Published online Mar 6, 2016. doi: 10.5527/wjn.v5.i2.204
Peer-review started: August 31, 2015
First decision: September 28, 2015
Revised: November 5, 2015
Accepted: December 18, 2015
Article in press: December 20, 2015
Published online: March 6, 2016
Processing time: 186 Days and 18.8 Hours
AIM: To investigate the efficacy of effluent biomarkers for peritoneal deterioration with functional decline in peritoneal dialysis (PD).
METHODS: From January 2005 to March 2013, the subjects included 218 PD patients with end-stage renal disease at 18 centers. Matrix metalloproteinase-2 (MMP-2), interleukin-6 (IL-6), hyaluronan, and cancer antigen 125 (CA125) in peritoneal effluent were quantified with enzyme-linked immunosorbent assay. Peritoneal solute transport rate was assessed by peritoneal equilibration test (PET) to estimate peritoneal deterioration.
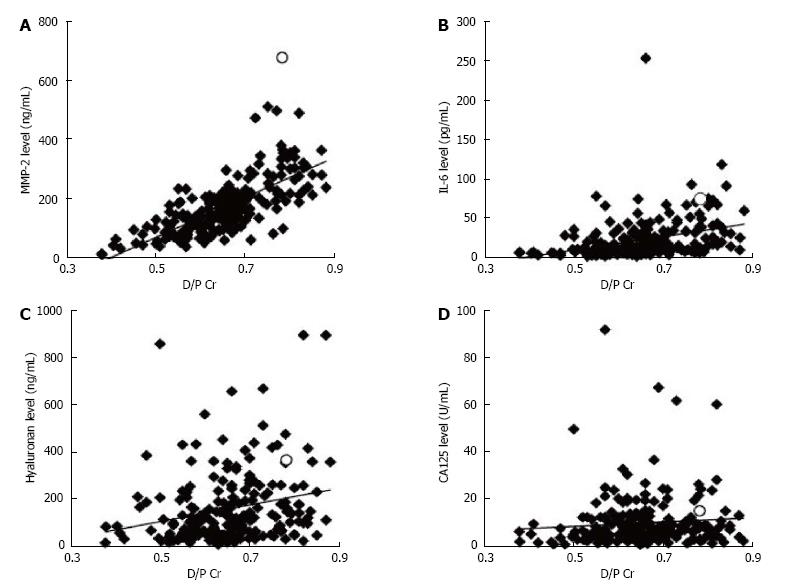
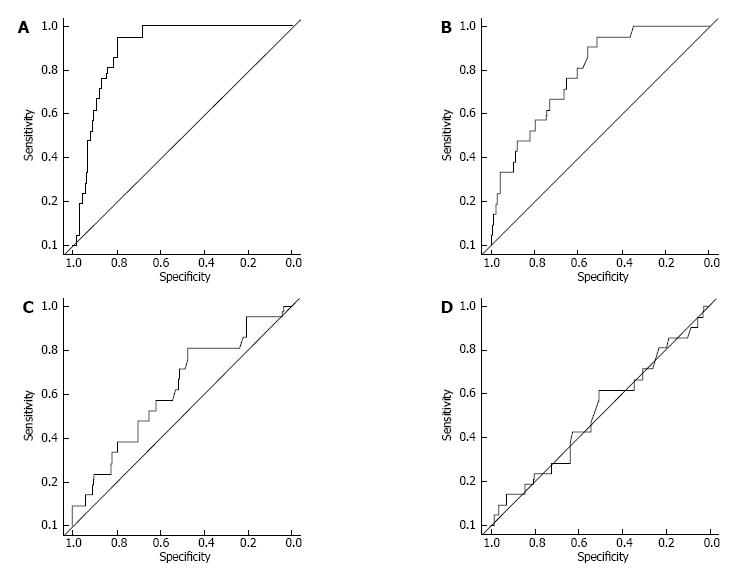
RESULTS: The ratio of the effluent level of creatinine (Cr) obtained 4 h after injection (D) to that of plasma was correlated with the effluent levels of MMP-2 (ρ = 0.74, P < 0.001), IL-6 (ρ = 0.46, P < 0.001), and hyaluronan (ρ = 0.27, P < 0.001), but not CA125 (ρ = 0.13, P = 0.051). The area under receiver operating characteristic curve for the effluent levels of MMP-2, IL-6, and hyaluronan against high PET category were 0.90, 0.78, 0.62, and 0.51, respectively. No patient developed new-onset encapsulating peritoneal sclerosis for at least 1.5 years after peritoneal effluent sampling.
CONCLUSION: The effluent MMP-2 level most closely reflected peritoneal solute transport rate. MMP-2 can be a reliable indicator of peritoneal deterioration with functional decline.
Core tip: Peritoneal effluent samples were obtained from 218 peritoneal dialysis (PD) patients with end-stage renal disease at 18 centers. The effluent levels of biomarkers matrix metalloproteinase-2 (MMP-2), interleukin-6, hyaluronan, and cancer antigen 125 were measured. Peritoneal solute transport rate was assessed by peritoneal equilibration test (PET) to estimate peritoneal deterioration. Among the biomarkers in the effluent, MMP-2 level correlated most significantly with peritoneal solute transport rate. The area under the receiver operating characteristic curve analysis for effluent MMP-2 level against high PET category was 0.90. MMP-2 may be a superior biomarker for peritoneal deterioration during PD.
- Citation: Hirahara I, Kusano E, Morishita Y, Inoue M, Akimoto T, Saito O, Muto S, Nagata D. Matrix metalloproteinase-2 as a superior biomarker for peritoneal deterioration in peritoneal dialysis. World J Nephrol 2016; 5(2): 204-212
- URL: https://www.wjgnet.com/2220-6124/full/v5/i2/204.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i2.204
Long-term peritoneal dialysis (PD) causes peritoneal deterioration with structural changes and functional decline such as ultrafiltration loss and increased solute transport rate, resulting in the cessation of PD treatment; thus, these are the major problems related to PD. At worst, peritoneal deterioration may even cause encapsulating peritoneal sclerosis (EPS), a serious but rare complication of PD with extremely high mortality rate[1-4]. To undergo PD safely and adequately, preventing the progression of peritoneal deterioration is important.
The mechanism of peritoneal injury is not well-known, but it may develop through multiple factors such as infectious peritonitis and continuous exposure to non-physiological PD fluid with low pH, high osmolarity, and high glucose and glucose degradation product levels[2-5].
Solute transport rate through the peritoneal membrane is often measured by peritoneal equilibration test (PET) to estimate PD efficiency and peritoneal deterioration[3,4,6,7]. Transport rate increases with peritoneal deterioration and a state of higher transporter membrane is a factor contributing to the development of EPS in patients on PD. However, the D/P creatinine (Cr) value from a single test of the PET is not sufficiently predictive of EPS, and monitoring the time-course changes are necessary[4]. In addition, PET is invasive because it requires blood sampling and patients need to spend half a day in hospital or clinic because the test takes a long time. Therefore, the PET is not performed routinely in many medical centers. It is thus necessary to evaluate peritoneal deterioration using an easy and non-invasive method.
Some biomarkers such as matrix metalloproteinase-2 (MMP-2), interleukin-6 (IL-6), hyaluronan, and cancer antigen 125 (CA125) in the peritoneal effluent are often measured non-invasively to estimate peritoneal deterioration[2,4,8-20].
MMP-2 degrades extracellular matrix components such as fibronectin and type IV collagen, which comprise the basement membrane. MMP-2 is produced by mesenchymal cells, macrophages, and endothelial cells in the peritoneum and plays important roles in angiogenesis, epithelial-to-mesenchymal transition (EMT) of mesothelial cells, inversion of transdifferentiated mesothelial cells, and migration of cells that promote inflammation or fibrosis[2,5,8,10,21-23]. Effluent MMP-2 level correlates with peritoneal solute transport rate and has a possibility to predict EPS[8-10]. On the other hand, Lopes Barreto et al[20] recently reported that the time course of MMP-2 appearance rates, studied by mean values from a model of repeated linear measures 4 years prior to EPS diagnosis, showed no difference between long-term controls and patients with EPS. IL-6 is a cytokine involved in acute phase inflammation and its effluent level is associated with high peritoneal solute transport rate[2,4,13,14]. Goodlad et al[19] reported that effluent levels of IL-6, monocyte chemotactic protein-1, and CCL15 (eukotactin) did not improve prediction of future EPS compared with a model that used known clinical risk factors although these cytokines were found at higher levels in the effluent of patients who subsequently developed EPS. Hyaluronan, a large glycosaminoglycan that is constitutively synthesized by mesothelial cells, plays important roles in the maintenance of mesothelial cell morphology, re-mesothelialization, and wound repair[15]. Intraperitoneal hyaluronan production increases with membrane permeability and length of time on PD[16]. Effluent hyaluronan level may be a useful biomarker to assess functional and morphological changes of peritoneum[4,16]. CA125 is a high molecular weight glycoprotein that is secreted from mesothelial cells; its concentration in effluent and appearance rate can indicate a change of the peritoneal mesothelial cell mass[4,16-18]. According to the guidelines for peritoneal dialysis of Japanese Society for Dialysis Therapy[4], it is very hard to evaluate peritoneal deterioration by a single biomarker, and, at present, no examination can be an absolutely reliable diagnostic method alone. For this reason, comprehensive judgment based on the results of multiple examinations is needed. Presently, the establishment of a simple and highly reproducible method with high sensitivity and specificity is extremely important.
The aim of this study was to compare many biomarkers and understand their individual properties and to confirm the efficacy of effluent biomarkers for peritoneal deterioration in peritoneal membranes with high solute transport rate.
The trial was conducted as a prospective, observational study at 18 centers in Japan. All patients were followed for at least 1.5 years after the measurement of effluent levels of the biomarkers.
PD patients with end-stage renal disease were analyzed based on peritoneal effluent biomarkers during the period of January 2005 through March 2013. Patients with bacterial peritonitis at the time of the analysis or in the preceding 4 wk were excluded from this analysis. After analysis of biomarkers, all patients were followed-up for more than 1.5 consecutive years to confirm for development of EPS.
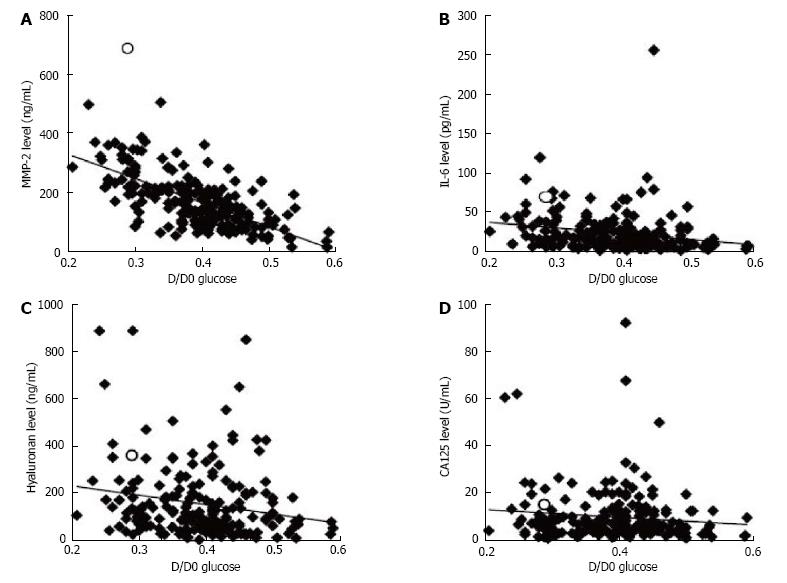
The peritoneal solute transport rate was assessed by PET[3,4,6]. Intraabdominal fluid was drained and 2 L of PD fluid containing 2.27%-2.5% glucose was injected intraperitoneally. The Cr level of peritoneal effluents obtained 4 h after the injection (D) was divided by that of plasma (P) to obtain the D/P Cr ratio. The glucose level of peritoneal effluents obtained 4 h after injection (D) was divided by that obtained immediately after injection (D0) to obtain the D/D0 glucose ratio. The effluent levels of MMP-2, IL-6, hyaluronan, and CA125 obtained at PET were measured by enzyme-linked immunosorbent assay (MMP-2: GE Healthcare, NJ, United States; IL-6: RD System, Inc., Minneapolis, MN, United States; hyaluronan: Seikagaku Biobusiness, Tokyo, Japan; CA125: Immunospec Co., CA, United States).
Statistical analyses were performed using R statistical software version 2.15.1 (R Foundation for Statistical Computing). Receiver operating characteristic (ROC) curve analyses were performed to evaluate the diagnostic accuracy of MMP-2, IL-6, hyaluronan, and CA125. Comparisons between two groups were performed by Wilcoxon’s test. Relationships between clinical variables and effluent biomarker levels were analyzed by Spearman’s correlation test. A P value of < 0.05 was considered to be significant.
This study was registered as the MAJOR IN PD study (Multicenter Analysis in Japan, ORiginal INdicator of Peritoneal Deterioration) in the University Hospital Medical Information Network-Clinical Trials Registry (UMIN-CTR), which was approved by the International Committee of Medical Journal Editors (No. UMIN000010572).
A total of 218 PD patients, including one patient with EPS, were analyzed and their characteristics are summarized in Table 1. Biomarker levels in the peritoneal effluent of all patients are shown in Table 2. The relationship between the effluent levels of the biomarkers and the characteristics of the patients is shown in Table 3. Effluent CA125 level was affected by sex and etiology of end-stage renal disease. Effluent IL-6 and hyaluronan levels correlated significantly with PD duration (P < 0.05, P < 0.01, respectively). Effluent MMP-2 and IL-6 levels significantly reflected the number of peritonitis episodes. The peritoneal solute transport rate determined by PET was correlated with MMP-2, IL-6, and hyaluronan levels in the effluent and the correlation coefficient between D/P Cr and MMP-2 level was significantly the highest (Figures 1 and 2, Table 4). The effluent level of each biomarker was correlated with that of the remaining biomarker (Table 4). In the patient with EPS, MMP-2, IL-6, hyaluronan, and CA125 levels were 681 ng/mL, 74.7 pg/mL, 362 ng/mL, and 15.1 U/mL, respectively; his effluent MMP-2 level was the highest among all PD patients. The highest MMP-2 level was 514 ng/mL among patients without EPS onset. In the patient with the highest IL-6 level (253.5 pg/mL), there was accumulation of ascitic fluid with C-reactive protein level of 1.3 mg/dL; his effluent MMP-2, hyaluronan, and CA125 levels were 236 ng/mL, 651 ng/mL, and 4.0 U/mL, respectively.
| Characteristics of patients | |
| Sex (male/female) | 122/96 (56% male) |
| Etiology (non-DM/DM) | 179/36 (17% diabetes) |
| Age (yr) | 56 (20, 46, 64, 84) |
| PD duration (mo) | 40 (1, 15, 63, 206) |
| Peritonitis episode (times) | 0 (0, 0, 1, 9) |
| D/P Cr | 0.65 (0.38, 0.59, 0.71, 0.88) |
| D/D 0 glucose | 0.40 (0.21, 0.34, 0.44, 0.59) |
| Biomarker | Level in the peritoneal effluent |
| Matrix metalloproteinase-2 (ng/mL) | 159 (14, 107, 220, 681) |
| Interleukin-6 (pg/mL) | 14.6 (1.5, 7.6, 29.6, 253.5) |
| Hyaluronan (ng/mL) | 109 (5.1, 53, 204, 889) |
| Cancer antigen 125 (U/mL) | 6.7 (0.7, 4.0, 12.1, 91.8) |
| Biomarker levels in the peritoneal effluents | ||||
| MMP-2 | IL-6 | Hyaluronan | CA125 | |
| Sex (male/female) | P = 0.92 | P = 0.54 | P = 0.48 | P < 0.05 |
| Etiology (non-DM/DM) | P = 0.18 | P = 1.00 | P = 0.56 | P < 0.05 |
| Age (yr) | ρ = 0.076 | ρ = 0.12 | ρ = 0.046 | ρ = -0.096 |
| P = 0.27 | P = 0.092 | P = 0.50 | P = 0.16 | |
| PD duration (mo) | ρ = 0.050 | ρ = 0.15 | ρ = 0.25 | ρ = -0.062 |
| P = 0.47 | P < 0.05 | P < 0.01 | P = 0.93 | |
| Peritonitis episode (times) | ρ = 0.17 | ρ = 0.25 | ρ = 0.092 | ρ = -0.012 |
| P < 0.05 | P < 0.001 | P = 0.20 | P = 0.86 | |
| D/P Cr | D/D0 glucose | MMP-2 | IL-6 | Hyaluronan | CA125 | |
| D/P Cr | ρ = 1 | ρ = -0.86 | ρ = 0.74 | ρ = 0.46 | ρ = 0.27 | ρ = 0.13 |
| P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P = 0.051 | ||
| D/D0 Glucose | ρ = 1 | ρ = -0.65 | ρ = -0.28 | ρ = -0.22 | ρ = -0.094 | |
| P < 0.001 | P < 0.001 | P < 0.005 | P = 0.19 | |||
| MMP-2 | ρ = 1 | ρ = 0.54 | ρ = 0.40 | ρ = 0.1 | ||
| P < 0.001 | P < 0.001 | P < 0.05 | ||||
| IL-6 | ρ = 1 | ρ = 0.45 | ρ = 0.26 | |||
| P < 0.001 | P < 0.001 | |||||
| Hyaluronan | ρ = 1 | ρ = 0.18 | ||||
| P < 0.01 | ||||||
| CA125 | ρ = 1 |
In the present study, the proportion of PD patients with high PET category was 9.6%. ROC curves were constructed to assess the ability of the biomarkers in distinguishing high category of PET (Figure 3). MMP-2 and IL-6 levels in the peritoneal effluents from high PET category patients were significantly higher than these levels from non-high PET category patients (MMP-2: P < 0.001, IL-6: P < 0.001, hyaluronan: P = 0.068, CA125: P = 0.876). The results from the ROC curve analysis and cut-off points for the high PET category are also shown in Table 5.
| Biomarkers | Cut-off level | Sensitivity (95%CI) | Specificity (95%CI) | AUC |
| MMP-2- | 214 ng/mL | 0.95 (0.86 to 1.00) | 0.79 (0.74 to 0.85) | 0.90 |
| 219 ng/mL | 0.86 (0.71 to 1.00) | 0.81 (0.76 to 0.86) | ||
| 228 ng/mL | 0.81 (0.62 to 0.95) | 0.84 (0.79 to 0.89) | ||
| 238 ng/mL | 0.76 (0.57 to 0.95) | 0.87 (0.82 to 0.91) | ||
| IL-6 | 16.8 pg/mL | 0.81 (0.62 to 0.95) | 0.60 (0.53 to 0.67) | 0.78 |
| 19.3 pg/mL | 0.76 (0.57 to 0.90) | 0.65 (0.58 to 0.73) | ||
| 20.0 pg/mL | 0.71 (0.52 to 0.90) | 0.66 (0.60 to 0.73) | ||
| 24.8 pg/mL | 0.67 (0.48 to 0.86) | 0.75 (0.66 to 0.79) | ||
| Hyaluronan | 94.5 ng/mL | 0.81 (0.62 to 0.95) | 0.47 (0.41 to 0.54) | 0.62 |
| 101.5 ng/mL | 0.71 (0.52 to 0.90) | 0.51 (0.44 to 0.58) | ||
| 108.0 ng/mL | 0.67 (0.48 to 0.86) | 0.52 (0.45 to 0.58) | ||
| 115.0 ng/mL | 0.62 (0.43 to 0.81) | 0.53 (0.46 to 0.53) | ||
| CA125 | 3.85 U/mL | 0.81 (0.62 to 0.95) | 0.23 (0.17 to 0.29) | 0.51 |
| 4.45 U/mL | 0.71 (0.52 to 0.90) | 0.30 (0.23 to 0.37) | ||
| 6.55 U/mL | 0.62 (0.43 to 0.81) | 0.50 (0.43 to 0.57) | ||
| 7.05 U/mL | 0.52 (0.33 to 0.71) | 0.53 (0.46 to 0.59) |
No patients developed new-onset EPS for at least 1.5 years after measurement of the effluent biomarkers.
For a safe and adequate PD, monitoring PD efficiency or peritoneal deterioration by increases in peritoneal solute transport rate is important. In addition to PET, some effluent biomarkers such as MMP-2, IL-6, hyaluronan, and CA125 are often measured to estimate peritoneal injury or progression to EPS during PD[2,4,8-20]. But there are no absolutely reliable diagnostic method[4]. Then, we analyzed the properties and efficacies of these biomarkers.
In the present multicenter clinical study, the results of PET most strongly correlated with effluent MMP-2 level among the biomarkers that we analyzed. High category of PET that is classified by high peritoneal solute transport rate was distinguished with high sensitivity and specificity by effluent MMP-2 level. In addition, effluent MMP-2 level of the patient with EPS was the highest among all PD patients that we analyzed. In our previous multicenter clinical studies, effluent MMP-2 level was high in patients with peritoneal injury[8,9]. Cho et al[11] have reported that high effluent MMP-2 level is associated with peritoneal solute transport rate. Barreto et al[12] also observed significant correlations between peritoneal transport parameters and effluent MMP-2 levels. Yamamoto et al[24] reported that high peritoneal membrane transport state may be a risk factor for EPS. Kawaguchi et al[3] summarized that an increase in D/P Cr ratio may constitute an independent and early marker of EPS. The Japanese Society for Dialysis Therapy Guidelines for Peritoneal Dialysis[4] has recommended that PET should be routinely performed to evaluate peritoneal deterioration. In patients showing serial increase and persistently high D/P Cr for over 12 mo, progression of peritoneal deterioration could be suspected and discontinuation of PD should be considered. We previously reported that among 13 patients with MMP-2 level > 600 ng/mL except the patients, who had been diagnosed as EPS when MMP-2 levels were analyzed, 7 patients (54%) developed EPS and 3 patients (23%) died in a prospective study[8]. In that study, only one patient with an MMP-2 level < 600 ng/mL developed EPS. In the present study, the effluent MMP-2 level of all patients without EPS onset was < 600 ng/mL and no patient developed EPS for at least 1.5 years after measurement of MMP-2 level. From these reports and results, MMP-2 may be expected to become a predictive marker for EPS. Recently, a retrospective clinical study by Lopes Barreto et al[20] was reported that the area under the ROC curve of MMP-2 appearance rate improved to 0.70 (95%CI: 0.51-0.899; P = 0.06) at one year prior to the diagnosis of EPS. They compared 11 PD patients who developed EPS with 33 control PD patients using a 1:3 case control design. The absence of statistically significant findings may be explained by insufficient sample size. In this time, we additionally conducted ROC curve analysis to predict EPS by using eight PD patients with EPS vs 452 control PD patients who have been registered in our database[8,9]. A summary of the results are shown in Table 6. In addition, as a result from ROC curve analysis using a 1:3 case control design as same as the analysis by Lopes Barreto et al[20], the area under the ROC curve for prediction of EPS in our study was 0.97 (95%CI: 0.92-1.00; P < 0.01) at 1 year prior to EPS diagnosis. The estimated sensitivity and specificity for prediction EPS development were 0.96 (95%CI: 0.88-1.00) and 1.00 (95%CI: 1.00-1.00), respectively, for a threshold effluent MMP-2 level of 414 ng/mL. Our data show higher sensitivity and specificity compared with the results by Lopes Barreto et al[20]; this discrepancy may be because of differences in the study designs (prospective and retrospective) or in methods of analysis. Our samples were prepared in the same manner at the 4-h PET and MMP-2 level was obtained as an absolute concentration. On the other hand, Lopes Barreto et al[20] analyzed effluent MMP-2 level as appearance rate. In the present study, effluent MMP-2 level significantly reflected the number of occurrence of peritonitis. Peritonitis, which induces peritoneal tissue injury, occurs 3.3 times more frequently in those who develop EPS than in those who do not[3]. This suggests a close relationship of peritonitis with peritoneal injury and developing EPS. Thus, effluent MMP-2 may be a superior indicator of peritoneal injury or a predictive marker for EPS.
| Cut-off level | Sensitivity (95%CI) | Specificity (95%CI) | AUC |
| 458.5 | 1.00 (1.00 to 1.00) | 0.96 (0.94 to 0.98) | 0.99 |
| 609.5 | 0.88 (0.63 to 1.00) | 0.99 (0.98 to 1.00) | |
| 863.5 | 0.63 (0.25 to 0.88) | 1.00 (0.99 to 1.00) |
IL-6 that plays a critical role in inflammatory processes is secreted in large quantities by peritoneal mesothelial cells in response to inflammatory stimuli and is modulated by exposure to PD solutions[13]. In the present study, effluent IL-6 level correlated with the results of PET and PD duration. On the other hand, although effluent IL-6 level in the EPS patient was high, the highest effluent IL-6 level was observed in another patient with accumulation of ascitic fluid and C-reacted protein-positive. This suggests that effluent IL-6 level reflected strong inflammation rather than EPS development. Cho et al[13] reported that effluent IL-6 level predicted increasing peritoneal solute transport rate and significantly increased with longer PD duration. Pecoits-Filho et al[14] also reported that effluent IL-6 level was correlated with high peritoneal solute transport rate. PD duration, in addition to high peritoneal solute transport rate, is a risk factor for EPS[25,26]. Abovementioned studies and our results suggest the efficacy of IL-6 as a biomarker of peritoneal deterioration. On the other hand, Goodlad et al[19] reported that although IL-6 was at higher levels in the effluent of patients who subsequently developed EPS, it did not improve prediction of future EPS compared with a model that used known clinical risk factors. Also in our study, a patient with the highest IL-6 level did not developed to EPS. As mentioned above, effluent IL-6 level may reflect deterioration of peritoneal membrane but it has to be kept in mind that effluent IL-6 level strongly reflects inflammation with or without EPS development.
Hyaluronan is constitutively synthesized by mesothelial cells and plays a crucial role in the maintenance of mesothelial cell morphology and re-mesothelialization. In particular, low molecular weight hyaluronan promotes angiogenesis, matrix protein synthesis, and transcription of MMPs[15]. In the present study, effluent hyaluronan level also significantly reflected PET results and PD duration. Yamagata et al[16] reported that intraperitoneal hyaluronan production increased with both higher membrane permeability and longer time on PD. Monitoring of hyaluronan in the peritoneal effluent may be useful as a marker to assess functional and morphological changes in the peritoneum in long-term PD patients. Effluent hyaluronan level may reflect deterioration of peritoneal membrane.
The concentration or appearance rate of CA125 in PD effluent has been used as a biomarker for mesothelial cell mass in patients on PD. In the present study, although there was no association between effluent CA125 level and peritoneal solute transport, effluent CA125 level correlated weakly with all other biomarkers. Effluent CA125 level of male patients was significantly lower than that of female patients; in addition, the level of diabetic patients was significantly lower than that of non-diabetic patients. In several previous studies, the concentration and appearance rate of CA125 in peritoneal effluent had significant negative correlation with the duration of dialysis[17]. In contrast, three cross-sectional studies found that duration of PD did not affect the CA125 level in the peritoneal effluent. Peritoneal transport parameters and a history of peritonitis in PD patients were not related to effluent CA125[16,17]. A few previous studies did not observe a relationship between patient sex and effluent CA125[17]. However, in a prospective study, effluent CA125 level was significantly lower in male patients than in female patients at 6 and 12 mo after PD initiation[17]. Ditsawanon et al[17] reported that effluent CA125 level and appearance rate can also be used to follow-up individual patients who are not infected to evaluate peritoneal fibrosis, which is characterized by loss of mesothelial cells. Krediet[18] described that effluent CA125 in stable PD patients without acute peritonitis is a marker of mesothelial cell mass, but has large inter-individual variability. Serial measurements over time can be used for assessment of peritoneal mesothelial mass in individual patients, but effluent CA125 level may depend on individual characteristics of patients.
In conclusion, the peritoneal solute transport rate was most strongly correlated with the effluent level of MMP-2 among the biomarkers that were measured in the present study. MMP-2 may be useful as an indicator of peritoneal deterioration and can also potentially become a predictive marker with high sensitivity and specificity for EPS. Future studies should examine the serial changes of effluent MMP-2 level in relation to the progression of peritoneal injury. More patients should be tested to confirm the efficacy of MMP-2 as a biomarker and to eliminate selection bias of patients.
This study was supported by Terumo Core Technology Center (Kanagawa, Japan). We would like to thank Dr. Taizo Iwasaki (Terumo Core Technology Center) for his help with statistical analysis and Dr. Hideaki Kagitani (Terumo Europe, Leuven, Belgium) for his review of statistical methods. The collaborators in the multicenter clinical study were as follows: Fumihiko Hatafuku (Department of Urology, Onoda Hospital), Hideki Takizawa (Department of Nephrology, Teine Keijinkai Hospital), Makoto Nishina (Metabolism, Department of Internal Medicine, Tokai University, School of Medicine), Masanobu Horie (Department of Urology, Daiyukaidaiichi Hospital), Morihiro Kondou (Department of Nephrology, Otowa Hospital), Naomi Yoshimune (Department of Internal Medicine, Kinashi Obayashi Hospital), Noriaki Yorioka (Department of Advanced Nephrology, Graduate School of Biomedical Sciences, Hiroshima University), Ryoichi Miyazaki (Department of Internal Medicine, Fujita Memorial Hospital), Ryouji Wakamatsu (Nishikatakai Clinic), Sukenari Koyabu (Department of Internal Medicine, Owase General Hospital), Tadashi Yamamoto (Kidney Center, Shirasagi Hospital), Takeyuki Hiramatsu (Department of Internal Medicine, Aihoku Hospital), Tetsurou Yanase (Dr. Yanase Internal Medicine Office), Tohru Mizumasa (Department of Nephrology, Fukuoka Red Cross Hospital), Tomoyoshi Kimura (Department of Nephrology, Sendai Social Insurance Hospital), Toyonori Saiki (Department of Nephrology, Saiki Jin Clinic), Yumiko Ikeda (Department of Nephrology, Yokohama Minami Kyousai Hospital).
Peritoneal dialysis (PD) is a blood purifying method for patients with end-stage renal disease. Long-term PD causes peritoneal deterioration with structural changes and functional decline, resulting in the cessation of PD treatment; these are the major problems associated with PD. At worst, peritoneal deterioration causes encapsulating peritoneal sclerosis (EPS), a serious complication of PD with extremely high mortality rate. To undergo PD safely and adequately, diagnosis of peritoneal deterioration is important.
Solute transport rate through the peritoneal membrane is often measured by peritoneal equilibration test (PET) to estimate PD efficiency and peritoneal deterioration. However, the results from PET are not sufficient predictors of EPS onset, and monitoring the time-course changes are necessary. In addition, PET is invasive and takes a long time. It is thus necessary to evaluate peritoneal deterioration using an easy and reliable non-invasive method.
In this study, the peritoneal solute transport rate most strongly correlated with the effluent level of matrix metalloproteinase-2 (MMP-2) among some biomarkers. MMP-2 could also predict EPS with high sensitivity and specificity at 1 year prior to EPS onset.
The data in this study suggested that effluent MMP-2 may be useful as a reliable indicator of peritoneal deterioration and a predictive biomarker with high sensitivity and specificity for EPS.
MMP-2 degrades Type IV collagen and fibronectin, which are components of extracellular matrix, and plays a critical role in cell migration, angiogenesis, and epithelial to mesenchymal transition of mesothelial cells.
This is a good article.
P- Reviewer: Friedman EA, Hsieh MJ S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Gandhi VC, Humayun HM, Ing TS, Daugirdas JT, Jablokow VR, Iwatsuki S, Geis WP, Hano JE. Sclerotic thickening of the peritoneal membrane in maintenance peritoneal dialysis patients. Arch Intern Med. 1980;140:1201-1203. [PubMed] |
| 2. | Schmidt DW, Flessner MF. Pathogenesis and treatment of encapsulating peritoneal sclerosis: basic and translational research. Perit Dial Int. 2008;28 Suppl 5:S10-S15. [PubMed] |
| 3. | Kawaguchi Y, Saito A, Kawanishi H, Nakayama M, Miyazaki M, Nakamoto H, Tranaeus A. Recommendations on the management of encapsulating peritoneal sclerosis in Japan, 2005: diagnosis, predictive markers, treatment, and preventive measures. Perit Dial Int. 2005;25 Suppl 4:S83-S95. [PubMed] |
| 4. | Working Group Committee for the Preparation of Guidelines for Peritoneal Dialysis, Japanese Society for Dialysis Therapy. 2009 Japanese Society for Dialysis Therapy guidelines for peritoneal dialysis. Ther Apher Dial. 2010;14:489-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Hirahara I, Kusano E, Yanagiba S, Miyata Y, Ando Y, Muto S, Asano Y. Peritoneal injury by methylglyoxal in peritoneal dialysis. Perit Dial Int. 2005;26:380-392. [PubMed] |
| 6. | Twardowski ZJ, Nolph KD, Khanna R, Prowant BF, Ryan LP, Moore HL, Nielsen MP. Peritoneal equilibration test. Perit Dial Bull. 1987;7:138-147. |
| 7. | Yamamoto R, Otsuka Y, Nakayama M, Maruyama Y, Katoh N, Ikeda M, Yamamoto H, Yokoyama K, Kawaguchi Y, Matsushima M. Risk factors for encapsulating peritoneal sclerosis in patients who have experienced peritoneal dialysis treatment. Clin Exp Nephrol. 2005;9:148-152. [PubMed] |
| 8. | Hirahara I, Inoue M, Okuda K, Ando Y, Muto S, Kusano E. The potential of matrix metalloproteinase-2 as a marker of peritoneal injury, increased solute transport, or progression to encapsulating peritoneal sclerosis during peritoneal dialysis--a multicentre study in Japan. Nephrol Dial Transplant. 2007;22:560-567. [PubMed] |
| 9. | Hirahara I, Inoue M, Umino T, Saito O, Muto S, Kusano E. Matrix metalloproteinase levels in the drained dialysate reflect the peritoneal solute transport rate: a multicentre study in Japan. Nephrol Dial Transplant. 2011;26:1695-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Hirahara I, Umeyama K, Urakami K, Kusano , Y . Masunaga, Y. Asano. Serial analysis of matrix metalloproteinase-2 in dialysate of rat sclerosing peritonitis models. Clin Exp Nephrol. 2001;5:103-108. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Cho Y, Johnson DW, Vesey DA, Hawley CM, Pascoe EM, Clarke M, Topley N. Higher dialysate matrix metalloproteinase-2 levels are associated with peritoneal membrane dysfunction. Perit Dial Int. 2016;36:16-25. [PubMed] |
| 12. | Barreto DL, Coester AM, Struijk DG, Krediet RT. Can effluent matrix metalloproteinase 2 and plasminogen activator inhibitor 1 be used as biomarkers of peritoneal membrane alterations in peritoneal dialysis patients? Perit Dial Int. 2013;33:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Cho Y, Johnson DW, Vesey DA, Hawley CM, Pascoe EM, Clarke M, Topley N; balANZ Trial Investigators. Dialysate interleukin-6 predicts increasing peritoneal solute transport rate in incident peritoneal dialysis patients. BMC Nephrol. 2014;15:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Pecoits-Filho R, Araújo MR, Lindholm B, Stenvinkel P, Abensur H, Romão JE, Marcondes M, De Oliveira AH, Noronha IL. Plasma and dialysate IL-6 and VEGF concentrations are associated with high peritoneal solute transport rate. Nephrol Dial Transplant. 2002;17:1480-1486. [PubMed] |
| 15. | Yung S, Chan TM. Pathophysiology of the peritoneal membrane during peritoneal dialysis: the role of hyaluronan. J Biomed Biotechnol. 2011;2011:180594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Yamagata K, Tomida C, Koyama A. Intraperitoneal hyaluronan production in stable continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 1999;19:131-137. [PubMed] |
| 17. | Ditsawanon P, Supasyndh O, Aramwit P. Dialysate cancer antigen 125 in long-term peritoneal dialysis patients. Clin Exp Nephrol. 2014;18:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Krediet RT. Cancer Antigen 125 as a biomarker in peritoneal dialysis: mesothelial cell health or death? Perit Dial Int. 2013;33:715-718. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Goodlad C, Tam FW, Ahmad S, Bhangal G, North BV, Brown EA. Dialysate cytokine levels do not predict encapsulating peritoneal sclerosis. Perit Dial Int. 2014;34:594-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Lopes Barreto D, Struijk DG, Krediet RT. Peritoneal effluent MMP-2 and PAI-1 in encapsulating peritoneal sclerosis. Am J Kidney Dis. 2015;65:748-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Hirahara I, Umeyama K, Shofuda K, Kusano E, Masunaga Y, Honma S, Asano Y. Increase of matrix metalloproteinase-2 in dialysate of rat sclerosing encapsulating peritonotis model. Nephrology. 2002;7:161-169. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 22. | Hirahara I, Ishibashi Y, Kaname S, Kusano E, Fujita T. Methylglyoxal induces peritoneal thickening by mesenchymal-like mesothelial cells in rats. Nephrol Dial Transplant. 2009;24:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Hirahara I, Ogawa Y, Kusano E, Asano Y. Activation of matrix metalloproteinase-2 causes peritoneal injury during peritoneal dialysis in rats. Nephrol Dial Transplant. 2004;19:1732-1741. [PubMed] |
| 24. | Yamamoto R, Nakayama M, Hasegawa T, Miwako N, Yamamoto H, Yokoyami K, Ikeda M, Kato N, Hayakawa H, Takahashi H. High-transport membrane is a risk factor for encapsulating peritoneal sclerosis developing after long-term continuous ambulatory peritoneal dialysis treatment. Adv Perit Dial. 2002;18:131-134. [PubMed] |
| 25. | Brown EA, Van Biesen W, Finkelstein FO, Hurst H, Johnson DW, Kawanishi H, Pecoits-Filho R, Woodrow G; ISPD Working Party. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis: position paper for ISPD. Perit Dial Int. 2009;29:595-600. [PubMed] |
| 26. | Nakayama M, Miyazaki M, Honda K, Kasai K, Tomo T, Nakamoto H, Kawanishi H. Encapsulating peritoneal sclerosis in the era of a multi-disciplinary approach based on biocompatible solutions: the NEXT-PD study. Perit Dial Int. 2014;34:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |