperparathyroidism [20-30 ng/mL (50 to 75 nmol/L)] may appear[18-20,35,36]. Being aware of their potential clinical significance, the present study examines the prognosis value of 25(OH)D levels in a cohort of 3-5 stage CKD subjects not on dialysis, trying to identify cut-off points for serum 25(OH)D levels to define VD sufficiency. These cut-offs were not based on biological abnormalities as classically noted[25,26], but on VD levels at highest risk for death, CKD progression and all-cause hospitalization.
Although randomized clinical trials are the best way for generating a high evidence for treatment decisions, trials are rare and suboptimal in nephrology[37]. Therefore, observational studies have an important role, particularly when the intervention, in this case vitamin D supplementation, is inexpensive and potentially effective. Although there are previous prospective observational studies which examined the prognosis value of 25(OH)D levels in CKD subjects not on dialysis[8,12], this is the first one, to our knowledge, in which 25OH(D) levels unequivocally reflect exposure to VD, given that patients on treatment with active VD were excluded, as well as including the biggest cohort of non-dialysis CKD subjects with data regarding emerging cardiovascular risk factors as vascular calcification scores and ABPI. In the main analysis of the OSERCE-2 study, low VD levels were associated to worse survival and CKD progression only in the univariate analysis[32]. However, 26% of patients of the study received activated VD, which may confer a protective effect and therefore may decrease any negative effect of VD levels observed, as it has been stated on dialysis population[38]. In this context, we conducted this post-hoc analysis of the OSERCE-2 dataset in patients without active VD treatment. In this selected cohort, the main findings were the independent predictor value of VD deficiency, but not insufficiency, for the 3-year incidence of death and CKD progression, which remained significant after multivariate adjustments, as previously published[8,12]. In a prospective study involving 94 CKD patients, those with 25(OH)D levels less than 16.7 ng/mL had a higher mortality rate[12]. 25(OH)D was confirmed as an independent inverse predictor of death in a 6-year follow-up study which included 168 CKD subjects[8]. In that study patients with ≥ 15 ng/mL of 25(OH)D showed a reduction in mortality by 33% to 60% in the different models, compared to patients with 25(OH)D < 15 ng/mL. Less CKD progression to end-stage renal disease was also reported in the groups of patients with better VD status. All these data are in agreement with our results, which show how low 25(OH)D levels predicted mortality and CKD progression independently of such traditional and non-traditional risk factors, as vascular calcification or inflammation. In this context, it is noteworthy that the lack of association between 25(OH)D levels and vascular calcification observed in our study, is in agreement with some[12], but not all[9,10], previously published data. These findings indicate that 25(OH)D may impact on CKD outcomes by additional mechanisms including the suppression of the renin-angiotensin system, albuminuria reduction or amelioration of left ventricular hypertrophy[6,9,16,31,39]. Of note, we have detected ABPI as an independent predictor of VD deficiency, which could contribute to vascular stiffness and high cardiovascular risk for this population.
More interestingly, our study, as the first prospective which analyzed the upper level associated to better improvement in survival and CKD progression on CKD patients, did not demonstrate additional benefits on these hard outcomes when patients reached the optimal target levels for VD suggested by current guidelines (≥ 30 ng/mL). It is noteworthy that all three cut-off points for serum 25(OH)D levels at highest risk for death, CKD progression and all-cause hospitalization were between 17 ng/mL and 19, which reinforces the threshold value for abnormally reduced 25(OH)D in 20 ng/mL. These findings confirm the data reported in the biggest retrospective observational study analyzing VD and mortality in CKD patients. Navaneethan et al[40] studied 12763 patients with 3-4 stage CKD, showing 25(OH)D level ≤ 15 ng/mL to be associated independently with a 33% increased risk of all-cause mortality, whereas the group with 25(OH)D levels of 15-29 ng/mL did not show a significantly increased risk of mortality compared with patients with 25(OH)D levels ≥ 30 ng/mL.
Taking all these data together, we agree with the Institute of Medicine recommendation to consider sufficient 25(OH)D levels of at least 20 ng/mL, given that serum 25(OH)D concentrations above 30 ng/mL are not consistently associated with increased benefit[27,40]. In addition, most clinical trials have only confirmed the neutral effect of VD supplementation on hard outcomes[41], whereas some controlled studies have shown positive results in spite of the mean VD concentration not reaching the optimal recommended levels of ≥ 30 ng/mL[16]. Moreover, VD might not be safe in all settings, and supplementing could cause harm in people with CKD, who have a high prevalence of vascular calcification, and a decreasing ability for renal excretion of calcium and phosphorous[32,42]. Excessive VD supplementation may be particularly harmful in those high risk individuals with serum 25(OH)D levels above 20 ng/mL which are classified as insufficient according to current guidelines, and who then are treated with high-dose supplements of VD containing many times the levels of intake recommended for adults (600-800 UI/d)[18,27,43]. Although some experts suggest that it is safe to carry higher vitamin D levels (40-70 ng/mL), this recommendation is based on acute and not long-term observations[44].
Lastly, our study confirmed the high prevalence of low VD status on CKD patients[1-5]. There are many factors which could contribute to the deficiency that are not related to GFR, including limited exposure to the sun, reduced dietary intake and urinary loss of 25(OH)D and VD-binding protein in proteinuric nephropathies[24,44,45]. The present study, as others[8,12,38], has shown significant correlation between 25(OH)D levels and body mass index and albumin, which emphasizes the relationship between nutritional status, VD levels and survival in chronic illness as CKD. Of note, the independent relationship observed, even after adjustment for chronic heart failure, between VD deficiency and diuretic use. VD deficiency is highly prevalent in heart failure patients, being a significant predictor of reduced survival. In addition, loop diuretics treatment may worsen osteoporosis on general population, but no data are available in CKD patients[46,47].
The strong points of the study include the relatively high number of patients included and the 3-year follow-up, which strengthens the study’s power. To minimize the inter-method and seasonal variability in VD and PTH measurements, blood samples were analyzed by a central laboratory, and patients’ recruitment was done in a short period of time (April-May)[32]. In contrast, there are several limitations to be commented. As a longitudinal study, it is still insufficient to determine whether the association between low 25(OH)D levels and worse CKD outcomes is causal and reversible, which should be tested in future randomized clinical trials. The results may not be valid to non-Caucasian populations living at other latitudes, or to patients on active VD treatment. The multivariate analysis of cardiovascular deaths was limited due to its low incidence. Lastly, it would be interesting to study other relevant bone-related clinical outcomes, such as bone-density changes or fracture risk.
In conclusion, in accordance with previously published data, the present study confirms: (1) a high prevalence of 25(OH)D deficiency and insufficiency in non-dialysis CKD patients; and (2) an independent association between serum 25(OH)D levels and worse clinical outcomes, such as death and CKD progression. The results of this study add to the knowledge of optimal VD status in non-dialysis CKD patients, identifying the threshold value for abnormally reduced 25(OH)D in 20 ng/mL, which is in agreement with the Institute of Medicine recommendations. Whereas high doses of VD supplementation on this population can lead to a calcium and phosphate overload, promoting vascular calcification and CKD progression, our results suggest that, with the limitations inherent to the observational studies, 25(OH)D levels between 20 to 30 ng/mL could be sufficient for CKD patients. Randomized clinical trials are warranted to know the most favorable 25(OH)D level for CKD patients.
The authors gratefully acknowledge their co-investigators in the OSERCE-2 Study. The authors thank to Jacqueline Clarke, B.Ed (Hons) for her collaboration in translating this text. For the figure editing, the authors thank to David Almenar, PhD. Portions of this work were presented and published in thesis form in fulfillment of the requirements for the PhD for Student Pablo Molina from University Autonoma of Barcelona.
Although knowledge of the skeletal and non-skeletal effects of nutritional vitamin D (VD) has expanded, no consensus currently exists within the medical community regarding the criteria for defining thresholds for VD supplementation in chronic kidney disease (CKD) patients.
Based on levels of 25(OH)D required to suppress parathyroid hormone (PTH), clinical guidelines most commonly recommend a serum concentration of 25(OH)D above 30-40 ng/mL (75-100 nmol/L), levels at which PTH is suppressed to a minimum in its relation to 25(OH)D. However, there is a lack of evidence regarding this target recommendation, and overuse of VD supplementation on this population can lead to a calcium and phosphate overload, promoting vascular calcification and CKD progression.
Being aware of both the therapeutic and iatrogenic power of VD supplementation, the present study examines the prognosis value of 25(OH)D levels in a cohort of 3-5 stage CKD subjects not on dialysis, trying to identify cut-off points for serum 25(OH)D levels to define VD sufficiency. These cut-offs were not based on biochemical abnormalities as classically noted, but on VD levels at highest risk for death, CKD progression and all-cause hospitalization. The results of this study add to the knowledge of optimal VD status in non-dialysis CKD patients, identifying the threshold value for abnormally reduced 25(OH)D in 20 ng/mL.
The data in this study suggested that the optimal VD level might be lower than is currently recommended, advocating that 25(OH)D levels at or above 20 ng/mL could be sufficient for CKD patients. The authors recommend caution when nutritional VD is prescribed.
25(OH)D, also known as calcifediol, is a prehormone that is produced in the liver by hydroxylation of vitamin D3 (cholecalciferol). Serum 25(OH)D levels are considered the best indicator of VD status.
The paper with the title: “What is the optimal level of vitamin D in non-dialysis CKD population?” is an interesting well written article and the authors claim that their study as the first prospective which analyzed the upper level of VD associated to better improvement in survival and CKD progression on CKD patients, did not demonstrate additional benefits on these hard outcomes when patients reached the optimal target levels for VD suggested by current guidelines (≥ 30 ng/mL).So with this study, despite the limitations, the authors provide a new option in this so controversial field of VD treatment in CKD patients.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Eirini G, Pontremoli R S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Górriz JL, Molina P, Bover J, Barril G, Martín-de Francisco AL, Caravaca F, Hervás J, Piñera C, Escudero V, Molinero LM. Characteristics of bone mineral metabolism in patients with stage 3-5 chronic kidney disease not on dialysis: results of the OSERCE study. Nefrologia. 2013;33:46-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 2. | Mehrotra R, Kermah D, Budoff M, Salusky IB, Mao SS, Gao YL, Takasu J, Adler S, Norris K. Hypovitaminosis D in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1144-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1008] [Cited by in RCA: 1011] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 4. | LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, Graves KL, Moe SM. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 561] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 6. | de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis. 2007;50:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Holden RM, Morton AR, Garland JS, Pavlov A, Day AG, Booth SL. Vitamins K and D status in stages 3-5 chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, Mallamaci F, Zoccali C. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 315] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 9. | Matias PJ, Ferreira C, Jorge C, Borges M, Aires I, Amaral T, Gil C, Cortez J, Ferreira A. 25-Hydroxyvitamin D3, arterial calcifications and cardiovascular risk markers in haemodialysis patients. Nephrol Dial Transplant. 2009;24:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | García-Canton C, Bosch E, Ramírez A, Gonzalez Y, Auyanet I, Guerra R, Perez MA, Fernández E, Toledo A, Lago M. Vascular calcification and 25-hydroxyvitamin D levels in non-dialysis patients with chronic kidney disease stages 4 and 5. Nephrol Dial Transplant. 2011;26:2250-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Mehrotra R, Kermah DA, Salusky IB, Wolf MS, Thadhani RI, Chiu YW, Martins D, Adler SG, Norris KC. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 2009;76:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Barreto DV, Barreto FC, Liabeuf S, Temmar M, Boitte F, Choukroun G, Fournier A, Massy ZA. Vitamin D affects survival independently of vascular calcification in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Pilz S, Tomaschitz A, Friedl C, Amrein K, Drechsler C, Ritz E, Boehm BO, Grammer TB, März W. Vitamin D status and mortality in chronic kidney disease. Nephrol Dial Transplant. 2011;26:3603-3609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1285] [Cited by in RCA: 1220] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 15. | de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376:1543-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 494] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 16. | Molina P, Górriz JL, Molina MD, Peris A, Beltrán S, Kanter J, Escudero V, Romero R, Pallardó LM. The effect of cholecalciferol for lowering albuminuria in chronic kidney disease: a prospective controlled study. Nephrol Dial Transplant. 2014;29:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Cozzolino M. Vitamin D: something new under the sun. Clin Kidney J. 2012;5:285-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6974] [Cited by in RCA: 6833] [Article Influence: 488.1] [Reference Citation Analysis (0)] |
| 19. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;S1-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 1075] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 20. | Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2655] [Cited by in RCA: 2839] [Article Influence: 202.8] [Reference Citation Analysis (0)] |
| 21. | Torregrosa JV, Cannata Andia J, Bover J, Caravaca F, Lorenzo V, Martín de Francisco AL, Martín-Malo A, Martínez I, González Parra E, Fernández Giráldez E. [SEN Guidelines. Recommendations of the Spanish Society of Nephrology for managing bone-mineral metabolic alterations in chronic renal disease patients]. Nefrologia. 2008;28 Suppl 1:1-22. [PubMed] |
| 22. | Mariani LH, White MT, Shults J, Anderson CA, Feldman HI, Wolf M, Reese PP, Denburg MR, Townsend RR, Lo JC. Increasing use of vitamin D supplementation in the chronic renal insufficiency cohort study. J Ren Nutr. 2014;24:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Scientific Committee for Food. Nutrient and energy intakes for the European Community. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Vitamin D. Available from: http://www.ec.europa.eu/food/fs/sc/scf/out157_en.pdf. |
| 24. | Inda Filho AJ, Melamed ML. Vitamin D and kidney disease: what we know and what we do not know. J Bras Nefrol. 2013;35:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Vieth R, Ladak Y, Walfish PG. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab. 2003;88:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 290] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 26. | Dusso AS, Tokumoto M. Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: a downward spiral in kidney disease. Kidney Int. 2011;79:715-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium: Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US) 2011; . [PubMed] [DOI] [Full Text] |
| 28. | Allen SH, Shah JH. Calcinosis and metastatic calcification due to vitamin D intoxication. A case report and review. Horm Res. 1992;37:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Moncrieff MW, Chance GW. Nephrotoxic effect of vitamin D therapy in vitamin D refractory rickets. Arch Dis Child. 1969;44:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Cardús A, Panizo S, Parisi E, Fernandez E, Valdivielso JM. Differential effects of vitamin D analogs on vascular calcification. J Bone Miner Res. 2007;22:860-866. [PubMed] |
| 31. | Melamed ML, Thadhani R. Low calcidiol levels and coronary artery calcification: true, true, and related? J Am Soc Nephrol. 2009;20:1663-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Górriz JL, Molina P, Cerverón MJ, Vila R, Bover J, Nieto J, Barril G, Martínez-Castelao A, Fernández E, Escudero V. Vascular calcification in patients with nondialysis CKD over 3 years. Clin J Am Soc Nephrol. 2015;10:654-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 33. | Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247-254. [PubMed] |
| 34. | Halfon M, Phan O, Teta D. Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. Biomed Res Int. 2015;2015:953241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 35. | McKenna MJ, Freaney R. Secondary hyperparathyroidism in the elderly: means to defining hypovitaminosis D. Osteoporos Int. 1998;8 Suppl 2:S3-S6. [PubMed] |
| 36. | Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18-28. [PubMed] |
| 37. | Palmer SC, Sciancalepore M, Strippoli GF. Trial quality in nephrology: how are we measuring up? Am J Kidney Dis. 2011;58:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Tonelli M. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004-1013. [PubMed] |
| 39. | London GM, Guérin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Mëtivier F. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613-620. [PubMed] |
| 40. | Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Jain A, Schreiber MJ, Simon JF, Srinivas TR, Nally JV. Low 25-hydroxyvitamin D levels and mortality in non-dialysis-dependent CKD. Am J Kidney Dis. 2011;58:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 775] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 42. | Craver L, Marco MP, Martínez I, Rue M, Borràs M, Martín ML, Sarró F, Valdivielso JM, Fernández E. Mineral metabolism parameters throughout chronic kidney disease stages 1-5--achievement of K/DOQI target ranges. Nephrol Dial Transplant. 2007;22:1171-1176. [PubMed] |
| 43. | Figuiredo-Dias V, Cuppari L, Garcia-Lopes MG, de Carvalho AB, Draibe SA, Kamimura MA. Risk factors for hypovitaminosis D in nondialyzed chronic kidney disease patients. J Ren Nutr. 2012;22:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Petchey WG, Johnson DW, Hawley CM, Isbel NM. Predictors of vitamin D status in predialysis chronic kidney disease patients: a cross-sectional analysis in a high ultraviolet climate. J Ren Nutr. 2012;22:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Gotsman I, Shauer A, Zwas DR, Hellman Y, Keren A, Lotan C, Admon D. Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin D supplementation improves outcome. Eur J Heart Fail. 2012;14:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 46. | Aluoch AO, Jessee R, Habal H, Garcia-Rosell M, Shah R, Reed G, Carbone L. Heart failure as a risk factor for osteoporosis and fractures. Curr Osteoporos Rep. 2012;10:258-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |