Revised: April 18, 2014
Accepted: June 10, 2014
Published online: August 6, 2014
Processing time: 215 Days and 18 Hours
Obesity is an important worldwide challenge that must be faced in most developed and developing countries because of unhealthy nutritional habits. The consequences of obesity and being overweight are observed in different organs, but the kidney is one of the most affected. Excess adipose tissue causes hemodynamic alterations in the kidney that can result in renal disease. However, obesity is also commonly associated with other comorbidities such as chronic inflammation, hypertension and diabetes. This association of several aggravating factors is still a matter of concern in clinical and basic research because the pathophysiologic mechanisms surrounding chronic kidney disease development in obese patients remain unclear. This review will discuss the consequences of obesity in the context of renal injury.
Core tip: Obesity is unquestionably one of the biggest health challenges the modern world will face this century. It has vast effects on systemic function including cardiovascular disease, metabolic dysfunction and chronic inflammation. All of these factors have a great impact on kidney function, and current data indicate a significant correlation between obesity and kidney disease because of irregular immune activation, altered renal hemodynamics and metabolic mediator signaling. This review focuses on the most recent findings that have begun to elucidate the relationship between obesity and its effect on the kidneys.
- Citation: Felizardo RJF, Silva MBD, Aguiar CF, Câmara NOS. Obesity in kidney disease: A heavyweight opponent. World J Nephrol 2014; 3(3): 50-63
- URL: https://www.wjgnet.com/2220-6124/full/v3/i3/50.htm
- DOI: https://dx.doi.org/10.5527/wjn.v3.i3.50
Obesity is unquestionably one of the biggest health challenges the world population faces this century. Statistics indicate that more than 1.4 billion adults over 20 (35%) are overweight, with 11% falling into the obesity category according to World Health Organization statistics. Although the state of being obese and overweight has usually been associated with developed countries and high income, globalization and widespread unhealthy nutritional habits have caused these phenomena to reach epidemic proportions. As a consequence to the rise in obesity rates, comorbidities linked to this disease such as diabetes, cardiovascular disease, and cancer have also increased[1].
Adipose tissue has a great impact on metabolic homeostasis and immunological function. The conjunction of the main obesity-related risk factors defines a clinical condition termed Metabolic Syndrome. This syndrome aggregates a variety of pathologies, including dyslipidemia, thrombosis, low-grade systemic inflammation, elevated blood pressure, hyperglycemia and insulin resistance. Adipose tissue possesses an important influence over the immune response profile via direct and indirect mechanisms through the secretion of nonesterified fatty acids, cytokines and endocrine mediators defined as adipokines. Together, these factors contribute to a systemic change in the way the body works, adapts and responds to challenges.
Although many studies have associated obesity with higher morbidity rates and obesity-related diseases[2], some groups argue the contrary. Overweight and obese patients reportedly display higher survival, while patients with low body mass are at a higher risk of general mortality and cardiovascular and many non-cardiovascular disease incidence, a phenomenon referred to as the “obesity paradox”[3,4]. These findings also highlight the complex relationship that obesity has with different pathologies and demonstrates that a closer look is needed to understand the particular effects of being obese and overweight on the organism.
Obesity affects the function of many organs. The heart is one of the main organs affected by metabolic syndrome, and obesity significantly increases the chances of cardiac dysfunction because of chronic hemodynamic burden, which causes dyspnea, edema, ongoing systemic inflammation, metabolic alterations and other related comorbidities[5]. Other organs such as the liver are also affected by this pathology, with lipid accumulation causing nonalcoholic fatty liver disease[6]. Lung function is also compromised by adipose tissue around the abdomen, rib cage and visceral cavity[7].
The kidney is also responsive to obesity. Several multicenter studies have identified a direct correlation between obesity and renal complications (Table 1). Obesity has a multifactorial mechanism and is considered an independent factor in chronic kidney disease (CKD) development and progression to end-stage renal disease (ESRD)[8]. Studies demonstrate that obesity-induced hypertension and diabetes are strong determinants of CKD. Analyses relating obesity and kidney transplantation revealed that in 1987, 11.6% of adults awaiting a kidney transplant were obese, and in 2001, obesity among adults rose to 25.1%[9]. Concomitantly, body mass index (BMI) among patients initiating dialysis increased from 25.7 kg/m2 to 27.5 kg/m2 from 1995 to 2002[10]; and when compared with normal weight persons (BMI, 18.5-24.9 kg/m2), there is a directly proportional relationship between increased BMI and increased CKD and ESRD risk[11,12]. A study conducted by Ejerblad et al[13] examined the association between degrees of obesity and CKD. After making adjustments for many covariates, the investigators found a 2.8-fold increased risk of nephrosclerosis and a 7-fold increased risk of diabetic nephropathy among adults who had a BMI of 35 kg/m2 or higher compared with a lifetime highest BMI lower than 25 kg/m2. In adults with no diabetes or hypertension, a lifetime highest BMI of 35 kg/m2 or higher was associated with a 2-fold increased risk of CKD. Conversely, obese patients had better recovery and benefitted from reduced body weight by diminishing proteinuria[14]. Obesity was recently demonstrated to accelerate IgA nephropathy progression[15]. In this scenario, obesity could be one of the few preventable risk factors for CKD development because it also mediates diabetes and hypertension, which are related to kidney disease progression[14,16,17].
| Cohort | Number of patients | Country | Result | Ref. |
| Dialysis patients | 1957 | Netherlands | Higher mortality with very high or low BMI (< 65 yr) | [157] |
| Kidney transplant | 1810 | Netherlands | Higher mortality and kidney graft failure | [158] |
| Native population | 1924 | Sweden | Higher Chronic Renal Failure | [13] |
| National Health and Nutrition Examination Survey III | 5659 | United States | Higher microalbuminuria with metabolic syndrome | [159] |
| Hipertension and obesity | 4585 | Spain | Higher risk of renal insufficiency | [160] |
| Native population | 2585 | United States | Higher risk of kidney disease | [12] |
| Native population | 5403 | Japan | Higher risk of proteinuria | [161] |
| Kidney transplant | 51927 | United States | Lower patient and graft survival. Higher chronic graft failure and delayed graft function | [162] |
The occurrence of obesity during early life is linked to low glomerular filtration rates (GFRs), while being overweight during adulthood doubles the chances of chronic kidney disease[18]. Many researchers have described the direct impacts obesity has on the kidneys, which include hyperfiltration, elevated glomerular tension, and podocyte stress[19]. Some researchers have also correlated obesity-related inflammation and adipokine deregulation to kidney function. The present review will focus on the impact of obesity on kidney function and discuss its influence on the progression of kidney disease.
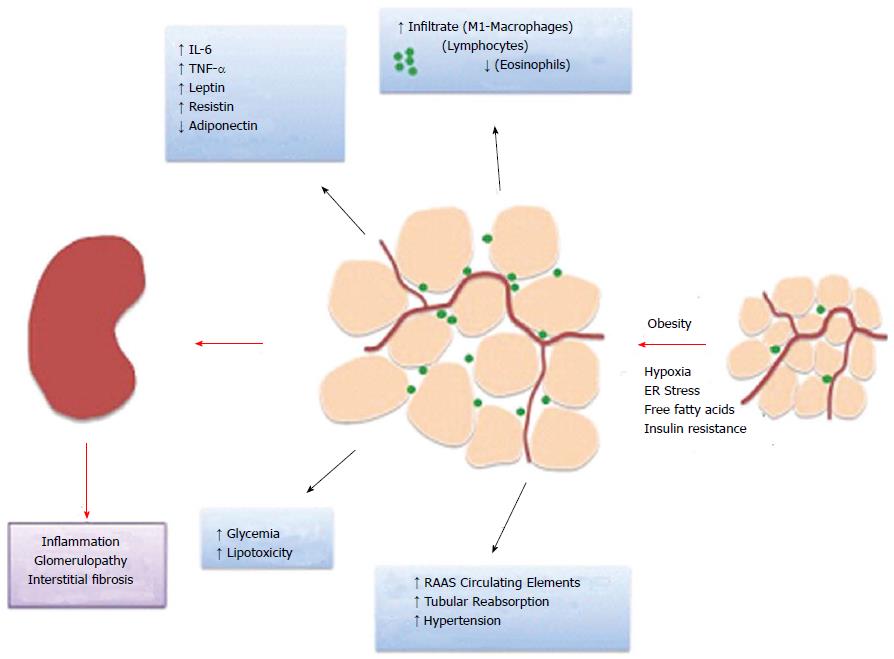
Adipose tissue is known for its roles in lipid storage, thermogenesis and metabolic regulation. However, in recent years, focus has been given to its endocrine properties such as cytokine and adipokine secretion (Figure 1).
As previously described, obesity and diabetes are conditions that present a state of low-grade inflammation. Significant evidence supports the concept of adipose tissue as an immunomodulatory organ. Adipose tissue harbors a considerable amount of immune cells such as macrophages, lymphocytes and eosinophils. In obesity, the frequency of infiltrated cells rises, and they acquire a pro-inflammatory profile[20]. Excess free fatty acids that are present in obesity activate diverse inflammatory pathways involving endoplasmic reticulum stress[21], toll-like receptor[22,23], inflammasome and nuclear factor-κB (NF-κB) signaling activation[24,25]. In parallel, adipose tissue becomes hypoxic with adipocyte hypertrophy, which induces a change from aerobic to anaerobic glycolysis and lactate production.
With obesity, adipocyte hypertrophy and hypoxia induce cell death and resident immune cell activation, which in turn promotes inflammatory cell recruitment[26]. Macrophages constitute the principal population of resident and recruited cells in adipose tissue, which have a role in maintaining tissue homeostasis by assisting with the clearance of dead cells and debris. Because of lipid accumulation and adipocyte cell death, non-inflammatory tissue-resident M2 type macrophages and recruited monocytes undergo proliferation and macrophage M1 polarization[27-29]. These cells in turn secrete higher levels of inflammatory cytokines such as TNF-α, IL-6 and MCP-1 and lower levels of anti-inflammatory mediators such as arginase 1[28-30]. IL-4-expressing eosinophil counts also decrease with obesity, which contributes to inflammation[31]. Furthermore, CD8+ and CD4+ Th1 lymphocyte counts also increase while Treg numbers reduce with obesity. In accordance, B cell pro-inflammatory immunoglobulin G2c (IgG2c) production also participates in cell activation[32-35].
Proinflammatory cytokines are also produced by the renal parenchyma in response to hyperglycemia as well as vasoactive peptides such as angiotensin II and endothelin[36]. These molecules activate signaling second messengers such as protein kinase C, MAP kinase and NF-κB, which alter the gene expression of several cytokines and growth factors.
Increased TNF-α levels reduce the expression of nephrin and podocin, causing podocytopathy[37]. Similarly, IL-6 promotes adhesion molecule expression, which increases oxidative stress[38], and IL-6 receptor blockade can inhibit the progression of proteinuria, renal lipid deposition and mesangial cell proliferation[39]. An additional important growth factor for renal injury is transforming growth factor (TGF)-β, which induces podocyte apoptosis, extracellular matrix synthesis and mesangial cell proliferation, thus exacerbating the development of the glomerular lesions associated with diabetes and obesity[40].
While many studies demonstrate the effect of metabolism on the immune system, studies have demonstrated that the reverse also happens; immune cell activation in adipose tissue is a determinant of obesity-linked metabolic changes such as insulin resistance[41]. For example, in response to inflammatory mediators, adipose tissue also down regulates glucose transporter GLUT4 expression, which increases insulin resistance.
In addition to cytokines, adipose tissue is also responsible for the production of many endocrine mediators termed adipokines, which regulate inflammation, food consumption and link immune and metabolic functions. Amongst these are leptin, adiponectin, visfatin, resistin, intelectin and others. These factors are mostly secreted by adipocytes and have imbalanced expression in obesity. Many studies have documented the importance of these cytokines in the regulation of metabolism and inflammation and suggest a role for these cytokines in obesity-related metabolic and inflammatory distortion. Although there is still much to elucidate regarding the role of adipokines in kidney disease, recent studies now have begun to clarify the influence of these mediators in kidney pathology.
Leptin was the first adipokine to be characterized and is the best described in the literature. It is secreted by different adipose compartments and induces signaling through Ob-a to Ob-f subtype receptor activation, and the Ob-Rb receptor is the most important. Its main actions are on the nervous system and kidneys. Leptin acts on the nervous system by stimulating neuropeptides that promote satiety and energy consumption. It has been suggested that one develops leptin resistance in obesity because of the absence of many of its effects despite elevated adipokine levels. Hyperleptinemia has also been associated with many cardiovascular and immunologic dysfunctions[42].
Many reports have linked obesity and leptin to hypertension. Studies indicate that this adipokine activates the sympathetic nervous system and may suppress parasympathetic nerve activity, which alters baroreflex control[43,44]. Leptin also increases renal sympathetic nerve activity, as demonstrated by studies on ObR deletion in the central nervous system[45]. Because the sympathetic nervous system contributes to CKD, leptin hypertensive actions may promote kidney disease.
Leptin also holds important pro-inflammatory activity. Its structure resembles other cytokines such as IL-2 and can stimulate many immune cells. Studies demonstrate that leptin induces the production of inflammatory cytokines as IL-6 and TNF-α by monocytes and additionally induces chemokine ligands, reactive oxygen species production and macrophage and monocyte proliferation[42]. Leptin also polarizes CD4+ lymphocytes toward a Th2 profile, which increases the production of inflammatory cytokines such as IL-2 and IFN-γ[42]. Therefore, excess leptin, which is characteristic during obesity, is an important mediator of obesity-related immune and metabolic dysfunction.
Recent studies have also suggested that leptin imposes an important action in the kidneys, as this mediator localizes mainly to the organ after injection[46]. CKD patients demonstrate high leptin levels, as do ESRD patients, and hemodialysis fails to lower these values[47,48]. The kidneys also express the Ob-Ra and Ob-Rb leptin receptor isoforms[49]. In vitro, leptin induces glomerular endothelial cell proliferation, which is augmented in the presence of angiotensin II and increases TGF-β1 production. Furthermore, leptin infusion into rats in vivo also induced proteinuria, glomerular endothelial cell proliferation and TGF-β1 production and increased collagen type IV expression[50]. This adipokine also induced type I collagen in mesangial cells, confirming data that link obesity, glomerulosclerosis and glomerulomegaly, which is defined as obesity-related glomerulopathy[51,52].
Adiponectin is another adipokine with immunomodulatory and metabolic actions. It is present in plasma at a considerable concentration[53], and its receptors R1, R2 and T cadherin are expressed by a wide range of tissues. Adiponectin is negatively correlated with hypertension[54]. It exerts its metabolic actions by increasing glucose uptake and fatty acid oxidation and inhibiting gluconeogenesis. In addition to improving insulin sensitivity, it also possesses potent anti-inflammatory properties[42].
Unlike leptin, low serum adiponectin levels are found in obese patients, and its production is reduced by hypoxia, inflammatory mediators such as IL-6 and oxidative stress[55-57]. Hypoadiponectinemia has been linked to diverse complications in obesity. Mice lacking adiponectin display increased susceptibility to high-fat diet-induced insulin resistance[58]. Moreover, adiponectin overexpression in high-fat diet-fed animals caused less fat accumulation and reduced adipose tissue macrophage infiltration, and it prevented premature death[59].
Recent studies have begun to elucidate the role of adiponectin in kidney injury. Current data demonstrate that adiponectin is secreted not only by adipocytes but also renal tubular cells[60]. Research indicates that plasma adiponectin is inversely correlated with albuminuria in obese patients[61]. Adiponectin-null mice also develop albuminuria and podocyte damage as well as glomerular oxidative stress[62]. These mice also display more expressive albuminuria, fibrosis and macrophage infiltration after 5/6 nephrectomy[63]. Moreover, mice overexpressing adiponectin recover more rapidly and exhibit less interstitial fibrosis after podocyte-specific damage[64]. Metabolic syndrome has also been associated with low adiponectin levels and worse prognosis after kidney transplantation[65]. These data are controversial, however, as some studies describe a direct link between adiponectin levels and mortality in advanced CKI and kidney transplant patients[66,67]. While recent work suggests that adiponectin causes less intense ischemia-reperfusion kidney injury[68], the contrary was observed when exogenous adiponectin was administered[69]. Furthermore, kidney function also influences adiponectin levels because the kidneys are responsible for its elimination, and kidney transplantation significantly reduces the adiponectin concentration[70].
Resistin is a recently discovered adipokine with inflammatory properties. Some works suggest that this mediator increases insulin resistance, while others fail to find this correlation[71,72]. Although in mice, it is expressed mainly by adipocytes, in humans it is produced principally by macrophages and monocytes. Although there are still few data on its impact on renal function, some research indicates that serum resistin levels are strongly associated with decreased GFRs and inflammatory biomarkers in CKD[71].
Adipose tissue and the kidneys also synthesize visfatin, and this is upregulated in type-2 diabetic rats, inducing fibrosis and inflammatory pathway activation[73]. In CKD patients, higher visfatin levels also are correlated with decreased GFR and endothelial dysfunction[74,75]. Furthermore, another study with human plasma determined that this mediator was also linked to creatinine levels, inflammation and endothelial damage in kidney recipients, which is negatively related to plasma albumin levels[19].
The pathophysiologic mechanism surrounding CKD development in obese patients remains unclear, but many events must be linked to ESRD such as altered renal hemodynamics, insulin resistance, hyperlipidemia, inflammation and oxidative stress (Figure 1). Hemodynamic alterations such as higher renal plasma flow, GFR and filtration fraction were linked to obesity when compared with the levels in non-obese patients[76,77]. The effect of BMI on renal hemodynamics was also proven by another work in which GFR and effective renal plasma flow (ERPF) were evaluated with a high-sodium diet. According to this study, ERPF and the GFR were statistically increased when individuals were exposed to a high-sodium diet and compared to another group that was exposed to a low-sodium diet without a change in filtration fraction (FF). However, increased sodium intake-induced changes in the GFR and FF were significantly greater in people with a BMI ≥ 25 kg/m2[78]. The hemodynamic effects of overweight on kidney function and albuminuria are enhanced with hypertension, which itself is a clinical complication of obesity. Chagnac et al[79] demonstrated that glomerular hyperfiltration could have a relevant role in development of hypertension in obese patients by increasing postglomerular oncotic pressure and proximal tubular sodium reabsorption.
As an individual gains weight, renal mass as well as the glomerular diameter increases[80]. Podocytes are highly specialized cells that support the glomerular basement membrane (GBM) and play an important role in the glomerular filtration barrier via their foot processes. With glomerular hypertrophy, podocytes must cover a larger area by expanding these processes. If this podocyte enlargement is not proportional to glomerular hypertrophy, this adaptation could cause podocyte detachment and consequently a loss selectivity of serum protein selectivity[81,82]. Considering that podocytes are cells with limited capacity for cell division and replacement, proteinuria may be detected as is commonly observed in obese patients. Supporting this hypothesis, individuals who reduced their body mass also had significant reductions in proteinuria[14,83].
Extensive studies demonstrate that a lack of podocytes covering the GBM results in the formation of denuded areas, which trigger matrix deposition resulting in glomerulosclerosis in experimental models as well as in human biopsies[84-87]. As kidney injury persists, kidney fibrosis becomes an inevitable outcome in which epithelial-mesenchymal and endothelial-mesenchymal transition events generate matrix-producing fibroblasts in the interstitial space that contribute to renal fibrosis. Accumulation of matrix elements caused by the fibrotic process progressively alters normal kidney architecture by contraction and increased stiffness, resulting in disrupted blood flow supply and nephron function[88,89].
Once a number of podocytes are injured, a vicious cycle starts in which other podocytes also become damaged, accelerating podocyte deterioration and glomerulosclerosis[90]. The extensive loss of glomeruli imposes excessive stress on the remaining glomeruli because of hemodynamic alterations and glomerular hypertrophy, which can subsequently cause further sclerosis of the remaining glomeruli[91]. This could explain the progressive spreading of glomerular damage in later disease stages in which patients develop chronic renal failure[90]. The approach of using new agents to avoid podocyte lesions in different models of acute and chronic kidney disease resulted in less matrix deposition and consequent glomerulosclerosis[92,93].
In obesity, the renin-angiotensin-aldosterone system (RAAS) is commonly activated and is one of the strongest links to renal injury. All of the major components necessary to generate angiotensin II (Ang II) are found in the kidney[94]. The RAAS is a well-known mechanism to regulate blood pressure, fluids and electrolyte balance[95], and its activation impairs normal pressure natriuresis, increases renal tubular sodium reabsorption, and causes volume expansion. Physical compression of kidneys by visceral adipose tissue in obesity exacerbates these responses and increases blood pressure, leading to hypertension in obese subjects.
RAAS effects are obtained when angiotensinogen (AGT), the precursor of bioactive angiotensin peptides, is cleaved by both renin and angiotensin converting enzyme (ACE) to generate Ang II. Ang II, which is the active peptide and is the main effector of RAAS, possesses a dual role in physiology. Ang II helps maintain long-term blood pressure and blood volume in the body; conversely, it has also been considered a multifunctional cytokine that plays a role in cell proliferation, hypertrophy, superoxide production, inflammation and extracellular matrix deposition[96]. Ang II plays an endocrine role, and its participation in the development of obesity was evidenced by several works in which AGT, ang II and ang II receptor-deficient mice were protected against high-fat diet-induced obesity[97-99].
There are several pathophysiological conditions, including hypertensive models, in which Ang II, in response to increased arterial blood pressure, increases efferent glomerular arteriole resistance and induces TGF-β production[100]. It also impairs the auto-regulation of afferent arterioles by avoiding vasoconstriction[101]. Taken together, Ang II directly and indirectly enhances capillary filtration pressure and promotes proteinuria, which is one of the most important factors involved in renal disease progression. Moreover, Ang II is also involved in nephrin dephosphorylation during podocyte apoptosis[102], which is a protein that is part of the slit diaphragm and binds to the adjacent nephrins of other podocytes. Ang II decreases the synthesis of negatively charged proteoglycans that are present on the glomerular basement membrane, which impairs the filtration of high molecular weight proteins by electrostatic repulsion[103].
Human adipose tissue expresses all of the RAAS components, including angiotensin, ACE, renin and the AT1 and AT2 receptors. Consequently, the AGT produced by adipose tissue contributes significantly to circulating AGT levels. In humans and mice, a strong relationship has been observed between increased AGT gene expression and obesity[104], supporting a role for adipose AGT in hypertensive obese patients. Weight reduction reduced blood pressure through systemic RAAS suppression and decreased AGT, renin and aldosterone levels in adipose tissue and plasma[105]. Mice with adipose tissue-restricted AGT expression were normotensive, whereas when adipose AGT was overexpressed, the mice became hypertensive[106]. Ang II is also involved in adipocyte metabolism by influencing leptin and adiponectin release. Once leptin levels are increased, Ang II promotes a number of cellular processes that attenuate leptin signaling and contribute to leptin resistance, which is common in obesity[107]. Conversely, adiponectin was upregulated when RAS was blocked by an ACE inhibitor or Ang II receptor blocker, suggesting Ang II participation in the inhibition of adiponectin release[108].
Not only AGT but also aldosterone levels are increased in obese patients. Aldosterone is a mineralocorticoid hormone that is produced in the adrenal glands in response to Ang II and a high extracellular potassium concentration, which increases blood pressure via sodium retention in the collecting duct. Aldosterone is correlated with increased blood pressure[109] and can also be produced by adipocytes through pathways that are dependent on the Ang II-ATI receptor axis and calcineurin signaling[110] as well as pathways that are independent of Ang II, in which adipocytes secrete factors that may stimulate the adrenal gland and increase circulating aldosterone levels, resulting in mineralocorticoid receptor activation and increasing blood pressure and hypertension[111]. Aldosterone binds to cytosolic mineralocorticoid receptors and promotes cell signaling pathways, endothelial dysfunction, inflammation and fibrosis independently and in concert with Ang II[112]. Moreover, Ang II activates the mineralocorticoid receptor in the absence of aldosterone and promotes kidney injury[113,114]. Blocking the mineralocorticoid receptors with antagonists attenuates obesity-induced hypertension and glomerular hyperfiltration[115].
Many clinical trials have been performed to mitigate the effects caused by RAAS. Multiple pharmacological strategies are used to treat CKD patients to diminish proteinuria and blood pressure. These strategies comprehend the use of RAAS-blocking agents alone or combined with ACE inhibitors, angiotensin-receptor blockers, direct renin inhibitors and mineralocorticoid-receptor antagonists[116]. The combination of a pharmacological therapy with reduced sodium intake was a better choice to diminish blood pressure and proteinuria than combined therapies[117]. Attempts to antagonize aldosterone receptors demonstrated promising results to diminish glomerulosclerosis[118].
In summary, the obesity-RAAS-hypertension axis is closely related to renal disease, as the increased release of adipose tissue derived-RAAS elements into the circulation can alter hemodynamic homeostasis. Increased Ang II, AGT and aldosterone levels promote increased tubular reabsorption, leading to arterial hypertension and renal vasodilation. These events contribute to glomerular hypertension, which is an important factor in glomerulosclerosis and CKD progression.
Obesity is an important risk factor for hypertension and type 2 diabetes development, which are the leading causes of end-stage renal disease. The relationship between obesity, diabetes and kidney disease is very close because obesity and diabetes alter renal function, leading to renal disease. These renal alterations in both cases include anatomical, physiological and pathological changes (Figure 1).
Physiological and hemodynamic alterations are largely responsible for the subsequent anatomical and histopathological modifications. Among the major hemodynamic changes in obese and/or diabetic patients are increased GFR and intraglomerular capillary pressure[119,120]. Such alterations lead to diabetic nephropathy, increases in kidney weight and size, increased glomerular size, podocyte hypertrophy and mesangial matrix expansion[121].
Diabetes-related renal injuries can be grouped into five stages that comprise the remodeling that occurs throughout diabetic nephropathy. These stages are summarized in Table 2.
| Stages | Features |
| 1 and 2 | Hyperfiltration and renal hypertrophy |
| 3 | Microalbuminuria and hypertension as clinical features. As histological features: arteriolar hyalinosis, glomerular basement membrane thickening and mesangial matrix expansion |
| 4 (Diabetic Ne phropathy) | Proteinuria, nephrotic syndrome and decreased GFR |
| 5 | End-stage renal disease |
Although obesity and diabetes per se are responsible for renal injury, some other factors usually present in these conditions significantly aggravate renal damage such as blood pressure, hyperlipidemia, hyperglycemia, genetic factors[122] and inflammation. Some of these conditions are described in the following sections.
Hypertension and diabetes are two important risk factors in the development of kidney diseases, and when they are present simultaneously, they aggravate renal injury.
Hypertension-induced kidney damage in obesity and diabetes follows a very similar sequence of events including increased renal tubular sodium reabsorption as well as RAAS and sympathetic nervous system activation[123-125]. Such an increase in blood pressure along with increased glomerular capillary pressure and GFR are main contributors to the initial renal damage in obesity and diabetic nephropathy[126].
Given the importance of hypertension in worsening renal injury, especially in diabetic nephropathy, many studies have been performed to demonstrate the importance of controlling blood pressure when treating diabetic nephropathy, and the recommended blood pressure is less than 130/80 mmHg[127]. Several clinical trials have also been developed and have demonstrated renal protection when low blood pressure is achieved[128].
Renin-angiotensin system blockade is an important treatment for controlling blood pressure and decreasing proteinuric kidney disease progression[129-131]. Angiotensin II-receptor blocker (ARB) therapy helps prevent the progression from normoalbuminuric (Albumin-Creatinine Ratio < 30 mg/g creatinine) to albuminuric stages (ACR 30-100 mg/g creatinine)[132]. Another important strategy is combined therapy with ARB and ACE inhibitors, which demonstrates a greater decrease in proteinuria than monotherapy[133].
Dyslipidemia is an important component of metabolic syndrome and is often directly related to obesity and diabetes. Patients with diabetic nephropathy usually have several changes in their lipid profile[134], and the presence of increased blood lipid levels is a risk factor for albuminuria[135]. Several studies have demonstrated a correlation between triglyceride and cholesterol levels with renal function markers. Ravid and colleagues[136] observed a significant and positive correlation between total cholesterol and albuminuria in type 2 diabetic patients in a five-year cohort. Similarly, Klein et al[137] noted that type 1 diabetic patients with elevated total cholesterol and low HDL levels also had higher incidence of renal failure.
Although these studies demonstrate significant correlations between dyslipidemia and impaired renal function in diabetic subjects, little is known about the mechanisms by which the increased lipid profile causes kidney damage. Studies have demonstrated lipid deposits in the glomeruli and in the mesangium of obese individuals, suggesting that these lipids may cause kidney damage and lipotoxicity[138]. This glomerular lipotoxicity would be because of renal sterol-regulatory element-binding protein (SREBP-1 and 2) expression, whereas lipotoxicity causes tubulointerstitial fibrosis and inflammation in the proximal tubule epithelial cells[139]. Furthermore, alterations in the coagulation-fibrinolytic system, increased atherosclerosis and endothelial cell damage can also cause or aggravate diabetic nephropathy[140].
Hence, the importance of lipid control in the maintenance of kidney function in diabetic patients has been postulated[141].
Vascular alterations in diabetes are largely due to increased blood glucose levels. Hyperglycemia promotes microvascular injury by several mechanisms. The most important mechanisms are as follows: increased intracellular advanced glycated end product (AGE) formation; interaction between AGEs and their receptors, with consequent disruption of cell signaling and function; constant protein kinase C activation[142]; and increased hexosamine pathway activity[143]. Renal endothelial and mesangial cells are susceptible to such hyperglycemia-induced changes[144]. Thus, the hyperglycemia-induced alterations that occur in the kidney are similar to those described above but generate characteristic damage to renal cells. Because of AGE-driven structural changes in extracellular matrix proteins, metalloproteinases lose their ability to degrade the matrix efficiently, which causes basement membrane thickening[145]. In the mesangium, AGE-induced changes include increased pericyte apoptosis and increased vascular endothelial growth factor expression, and these changes in turn cause glomerular hyperfiltration[146].
Because hyperglycemia causes severe damage to the kidneys and other organs, several studies were developed to demonstrate the importance of glycemic control to prevent diabetic nephropathy. One of these clinical trials, the Diabetes Control and Complications Trial, compared conventional and intensive insulin therapy in type 1 diabetic patients. Over approximately 6.5 years, decreased risks for microalbuminuria and overt nephropathy were observed with intensive glycemic control[147,148]. The Action in Diabetes and Vascular disease: Preterax and Diamicron-MR Controlled Evaluation clinical trial, which was based on type 2 diabetic patients, also observed a reduction in albuminuria and nephropathy progression with insulin therapy intensification in late disease[149].
Most treatments and approaches to reduce kidney injury in obese patients focus on managing associated risk factors such as hypertension, diabetes and hyperlipidemia using strategies as nutritional counseling, pharmacological interference and in some cases, surgery.
Dietary treatment consists on the change in nutritional habits and lifestyle. Eating smaller portions, increasing water consumption, minimizing salt ingestion and practicing physical activities are essential for weight reduction. Such practices can prevent and treat obesity which in turn reduces the risk of CKD. However this is a measure that brings long-term results. Treatment of patients with severe obesity focuses on reduction of proteinuria levels. Currently, several studies point out to the combined therapy of RAS inhibitors (ACE inhibitors and Ang II receptor antagonists); low calories and low salt diets as presumably the best therapeutic options for obese patients with high levels of proteinuria[117].
Weight loss is also an important factor in this treatment regimen. Surgical intervention to treat obesity is a strategic option that can diminish levels of proteinuria in obese patients by mainly reducing hyperfiltration, attenuating obesity-mediated dyslipidemia and insulin resistance, reducing blood pressure and altering adipokine levels such as leptin and adiponectin which have direct a effect on podocytes, therefore improving kidney function[14,151,152]. Even modest weight loss has been associated with a substantial reduction in blood pressure and risk of diabetes[153]. The benefits of bariatric surgery are attributed to sympathetic nervous system suppression, decreasing therefore overall renal sympathetic activity and reduction on sodium reabsorption[154].
Once patients begin to lose weight, longer-term maintenance is difficult and even with continued treatment, patients may regain their normal condition. To prevent this, there is a need for adjunctive therapies for patients who are not able to lose weight or sustain weight loss solely with lifestyle changes[155]. In this scenario, the introduction of pharmacological treatment by the use of, for instance, noradrenergic agents, gastrointestinal lipase inhibitors and serotonin receptor agonists become an alternative and efficient strategy towards weight loss[156].
Obesity has great influence on end-stage renal disease, and it can be either the cause of renal alterations and kidney injury or an aggravating factor when other conditions such as hypertension and diabetes are established. All of these factors represent severe insults to the kidney, resulting in high costs to health systems to manage dialysis patients as well as those with post- cardiovascular events. Therefore, studies that relate these factors are important for developing new strategies to treat obese patients with renal disease to reduce patient mortality and improve quality of life.
P- Reviewer: Aramwit P, Landry DL S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Jones Nielsen JD, Laverty AA, Millett C, Mainous AG, Majeed A, Saxena S. Rising obesity-related hospital admissions among children and young people in England: national time trends study. PLoS One. 2013;8:e65764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2601] [Cited by in RCA: 2471] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 3. | Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1088] [Cited by in RCA: 1162] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 4. | Pickkers P, de Keizer N, Dusseljee J, Weerheijm D, van der Hoeven JG, Peek N. Body mass index is associated with hospital mortality in critically ill patients: an observational cohort study. Crit Care Med. 2013;41:1878-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 5. | Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 570] [Cited by in RCA: 541] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 6. | Marchesini G, Moscatiello S, Di Domizio S, Forlani G. Obesity-associated liver disease. J Clin Endocrinol Metab. 2008;93:S74-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 7. | Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol (1985). 2010;108:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 471] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 8. | Alicic RZ, Patakoti R, Tuttle KR. Direct and indirect effects of obesity on the kidney. Adv Chronic Kidney Dis. 2013;20:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Friedman AN, Miskulin DC, Rosenberg IH, Levey AS. Demographics and trends in overweight and obesity in patients at time of kidney transplantation. Am J Kidney Dis. 2003;41:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Kramer HJ, Saranathan A, Luke A, Durazo-Arvizu RA, Guichan C, Hou S, Cooper R. Increasing body mass index and obesity in the incident ESRD population. J Am Soc Nephrol. 2006;17:1453-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 984] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 12. | Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 903] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 13. | Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17:1695-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 462] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 14. | Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14:1480-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 388] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 15. | Kataoka H, Ohara M, Shibui K, Sato M, Suzuki T, Amemiya N, Watanabe Y, Honda K, Mochizuki T, Nitta K. Overweight and obesity accelerate the progression of IgA nephropathy: prognostic utility of a combination of BMI and histopathological parameters. Clin Exp Nephrol. 2012;16:706-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3146] [Cited by in RCA: 3638] [Article Influence: 202.1] [Reference Citation Analysis (0)] |
| 17. | He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension. 2000;35:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 226] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Othman M, Kawar B, El Nahas AM. Influence of obesity on progression of non-diabetic chronic kidney disease: a retrospective cohort study. Nephron Clin Pract. 2009;113:c16-c23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Malyszko J, Malyszko JS, Mysliwiec M. Visfatin, a new adipocytokine, is predominantly related to inflammation/endothelial damage in kidney allograft recipients. Transplant Proc. 2009;41:150-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 2502] [Article Influence: 119.1] [Reference Citation Analysis (0)] |
| 21. | Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137-1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1994] [Cited by in RCA: 1957] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 22. | Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2565] [Cited by in RCA: 2743] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 23. | Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araújo EP, Vassallo J, Curi R, Velloso LA. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 629] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 24. | Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2148] [Cited by in RCA: 2049] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 25. | Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci USA. 2011;108:15324-15329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 579] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 26. | Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 1769] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 27. | Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 612] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 28. | Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3164] [Cited by in RCA: 3572] [Article Influence: 198.4] [Reference Citation Analysis (0)] |
| 29. | Prieur X, Mok CY, Velagapudi VR, Núñez V, Fuentes L, Montaner D, Ishikawa K, Camacho A, Barbarroja N, O’Rahilly S. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60:797-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 276] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 30. | Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O’Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648-1656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 474] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 31. | Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1139] [Cited by in RCA: 1063] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 32. | Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 797] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 33. | Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab. 2012;23:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 34. | Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1571] [Cited by in RCA: 1718] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 35. | Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1155] [Cited by in RCA: 1101] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 36. | Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl. 2002;S12-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 302] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 37. | Ikezumi Y, Suzuki T, Karasawa T, Kawachi H, Nikolic-Paterson DJ, Uchiyama M. Activated macrophages down-regulate podocyte nephrin and podocin expression via stress-activated protein kinases. Biochem Biophys Res Commun. 2008;376:706-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Patel NS, Chatterjee PK, Di Paola R, Mazzon E, Britti D, De Sarro A, Cuzzocrea S, Thiemermann C. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J Pharmacol Exp Ther. 2005;312:1170-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 39. | Tomiyama-Hanayama M, Rakugi H, Kohara M, Mima T, Adachi Y, Ohishi M, Katsuya T, Hoshida Y, Aozasa K, Ogihara T. Effect of interleukin-6 receptor blockage on renal injury in apolipoprotein E-deficient mice. Am J Physiol Renal Physiol. 2009;297:F679-F684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Ziyadeh FN. Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J Am Soc Nephrol. 2004;15 Suppl 1:S55-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 342] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 41. | Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 517] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 42. | Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne). 2013;4:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 428] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 43. | Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension. 2013;61:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 44. | Li B, Shi Z, Cassaglia PA, Brooks VL. Leptin acts in the forebrain to differentially influence baroreflex control of lumbar, renal, and splanchnic sympathetic nerve activity and heart rate. Hypertension. 2013;61:812-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Young CN, Morgan DA, Butler SD, Mark AL, Davisson RL. The brain subfornical organ mediates leptin-induced increases in renal sympathetic activity but not its metabolic effects. Hypertension. 2013;61:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Ceccarini G, Flavell RR, Butelman ER, Synan M, Willnow TE, Bar-Dagan M, Goldsmith SJ, Kreek MJ, Kothari P, Vallabhajosula S. PET imaging of leptin biodistribution and metabolism in rodents and primates. Cell Metab. 2009;10:148-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Widjaja A, Kielstein JT, Horn R, von zur Mühlen A, Kliem V, Brabant G. Free serum leptin but not bound leptin concentrations are elevated in patients with end-stage renal disease. Nephrol Dial Transplant. 2000;15:846-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Sharma K, Considine RV, Michael B, Dunn SR, Weisberg LS, Kurnik BR, Kurnik PB, O’Connor J, Sinha M, Caro JF. Plasma leptin is partly cleared by the kidney and is elevated in hemodialysis patients. Kidney Int. 1997;51:1980-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 157] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 49. | Frühbeck G, Gómez-Ambrosi J, Martínez JA. Pre- and postprandial expression of the leptin receptor splice variants OB-Ra and OB-Rb in murine peripheral tissues. Physiol Res. 1999;48:189-195. [PubMed] |
| 50. | Wolf G, Hamann A, Han DC, Helmchen U, Thaiss F, Ziyadeh FN, Stahl RA. Leptin stimulates proliferation and TGF-beta expression in renal glomerular endothelial cells: potential role in glomerulosclerosis [seecomments]. Kidney Int. 1999;56:860-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 261] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 855] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 52. | Wolf G, Ziyadeh FN. Leptin and renal fibrosis. Contrib Nephrol. 2006;151:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 53. | Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3416] [Cited by in RCA: 3426] [Article Influence: 131.8] [Reference Citation Analysis (0)] |
| 54. | Chow WS, Cheung BM, Tso AW, Xu A, Wat NM, Fong CH, Ong LH, Tam S, Tan KC, Janus ED. Hypoadiponectinemia as a predictor for the development of hypertension: a 5-year prospective study. Hypertension. 2007;49:1455-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 55. | Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 895] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 56. | Hattori Y, Akimoto K, Gross SS, Hattori S, Kasai K. Angiotensin-II-induced oxidative stress elicits hypoadiponectinaemia in rats. Diabetologia. 2005;48:1066-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 370] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 58. | Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1564] [Cited by in RCA: 1532] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 59. | Otabe S, Yuan X, Fukutani T, Wada N, Hashinaga T, Nakayama H, Hirota N, Kojima M, Yamada K. Overexpression of human adiponectin in transgenic mice results in suppression of fat accumulation and prevention of premature death by high-calorie diet. Am J Physiol Endocrinol Metab. 2007;293:E210-E218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Perri A, Vizza D, Lofaro D, Gigliotti P, Leone F, Brunelli E, Malivindi R, De Amicis F, Romeo F, De Stefano R. Adiponectin is expressed and secreted by renal tubular epithelial cells. J Nephrol. 2013;26:1049-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Yano Y, Hoshide S, Ishikawa J, Hashimoto T, Eguchi K, Shimada K, Kario K. Differential impacts of adiponectin on low-grade albuminuria between obese and nonobese persons without diabetes. J Clin Hypertens (Greenwich). 2007;9:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 62. | Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 298] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 63. | Ohashi K, Iwatani H, Kihara S, Nakagawa Y, Komura N, Fujita K, Maeda N, Nishida M, Katsube F, Shimomura I. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:1910-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 64. | Rutkowski JM, Wang ZV, Park AS, Zhang J, Zhang D, Hu MC, Moe OW, Susztak K, Scherer PE. Adiponectin promotes functional recovery after podocyte ablation. J Am Soc Nephrol. 2013;24:268-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 65. | Kulshrestha S, Ojo AO, Luan FL. Metabolic syndrome, vitamin D deficiency and hypoadiponectinemia among nondiabetic patients early after kidney transplantation. Am J Nephrol. 2013;37:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Menon V, Li L, Wang X, Greene T, Balakrishnan V, Madero M, Pereira AA, Beck GJ, Kusek JW, Collins AJ. Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2599-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 217] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 67. | Alam A, Molnar MZ, Czira ME, Rudas A, Ujszaszi A, Kalantar-Zadeh K, Rosivall L, Mucsi I. Serum adiponectin levels and mortality after kidney transplantation. Clin J Am Soc Nephrol. 2013;8:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Jin X, Chen J, Hu Z, Chan L, Wang Y. Genetic deficiency of adiponectin protects against acute kidney injury. Kidney Int. 2013;83:604-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 69. | Cheng CF, Lian WS, Chen SH, Lai PF, Li HF, Lan YF, Cheng WT, Lin H. Protective effects of adiponectin against renal ischemia-reperfusion injury via prostacyclin-PPARα-heme oxygenase-1 signaling pathway. J Cell Physiol. 2012;227:239-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 70. | Idorn T, Hornum M, Bjerre M, Jørgensen KA, Nielsen FT, Hansen JM, Flyvbjerg A, Feldt-Rasmussen B. Plasma adiponectin before and after kidney transplantation. Transpl Int. 2012;25:1194-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Axelsson J, Bergsten A, Qureshi AR, Heimbürger O, Bárány P, Lönnqvist F, Lindholm B, Nordfors L, Alvestrand A, Stenvinkel P. Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney Int. 2006;69:596-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 72. | Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3205] [Cited by in RCA: 3210] [Article Influence: 133.8] [Reference Citation Analysis (1)] |
| 73. | Kang YS, Song HK, Lee MH, Ko GJ, Han JY, Han SY, Han KH, Kim HK, Cha DR. Visfatin is upregulated in type-2 diabetic rats and targets renal cells. Kidney Int. 2010;78:170-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 74. | Mu J, Feng B, Ye Z, Yuan F, Zeng W, Luo Z, Qi W. Visfatin is related to lipid dysregulation, endothelial dysfunction and atherosclerosis in patients with chronic kidney disease. J Nephrol. 2011;24:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Yilmaz MI, Saglam M, Carrero JJ, Qureshi AR, Caglar K, Eyileten T, Sonmez A, Cakir E, Yenicesu M, Lindholm B. Serum visfatin concentration and endothelial dysfunction in chronic kidney disease. Nephrol Dial Transplant. 2008;23:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 76. | Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U. Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol. 2000;278:F817-F822. [PubMed] |
| 77. | Pecly IM, Genelhu V, Francischetti EA. Renal functional reserve in obesity hypertension. Int J Clin Pract. 2006;60:1198-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Krikken JA, Lely AT, Bakker SJ, Navis G. The effect of a shift in sodium intake on renal hemodynamics is determined by body mass index in healthy young men. Kidney Int. 2007;71:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 79. | Chagnac A, Herman M, Zingerman B, Erman A, Rozen-Zvi B, Hirsh J, Gafter U. Obesity-induced glomerular hyperfiltration: its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol Dial Transplant. 2008;23:3946-3952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 80. | Kasiske BL, Napier J. Glomerular sclerosis in patients with massive obesity. Am J Nephrol. 1985;5:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005;16:2953-2966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 254] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 82. | Kriz W, Elger M, Mundel P, Lemley KV. Structure-stabilizing forces in the glomerular tuft. J Am Soc Nephrol. 1995;5:1731-1739. [PubMed] |
| 83. | Afshinnia F, Wilt TJ, Duval S, Esmaeili A, Ibrahim HN. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant. 2010;25:1173-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 84. | Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005-3015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 515] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 85. | Kriz W. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech. 2002;57:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 86. | Laurens WE, Vanrenterghem YF, Steels PS, Van Damme BJ. A new single nephron model of focal and segmental glomerulosclerosis in the Munich-Wistar rat. Kidney Int. 1994;45:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Barisoni L, Kriz W, Mundel P, D’Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51-61. [PubMed] |
| 88. | Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 2010;21:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 706] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 89. | Kaissling B, Lehir M, Kriz W. Renal epithelial injury and fibrosis. Biochim Biophys Acta. 2013;1832:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 90. | Matsusaka T, Sandgren E, Shintani A, Kon V, Pastan I, Fogo AB, Ichikawa I. Podocyte injury damages other podocytes. J Am Soc Nephrol. 2011;22:1275-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 91. | Nagata M, Kriz W. Glomerular damage after uninephrectomy in young rats. II. Mechanical stress on podocytes as a pathway to sclerosis. Kidney Int. 1992;42:148-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 172] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 92. | Van Beneden K, Geers C, Pauwels M, Mannaerts I, Verbeelen D, van Grunsven LA, Van den Branden C. Valproic acid attenuates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:1863-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 93. | Pereira RL, Buscariollo BN, Corrêa-Costa M, Semedo P, Oliveira CD, Reis VO, Maquigussa E, Araújo RC, Braga TT, Soares MF. Bradykinin receptor 1 activation exacerbates experimental focal and segmental glomerulosclerosis. Kidney Int. 2011;79:1217-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 94. | Zhuo JL, Li XC. New insights and perspectives on intrarenal renin-angiotensin system: focus on intracrine/intracellular angiotensin II. Peptides. 2011;32:1551-1565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 95. | Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 892] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 96. | Rüster C, Wolf G. Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J Am Soc Nephrol. 2011;22:1189-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 97. | Massiera F, Seydoux J, Geloen A, Quignard-Boulange A, Turban S, Saint-Marc P, Fukamizu A, Negrel R, Ailhaud G, Teboul M. Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology. 2001;142:5220-5225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Yvan-Charvet L, Even P, Bloch-Faure M, Guerre-Millo M, Moustaid-Moussa N, Ferre P, Quignard-Boulange A. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes. 2005;54:991-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 99. | Kouyama R, Suganami T, Nishida J, Tanaka M, Toyoda T, Kiso M, Chiwata T, Miyamoto Y, Yoshimasa Y, Fukamizu A. Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinology. 2005;146:3481-3489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 100. | Wolf G. Link between angiotensin II and TGF-beta in the kidney. Miner Electrolyte Metab. 1998;24:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 101. | Sharma K, Cook A, Smith M, Valancius C, Inscho EW. TGF-beta impairs renal autoregulation via generation of ROS. Am J Physiol Renal Physiol. 2005;288:F1069-F1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 102. | Ren Z, Liang W, Chen C, Yang H, Singhal PC, Ding G. Angiotensin II induces nephrin dephosphorylation and podocyte injury: role of caveolin-1. Cell Signal. 2012;24:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 103. | Brinkkoetter PT, Holtgrefe S, van der Woude FJ, Yard BA. Angiotensin II type 1-receptor mediated changes in heparan sulfate proteoglycans in human SV40 transformed podocytes. J Am Soc Nephrol. 2004;15:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Yasue S, Masuzaki H, Okada S, Ishii T, Kozuka C, Tanaka T, Fujikura J, Ebihara K, Hosoda K, Katsurada A. Adipose tissue-specific regulation of angiotensinogen in obese humans and mice: impact of nutritional status and adipocyte hypertrophy. Am J Hypertens. 2010;23:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 105. | Engeli S, Böhnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 457] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 106. | Massiéra F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 333] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 107. | Myers MG, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21:643-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 587] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 108. | Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, Yoshida D, Shimamoto K. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 346] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 109. | Bochud M, Nussberger J, Bovet P, Maillard MR, Elston RC, Paccaud F, Shamlaye C, Burnier M. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 110. | Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Corrêa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 111. | Ehrhart-Bornstein M, Arakelyan K, Krug AW, Scherbaum WA, Bornstein SR. Fat cells may be the obesity-hypertension link: human adipogenic factors stimulate aldosterone secretion from adrenocortical cells. Endocr Res. 2004;30:865-870. [PubMed] |
| 112. | Nishiyama A, Abe Y. Molecular mechanisms and therapeutic strategies of chronic renal injury: renoprotective effects of aldosterone blockade. J Pharmacol Sci. 2006;100:9-16. [PubMed] |
| 113. | Kawarazaki W, Nagase M, Yoshida S, Takeuchi M, Ishizawa K, Ayuzawa N, Ueda K, Fujita T. Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J Am Soc Nephrol. 2012;23:997-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 114. | Luther JM, Luo P, Wang Z, Cohen SE, Kim HS, Fogo AB, Brown NJ. Aldosterone deficiency and mineralocorticoid receptor antagonism prevent angiotensin II-induced cardiac, renal, and vascular injury. Kidney Int. 2012;82:643-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 115. | de Paula RB, da Silva AA, Hall JE. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension. 2004;43:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 116. | Lambers Heerspink HJ, de Borst MH, Bakker SJ, Navis GJ. Improving the efficacy of RAAS blockade in patients with chronic kidney disease. Nat Rev Nephrol. 2013;9:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 117. | Slagman MC, Waanders F, Hemmelder MH, Woittiez AJ, Janssen WM, Lambers Heerspink HJ, Navis G, Laverman GD. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomised controlled trial. BMJ. 2011;343:d4366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 118. | Aldigier JC, Kanjanbuch T, Ma LJ, Brown NJ, Fogo AB. Regression of existing glomerulosclerosis by inhibition of aldosterone. J Am Soc Nephrol. 2005;16:3306-3314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 119. | Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol. 2004;286:F8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 120. | Hostetter TH. Hyperfiltration and glomerulosclerosis. Semin Nephrol. 2003;23:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 121. | Kopple JD, Feroze U. The effect of obesity on chronic kidney disease. J Ren Nutr. 2011;21:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 122. | Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 123. | Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12:1211-1217. [PubMed] |
| 124. | Kotchen TA. Obesity-related hypertension: epidemiology, pathophysiology, and clinical management. Am J Hypertens. 2010;23:1170-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 125. | Hall JE, Henegar JR, Dwyer TM, Liu J, Da Silva AA, Kuo JJ, Tallam L. Is obesity a major cause of chronic kidney disease? Adv Ren Replace Ther. 2004;11:41-54. [PubMed] |
| 126. | Maric-Bilkan C. Obesity and diabetic kidney disease. Med Clin North Am. 2013;97:59-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 127. | Van Buren PN, Toto R. Hypertension in diabetic nephropathy: epidemiology, mechanisms, and management. Adv Chronic Kidney Dis. 2011;18:28-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 128. | Grossman E, Messerli FH. Management of blood pressure in patients with diabetes. Am J Hypertens. 2011;24:863-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 129. | Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5059] [Cited by in RCA: 5069] [Article Influence: 211.2] [Reference Citation Analysis (0)] |
| 130. | Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3760] [Cited by in RCA: 3555] [Article Influence: 111.1] [Reference Citation Analysis (2)] |
| 131. | Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4068] [Cited by in RCA: 3988] [Article Influence: 166.2] [Reference Citation Analysis (1)] |
| 132. | Haller H, Ito S, Izzo JL, Januszewicz A, Katayama S, Menne J, Mimran A, Rabelink TJ, Ritz E, Ruilope LM. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 555] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 133. | Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148:30-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 443] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 134. | Shoji T, Emoto M, Kawagishi T, Kimoto E, Yamada A, Tabata T, Ishimura E, Inaba M, Okuno Y, Nishizawa Y. Atherogenic lipoprotein changes in diabetic nephropathy. Atherosclerosis. 2001;156:425-433. [PubMed] |
| 135. | Rutledge JC, Ng KF, Aung HH, Wilson DW. Role of triglyceride-rich lipoproteins in diabetic nephropathy. Nat Rev Nephrol. 2010;6:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 136. | Ravid M, Neumann L, Lishner M. Plasma lipids and the progression of nephropathy in diabetes mellitus type II: effect of ACE inhibitors. Kidney Int. 1995;47:907-910. [PubMed] |
| 137. | Klein R, Klein BE, Moss SE, Cruickshanks KJ, Brazy PC. The 10-year incidence of renal insufficiency in people with type 1 diabetes. Diabetes Care. 1999;22:743-751. [PubMed] |
| 138. | Griffin KA, Kramer H, Bidani AK. Adverse renal consequences of obesity. Am J Physiol Renal Physiol. 2008;294:F685-F696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 139. | Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, Lucia MS, Li J, Levi M. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280:32317-32325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 293] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 140. | Misra A, Kumar S, Kishore Vikram N, Kumar A. The role of lipids in the development of diabetic microvascular complications: implications for therapy. Am J Cardiovasc Drugs. 2003;3:325-338. [PubMed] |
| 141. | Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 450] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 142. | Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859-866. [PubMed] |
| 143. | Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J Clin Invest. 1998;101:160-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 290] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 144. | Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615-1625. [PubMed] |
| 145. | Thomas MC, Forbes JM, Cooper ME. Advanced glycation end products and diabetic nephropathy. Am J Ther. 2005;12:562-572. [PubMed] |
| 146. | Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol. 2003;162:1123-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 426] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 147. | The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. The DCCT Research Group. Diabetes. 1986;35:530-545. [PubMed] |
| 148. | The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16271] [Article Influence: 508.5] [Reference Citation Analysis (3)] |
| 149. | Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4759] [Cited by in RCA: 4888] [Article Influence: 287.5] [Reference Citation Analysis (0)] |
| 150. | Amann K, Benz K. Structural renal changes in obesity and diabetes. Semin Nephrol. 2013;33:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 151. | Currie A, Chetwood A, Ahmed AR. Bariatric surgery and renal function. Obes Surg. 2011;21:528-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 152. | Schuster DP, Teodorescu M, Mikami D, Foreman K, Rogers P, Needleman BJ. Effect of bariatric surgery on normal and abnormal renal function. Surg Obes Relat Dis. 2011;7:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 153. | Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1082] [Cited by in RCA: 1246] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 154. | van de Borne P, Watrin I, Bouquegneau M, Gilles A, Houben JJ, Fery F, Degaute JP. Ambulatory blood pressure and neuroendocrine control after diet-assisted gastric restrictive surgery. J Hypertens. 2000;18:301-306. [PubMed] |
| 155. | Middleton KM, Patidar SM, Perri MG. The impact of extended care on the long-term maintenance of weight loss: a systematic review and meta-analysis. Obes Rev. 2012;13:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 156. | Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 700] [Cited by in RCA: 597] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 157. | Hoogeveen EK, Halbesma N, Rothman KJ, Stijnen T, van Dijk S, Dekker FW, Boeschoten EW, de Mutsert R. Obesity and mortality risk among younger dialysis patients. Clin J Am Soc Nephrol. 2012;7:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 158. | Hoogeveen EK, Aalten J, Rothman KJ, Roodnat JI, Mallat MJ, Borm G, Weimar W, Hoitsma AJ, de Fijter JW. Effect of obesity on the outcome of kidney transplantation: a 20-year follow-up. Transplantation. 2011;91:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 159. | Palaniappan L, Carnethon M, Fortmann SP. Association between microalbuminuria and the metabolic syndrome: NHANES III. Am J Hypertens. 2003;16:952-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 219] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 160. | Gomez P, Ruilope LM, Barrios V, Navarro J, Prieto MA, Gonzalez O, Guerrero L, Zamorano MA, Filozof C. Prevalence of renal insufficiency in individuals with hypertension and obesity/overweight: the FATH study. J Am Soc Nephrol. 2006;17:S194-S200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 161. | Tozawa M, Iseki K, Iseki C, Oshiro S, Ikemiya Y, Takishita S. Influence of smoking and obesity on the development of proteinuria. Kidney Int. 2002;62:956-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 166] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 162. | Meier-Kriesche HU, Arndorfer JA, Kaplan B. The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation. 2002;73:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 394] [Article Influence: 17.1] [Reference Citation Analysis (0)] |